Novel compatible system of [C2OHmim][OAc]-cellulases for the in situ hydrolysis of lignocellulosic biomass
Lu
Li
a,
Juan
Xie
a,
Shitao
Yu
*a,
Zhongliang
Su
a,
Shiwei
Liu
a,
Fusheng
Liu
a,
Congxia
Xie
b and
Baoquan
Zhang
c
aCollege of Chemical Engineering, Qingdao University of Science and Technology, 53 Zhengzhou Road, Qingdao 266042, P. R. China. E-mail: yushitaoqust@126.com; Fax: +86 0532 84022719; Tel: +86 0532 84022864
bKey Laboratory of Eco-Chemical Engineering, Ministry of Education, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, 53 Zhengzhou Road, Qingdao 266042, P. R. China
cCollege of Materials Science and Engineering, Qingdao University of Science and Technology, 53 Zhengzhou Road, Qingdao 266042, P. R. China
First published on 27th September 2012
Abstract
The ionic liquid (IL) 1-(2-hydroxyethyl)-3-methylimidazolium acetate ([C2OHmim][OAc]) was designed and used to provide a compatible IL-cellulase system in which lignocellulosic biomass can be effectively solubilized and activated, while maintaining the high stability and activity of the cellulase. In this study, we investigated the stability and activity of a commercially available cellulase (optimum pH 4.8) in the presence of different concentrations of a [C2OHmim][OAc]/buffer solution, using CMC (carboxy-methylcellulose) as substrate. The cellulase retained 87% and 79% of its original activity after being pre-incubated in 15% (pH = 5.79) and 20% (pH = 6.0) (w/v) IL solutions at 50 °C for 1.5 h, respectively. Similar results obtained for both the IL and traditional buffer solutions led us to conclude that the reduction in the enzymatic activity may have been caused by the deviation of the solutions' pH from the optimum pH for cellulase; the IL showed improved enzymatic hydrolysis of cellulose compared with the traditional buffer solution. The cellulases also retained high activity in [C2OHmim][OAc] (15%) to hydrolyze MCC (microcrystalline cellulose), a model substrate for cellulase analysis, with a conversion efficiency of approximately 99.67%. Using this IL-cellulase system, the saccharification of straw, cotton and filter paper, which have different compositions of cellulose, hemicellulose and ligin, was also significantly improved during the first hour of enzymatic hydrolysis. The IL can be separated from the reducing sugar using affinity solvent extraction, and can then be reused. Together, these findings provide compelling evidence that [C2OHmim][OAc] was compatible with the cellulase, and this compatible IL-cellulase system is promising for the efficient activation and hydrolysis of native biomass to produce biofuels.
1. Introduction
Ionic liquids (ILs) have emerged as exceptionally interesting non-aqueous reaction media for enzymatic transformations because of their unique solvent properties, their independence from the effects of vapor pressure, their ease of recycling, 1 and their exceptional ability to maintain enzymes in active and stable conformations.2 In 2002, Rogers published a pioneering work showing that some ILs, including 1-butyl-3-methylimidazolium chloride ([Bmim][Cl]), 1-hexyl-3-methylimidazolium chloride ([Hmim][Cl]), were able to dissolve cellulose, consistent with reports using 1-allyl-3-methylimidazolium chloride ([Amim]Cl).1,3 This discovery has opened new opportunities for the use of large amounts of waste cellulose-containing materials (e.g., forest biomass); the depolymerization of cellulose into its glucose units, followed by their transformation into bioethanol through fermentation, is one of the most important processes in which this could have an impact.4 Recently, several studies have demonstrated that ILs can effectively solubilize lignocellulosic biomass such as switchgrass, poplar, pine wood, wheat straw, and corn cobs,5 suggesting their potential for the pretreatment of lignocellulosics for biofuels and co-product production. However, it has been widely reported that ILs that are excellent for dissolving cellulose—such as [Bmim][Cl], [Amim][Cl], etc.—induce fast enzyme deactivation by protein unfolding.6 The reason for this might be that the high Cl− ion concentration in [Bmim][Cl] or [Amim][Cl] is similar to that in concentrated brine, and denatures the enzyme.7,8There is no doubt that the ability to perform cellulase-catalyzed reactions in cellulose solutions is highly desirable. Cellulase provides the highest selectivity for the hydrolysis of the β-glycosidic bonds of the cellulose backbone, compared with any other chemical catalyst (i.e., acids, alkalis, etc.).9 In this context, one approach to solve this problem has been to use an extensive process to clean regenerated cellulose, to remove residual ILs.10 However, the cleaning protocol is cumbersome and expensive.8 To avoid the extensive clean-up process, it is critical to develop a compatible IL-cellulase system in which the IL is able to effectively solubilize and activate the lignocellulosic biomass, and the cellulase still remains stable and highly active. The feasibility of such a system is supported by a previous study showing that [C2mim][OAc] (1-ethyl-3-methylimidazolium acetate) performs well, as mentioned above.11 Although [C2mim][OAc] provides conditions compatible with the enzymatic in situ saccharification of yellow poplar, the pretreatment of lignocellulosic biomass must be carried out before the enzymatic saccharification, and the time taken for this pretreatment is more than 24 h.11 To improve the solubility for lignocellulosic biomass, and to lessen the inconvenience of pretreatment, new ILs must be designed. Feng et al. suggested that the hydroxyl group in the cation side chain could form hydrogen bonds with cellulose hydroxyl groups, thus enhancing the solubility of cellulose, allowing more to be dissolved.12 In addition, Zhao pointed out that hydrogen bond-forming anions, oxygen-containing cations, and low cation bulkiness are beneficial for the dissolution of carbohydrates, while low anion concentrations are necessary for enzyme stabilization.13 Based on this idea, we designed [C2OHmim][OAc], in which the –OH group is in the cation; this was done to improve the solubility of cellulose and the compatibility with cellulase, compared with [C2mim][OAc]. Due to the mechanism of solubility for lignocellulosic biomass and the compatibility with cellulase, this design was able to effectively realize a high solubility for lignocellulosic biomass while maintaining excellent compatibility with cellulase.
As discussed earlier, it has been determined that the primary mechanism of cellulose dissolution by ILs is the basicity of the anion, which disrupts the inter- and intramolecular hydrogen bonds in cellulose.14 However, the optimum pH of commercial grade cellulase is approximately 4.8, which means that cellulase has excellent activity in weak acidic conditions. Based on the above analysis, the pH of the IL-cellulose solution is a main factor affecting the activity of cellulase. Therefore, in this study, the cellulase activity at various pH values and in various systems (traditional buffer solution and ILs) was investigated. For the same pH value, the relative activity of cellulase was higher in [C2OHmim][OAc]/cellulose than in traditional buffer solution. The enzymatic hydrolysis of native biomass such as straw, cotton and filter paper, was also researched in the [C2OHmim][OAc]/cellulose system. The IL could be separated from the reducing sugar and reused, using affinity solvent extraction. Together, these findings provide compelling evidence that [C2OHmim][OAc] was compatible with cellulase, and this compatible IL-cellulase system holds promise for the efficient activation and hydrolysis of native biomass to produce biofuels.
2. Experimental
2.1 Materials and equipment
1-methylimidazole, 1-chloroethanol, potassium acetate, ethanol, acetone, citric acid monohydrate, sodium citrate, 3,5-dinitrosalicacid (DNS), sodium hydroxide, sodium potassium tartrate, phenol, sodium hydrogensulfite, carboxymethylcellulose (CMC), and microcrystalline cellulose (MCC, average molecular weight: 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were purchased from Sigma-Aldrich (St. Louis, MO), and all materials were used directly after drying without further purification. Cellulase (powder, 1800 units/mg solid, from Shanghai Source Leaf Biological Technology Co., LTD) activity was expressed as CMC units per mg of original enzyme, determined using a standard protocol from the National Renewable Energy Laboratory (NREL). Straw, cotton, and filter paper, supplied by our laboratory, were ground using a Wiley mill fitted with a 40-mesh screen. After grinding, an exhaustive extraction process using water followed by ethanol was performed (using the procedure documented by NREL), to remove non-structural extractives. The physical parameters of these natural celluloses are shown in Table 1.
000) were purchased from Sigma-Aldrich (St. Louis, MO), and all materials were used directly after drying without further purification. Cellulase (powder, 1800 units/mg solid, from Shanghai Source Leaf Biological Technology Co., LTD) activity was expressed as CMC units per mg of original enzyme, determined using a standard protocol from the National Renewable Energy Laboratory (NREL). Straw, cotton, and filter paper, supplied by our laboratory, were ground using a Wiley mill fitted with a 40-mesh screen. After grinding, an exhaustive extraction process using water followed by ethanol was performed (using the procedure documented by NREL), to remove non-structural extractives. The physical parameters of these natural celluloses are shown in Table 1.
The NMR spectra of the ionic liquid were recorded in DMSO or CDCl3 using a 500 MHz Bruker spectrometer, and a calibration was performed with tetramethylsilane (TMS) as the internal reference. FT-IR spectra were recorded using a Nicolet-510P instrument. The spectra were collected over the range of 4000–400 cm−1. Scanning electron microscopy (SEM) was performed using a HITACHI S-2600HS instrument, with a 15 kV accelerating voltage. Before the observations, the samples were coated with gold using ion sputtering, using a JEOL JFC-1100-E with a current of 10 mA, for 90 s. X-ray powder diffraction patterns were obtained for the samples on an XB-3A instrument operated at 40 kV and 100 mA, using monochromatic Cu-Kα radiation (λ = 0.15418 nm). The experimental conditions corresponded to a step width of 0.02° and a scan speed of 2θ min−1, in the diffraction region of 2θ = ∼10–60°. The decomposition temperatures (Tdec) were determined using thermogravimetric analysis (NETZSCH STA 409PC, Germany) from 25 °C to 450 °C, at a heating rate of 10 °C min−1. The Tdec was taken as the temperature at the 10% weight loss point on the TG chart. The reaction products were monitored using an Agilent 1200 HPLC equipped with Varian 380-LC Evaporative Light Scattering Detector. The unfolding temperature of the cellulase was determined using a differential scanning calorimeter (DSC), Calorimetry Sciences Corporation.
2.2 Synthesis of 1-ethoxyl-3-methylimidazolium acetate ([C2OHmim][OAc])
[C2OHmim][OAc] was synthesized according to the process illustrated in Scheme 1. 1-Chloroethanol (0.5 mol, 40.25 g) and 1-methylimidazole (0.5 mol, 41.05 g) were added to a round-bottomed flask fitted with a reflux condenser, and were heated for 8 h at 80 °C, under stirring. The reaction liquid was washed with acetone (3 × 50 mL), heated at 80 °C, and then stirred under vacuum (0.5 mmHg) for 6 h. The product [C2OHmim]Cl was obtained as a slightly yellow liquid, which solidified on cooling (80.0 g, 98%). [C2OHmim]Cl (0.5 mol, 80 g) and potassium acetate (0.55 mol, 51.80 g) were dissolved in 200 mL of alcohol, and then were added to a round-bottomed flask fitted with a reflux condenser; they were then heated for 5 h at 50 °C under stirring, to achieve ion-exchange. The generated and unreacted salt was removed via filtration, and the filtrate was washed with acetone (3 × 100 mL). The completeness of the washing was checked by adding a silver nitrate solution to a solution of the IL in water.15 The organic layer was collected and filtered, and the solvent was removed under vacuum, giving [C2OHmim][OAc] (92.68 g 99.95%). The ionic liquid [C2OHmim] [OAc] was stored with a desiccant and under vacuum, to limit the absorption of water. The synthetic process is illustrated in Scheme 1. The structure of the ILs was verified using 1H-NMR, 13C-NMR, FT-IR, and thermogravimetry/differential thermal analysis (TG/DTA). FT-IR (KBr cm−1): 3364.38, 3153.41, 1571.93, 1400.13, 1252.94, 1169.00, 1076.42; 1H-NMR (500 MHz, D2O, ppm): δ 8.57 (s, H), 7.30–7.25 (d, 3H), 4.09–3.34 (d, 10H); 13C-NMR (500 MHz, D2O, ppm): δ 182.82, 126.18, 62.29, 54.20, 38.35, 26.13. According to thermogravimetry/differential thermal analysis (TG/DTA) of [C2OHmim][OAc], the decomposition temperature, determined at 10% weight loss, was approximately 255 °C. This decomposition temperature was high enough to allow the dissolution process of cellulose.16![The synthesis of [C2OHmim][OAc].](/image/article/2012/RA/c2ra22128d/c2ra22128d-s1.gif) | ||
| Scheme 1 The synthesis of [C2OHmim][OAc]. | ||
2.3 Dissolution of cellulose in ionic liquids
10 g of pure and dry [C2OHmim][OAc] was added to a 50 ml flask, and dry MCC, straw, filter paper, and cotton were added slowly (in portions of 1 wt% of the IL weight each time), to allow the solids to dissolve. The dissolution process was observed using a polarizing microscope. When the MCC was completely dissolved in the IL, a black field could be observed through a polarizing microscope (Fig. 1).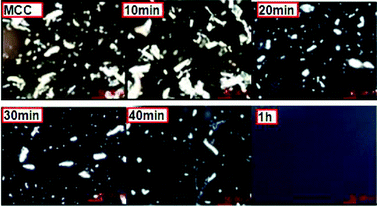 | ||
| Fig. 1 General morphology of MCC dissolution under polarizing microscope. | ||
2.4 Regeneration of cellulose from ionic liquids
The ionic liquid described in this paper was water soluble, so we choose deionized water as the solvent to achieve the regeneration of cellulose. The cellulose-ionic liquid solution was dried naturally in a well-ventilated place at room temperature for 6 h, after repeated washing with deionized water. The regenerated cellulose was placed in a vacuum drying oven and dried for 12 h at 30 °C, after which the surface water of the regenerated cellulose had completely evaporated. Ultimately, the regenerated cellulose was obtained as a light yellow powder, and was placed in a vacuum drying oven for storage.2.5 Thermostability of cellulases in [C2OHmim][OAc]
Samples with a total volume of 20 mL were prepared, containing the cellulase (10 mg) and various concentrations (0%, 5%, 10%, 15%, 20% and 25% v/v) of the IL in citrate buffer (0.10 M, pH 4.8). The unfolding temperature of the cellulase was then determined using DSC. The [C2OHmim][OAc]-containing samples were dialyzed overnight against a 100 mM sodium acetate buffer (pH 4.8) in the presence of [C2OHmim][OAc] in amounts similar to that in the sample. The dialyzed enzyme was scanned between 25 and 100 °C, using a scan rate of 0.5 °C min−1. The enzyme scans were corrected with a buffer baseline.2.6 Stability of cellulases in [C2OHmim][OAc]
The stability of cellulase was determined in [C2OHmim][OAc]. The stability experiments were performed in a total volume of 20 mL, which contained the cellulase (1 mg) and various concentrations (0%, 5%, 10%, 15%, 20% and 25% v/v) of the IL in citrate buffer (0.10 M, pH 4.8), using CMC (10 mg) as a substrate. The mixture was incubated at 50 °C. Aliquots were taken at different points following incubation (1.5, 3, 24 h), to allow the determination of residual enzyme activity. Cellulase in a citrate buffer without any ionic liquid was used as a control. The activity of the cellulase was investigated in the citrate buffer at various pH values (pH = 3.96, 4.8, 5.35, 5.83, 6, 6.5), under the same conditions. All experiments were performed in triplicate. The activity of the cellulase was analyzed by measuring the amount of reducing sugars. Those reducing sugars were produced from CMC using the 3,5-dinitrosalic acid (DNS) reducing sugar assay, and HPLC.We conducted the DNS assay for the measurement of cellulase activity as follows: 1 mL of the mixture was taken, and 1% CMC (w/v) in 0.1 M citrate buffer (pH 4.8) was added with the cellulase, which was incubated at 50 °C for 1 h; the two volumes of the DNS reagent were then mixed together. The reaction mixture was further incubated at 100 °C for 5 min and cooled in an ice-water bath before the absorbance was measured at 540 nm. Control reactions were performed with substrate CMC without any IL (0%).
2.7 Enzymatic saccharification of MCC
A 14% (w/v) MCC solution was prepared by combining 1050 mg of MCC with 7.5 mL of [C2OHmim][OAc] in a sterile flask. The MCC/IL solution was incubated and stirred at 50 °C for 30 min.Following incubation, the MCC/[C2OHmim][OAc] solution was diluted with 42.5 mL of 0.1 M sodium acetate buffer (pH 4.8). The final concentrations of MCC and [C2OHmim][OAc] were 2.1% (w/v) and 15% (v/v). 20 mg of cellulase was added to the MCC/[C2OHmim][OAc] solution, and the enzymatic hydrolysis was carried out at 50 °C under shaking at 180 rpm. The reaction was monitored over time (1, 3, 6, 9, 12, and 24 h) by quantifying the reducing sugar concentration using the DNS assay and HPLC, as described above. All assays were performed in triplicate.
2.8 Enzymatic saccharification of natural cellulose
6% (w/v) straw, cotton, and filter paper solutions were prepared by combining 450 mg of cellulose with 7.5 mL of [C2OHmim][OAc] in a sterile flask. The straw, cotton, or filter paper/IL solution was incubated and stirred at 70 °C for 60 min.Following incubation, the straw, cotton, or filter paper/[C2OHmim][OAc] solution was diluted with 42.5 mL of 0.1 M sodium acetate buffer (pH 4.8). The final concentrations of straw, cotton, or filter paper and [C2OHmim][OAc] were 0.9% (w/v) and 15% (v/v), respectively. The cellulase was added to the straw, cotton or filter paper/[C2OHmim][OAc] solutions, and enzymatic hydrolysis was carried out at 50 °C under shaking at 180 rpm. The reaction was monitored over time (1, 3, 6, 9, 12, and 24 h) by quantifying the reducing sugar concentration using the DNS assay and HPLC, as described above. All assays were performed in triplicate.
2.9 Regeneration of ionic liquids
The affinity extraction of sugars was adapted from the method of Griffin and Shu.17 Extraction experiments were conducted for the IL/hydrolysate solutions. After the reaction, the IL/hydrolysate solutions were collected; the amount of solution was typically approximately 48 mL. The solution was then centrifuged to remove any non-hydrolytic cellulose. Following centrifugation, the solution was distilled, and a viscous solution with a volume of approximately 10 ml was obtained. Equal volumes of the IL/hydrolysate solution and the organic phase (Napthelene-2-boronic acid dissolved in an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v) solution of n-hexane) were vigorously mixed at 1500 rpm at 20 °C for 2 h (YNT Thermomixer). The tubes were then transferred to a centrifuge (Eppendorf centrifuge 5434) and spun at 20
20 (v/v) solution of n-hexane) were vigorously mixed at 1500 rpm at 20 °C for 2 h (YNT Thermomixer). The tubes were then transferred to a centrifuge (Eppendorf centrifuge 5434) and spun at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to separate the two phases. Samples of the IL/water phase were then analyzed using HPLC to determine the content of residual sugar. All trials were conducted in triplicate. The final amount of the aqueous IL solution was approximately 8 mL, and very little of the IL was lost; the amount extracted reached 95%. All experiments were performed at 20 °C.18
000 rpm for 5 min to separate the two phases. Samples of the IL/water phase were then analyzed using HPLC to determine the content of residual sugar. All trials were conducted in triplicate. The final amount of the aqueous IL solution was approximately 8 mL, and very little of the IL was lost; the amount extracted reached 95%. All experiments were performed at 20 °C.18
3. Results and discussion
3.1 The solubilizing ability of [C2OHmim][OAc]
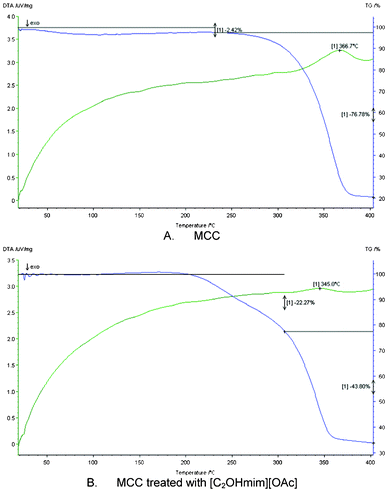
The solubility of microcrystalline cellulose (MCC), straw, filter paper, and cotton in [C2OHmim][OAc] is shown in Table 2. It can be seen from Table 2 that [C2OHmim][OAc] showed good ability to solubilize MCC, which is an insoluble pure cellulose and is widely accepted as a model substrate for cellulytic enzymatic analysis.11 [C2OHmim][OAc] could also dissolve native lignocellulosic biomass such as straw, filter paper, and cotton, which have different compositions of cellulose, hemicellulose, and lignin (Table 1). In brief, [C2OHmim][OAc] provides a benign method for the processing of native biomass.The process of the dissolution of MCC in [C2OHmim][OAc] and performance of MCC regenerated from [C2OHmim][OAc] were studied in this paper, to illustrate the mechanism of the solubilizing ability of [C2OHmim][OAc] for cellulose. Polarization microscopy was used to observe the changes in the features of MCC during the process of dissolution (Fig. 1). From Fig. 1 it can be seen that with increasing dissolution time, the MCC first swelled (Fig. 1: 20 min), then slowly dissolved, and the particles of MCC gradually diminished and disappeared (Fig. 1: 1 h). The FT-IR spectra (Fig. 2) showed peaks with similar features for MCC (b1) and MCC regenerated from [C2OHmim][OAc] (b2), which indicated that the dissolution of MCC in [C2OHmim][OAc] was a physical process.15 The 1430 and 897 cm−1 transmittance bands can be used to study the type of crystalline cell and the changes in crystallinity, because these bands in the spectrum of crystalline cellulose I clearly differ from those in the spectra of cellulose II and amorphous cellulose.19 The absorption band at 1430 cm−1 was assigned to CH2 symmetric bending in cellulose, being specific to the mixture of crystalline celluloses I and II. The absorption band at 897 cm−1 was assigned to C–O–C stretching at the β-(1→4)-glycosidic linkage in cellulose I.20 As shown in Fig. 2, the transmittance band at approximately 1430 cm−1 was strong for MCC, but weak for the treated one. An increase in the width and strength of this band would represent an increase in the cellulose I content, and a decrease in the cellulose II content. Furthermore, the most significant and best-defined absorption band, which was at approximately 897 cm−1 confirmed the presence of crystalline cellulose I. This transmittance band was intense for the untreated MCC, but weak for that treated with [C2OHmim][OAc] (Fig. 2).21,22 X-Ray diffraction was used to detect the transformation of the MCC during the dissolution step (Fig. 3). Before dissolution, the MCC exhibited a diffraction pattern typical for type-I cellulose. However, the images for the dissolved and precipitated MCCs suggested an amorphous state. This result suggests that [C2OHmim][OAc] breaks the hydrogen bonding network of MCC, and dissolves the MCC chains at a molecular level.16 In addition, the decomposition temperature of MCC changed from 366 °C to 345 °C after dissolution (Fig. 4). This observation also confirmed that the microfibril arrangements were destroyed during dissolution. According to the results described here, [C2OHmim][OAc] dissolved cellulose under mild conditions. These characteristics make [C2OHmim][OAc] a suitable choice to enhance the enzymatic conversion of cellulose, because the structure of cellulose changes to become looser during the dissolution process in [C2OHmim][OAc].
![FT-IR spectra of original MCC (b1), and MCC (b2) treated with [C2OHmim][OAc].](/image/article/2012/RA/c2ra22128d/c2ra22128d-f2.gif) | ||
| Fig. 2 FT-IR spectra of original MCC (b1), and MCC (b2) treated with [C2OHmim][OAc]. | ||
![XRD patterns for (a) original MCC, and (b) MCC treated with [C2OHmim][OAc].](/image/article/2012/RA/c2ra22128d/c2ra22128d-f3.gif) | ||
| Fig. 3 XRD patterns for (a) original MCC, and (b) MCC treated with [C2OHmim][OAc]. | ||
3.2 The stability of cellulase in [C2OHmim][OAc]
The cellulase was stable in the presence of [C2OHmim][OAc] when the temperature was maintained at 50 °C (Fig. 5). Following 1.5 h of incubation in 5%, 10%, 15%, 20%, and 25% solutions of [C2OHmim][OAc], the cellulase retained 93%, 90%, 87%, 79%, and 72% of its initial activity, respectively. The enzymatic activity decreased with increasing incubation time; following 24 h of incubation at 50 °C, the cellulase retained 91% and 88% of its activity in the presence of 5% and 10% [C2OHmim][OAc], respectively. The cellulase displayed 64% of the original activity after 24 h of incubation in 20% IL, but the activity significantly decreased to 56% when the cellulase was in a 25% IL solution for 24 h. With increasing amount of IL, the pH value of the system increased from 4.8 to 6.18, and the viscosity changed a little (Table 3). Judging from the results, the pH value was the main factor affecting the activity of cellulase. Next, the cellulase activity was examined in buffer solutions with various pH values (Fig. 6). It can be seen from Fig. 6 that when the pH value was higher than the optimum pH value for cellulase, the activity of the cellulase decreased, and at the same pH value the activity of the cellulase was higher in the IL than in the buffer solution. The results clearly showed that [C2OHmim][OAc] had excellent compatibility with the cellulase. Meanwhile, cellulose had lower or no crystallinity and could thus be enzymatically hydrolyzed to glucose in the [C2OHmim][OAc] system much more easily.16 The unfolding temperatures of cellulase at various concentrations of [C2OHmim][OAc] are shown in Table 3. The unfolding temperature of cellulase increased in the [C2OHmim][OAc] system compared with the buffer solution, so the thermostability of the cellulase was enhanced in the [C2OHmim][OAc] system. From the above results, we believe that the pH is the main factor affecting the activity of cellulase in ILs, in addition to the viscosity and type of IL.![Cellulase activity after pre-incubation with various concentrations of [C2OHmim][OAc]. The error bars indicate the standard deviation.](/image/article/2012/RA/c2ra22128d/c2ra22128d-f5.gif) | ||
| Fig. 5 Cellulase activity after pre-incubation with various concentrations of [C2OHmim][OAc]. The error bars indicate the standard deviation. | ||
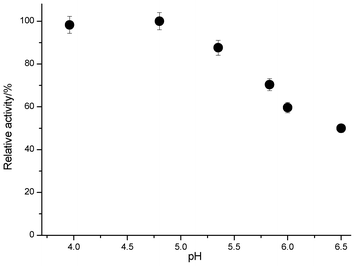 | ||
| Fig. 6 Activity of cellulase in buffer solutions with various pH values. | ||
| Concentration/% | 0 | 5 | 10 | 15 | 20 | 25 |
| pH | 4.8 | 5.39 | 5.61 | 5.79 | 6.00 | 6.18 |
| Unfolding T/°C | 64.3 | 74.4 | 76.3 | 76.9 | 76.0 | 75.8 |
| Viscosity/cP (50 °C) | 6.57 | 6.62 | 6.68 | 6.73 | 6.77 | 6.80 |
3.3 The enzymatic hydrolysis of MCC in [C2OHmim][OAc]
Despite the fact that increasing amounts of IL in the IL/buffer solution resulted in decreased cellulase activity, if the IL content was too low, the solubility of cellulose was similarly low, which resulted in problems in post processing. We therefore chose the system with a 15% [C2OHmim][OAc]/buffer solution to investigate the enzymatic hydrolysis of cellulose. The homogeneous MCC-IL system was diluted with citrate buffer to a final IL concentration of 15% (v/v). When the buffer solution was added, MCC was separated from the IL step by step, and at the same time the reaction system was changed from a homogeneous phase into a heterogeneous system. The cellulase was then added to the MCC-IL solution, and the released sugars were monitored at different time points (Fig. 7). In the presence of 15% IL, approximately 77.5% of the MCC was hydrolyzed during the first 3 h. After the reaction had continued for 24 h, 99.14% of the MCC had been converted to glucose and cellobiose. These findings clearly showed that the aqueous IL cellulase system worked effectively for the hydrolysis of pure cellulose (MCC). In addition, the mechanism of the enzymatic hydrolysis of cellulose was studied. In a celloluse/[C2OHmim][OAc] solution, [C2OHmim][OAc] is a strong electrolyte, and [C2OHmim] + and [OAc]− distribute uniformly in their ionic form. More importantly, [C2OHmim]+ and [OAc]− can easily enter the network structure of cellulose to disrupt the inter- and intra-molecular hydrogen-bonding, according to the theory of EDA.3,23 With the [C2OHmim]+ and [OAc]− having access to the cellulose framework, the structure of the cellulose became loose. After the dilution with buffer solution, the cellulose was separated from the IL, and the regenerated cellulose had a less compact structure and weaker binding forces between the hydrogen atoms than in the untreated cellulose,3 according to the XRD results. At the same time, the β-(1→4)-glycosidic linkage was exposed, and the accessibility between the cellulase and cellulose increased, so enzymatic saccharification was accelerated. The hydrolysis of the regenerated MCC and untreated MCC were also investigated (Fig. 7). From the results, it can be seen that the enzymatic hydrolysis of untreated MCC was very low, which suggested that the IL decreased the crystallinity of the MCC, improving the accessibility of the MCC to the cellulases. On the other hand, the enzymatic hydrolysis of the regenerated MCC was higher than that in the aqueous IL, indicating that the incomplete conversion was caused by the inactivation of the cellulases in the aqueous IL mixtures, and that the pH of the solution deviated from the optimum pH after addition of the IL, which agreed with the previous results.![Cellulase-catalyzed hydrolysis of MCC in the presence of 15% [C2OHmim][OAc] (v/v). The error bars indicate the standard deviation.](/image/article/2012/RA/c2ra22128d/c2ra22128d-f7.gif) | ||
| Fig. 7 Cellulase-catalyzed hydrolysis of MCC in the presence of 15% [C2OHmim][OAc] (v/v). The error bars indicate the standard deviation. | ||
3.4 The enzymatic hydrolysis of lignocellulosic biomass in [C2OHmim][OAc]
However, previous studies found that cellulase activity was reduced during the hydrolysis of lignocellulosic biomass, due to the interaction with lignin or lignin-carbohydrate complexes.24,25 To evaluate the efficiency of our IL-aqueous system in the hydrolysis of native biomass, straw, filter paper, and cotton were used as substrates (Fig. 8). These native biomass materials had different cellulose, hemicellulose, and lignin compositions (Table 1). The results from this study showed that the conversion efficiency of the native biomass was lower than that of MCC, which indicated that the incomplete conversion was not caused by the inactivation of cellulase in the aqueous-IL mixtures.6 It is likely that the incomplete conversion was due to the reduction of the cellulase-accessible surface area during the interaction of the cellulase with lignin or hemicellulose complexes in the biomass.25 After 24 h of reaction, the amount of reducing sugar was only 1.192 mg mL−1 for straw, which was lower than the amounts for filter paper or cotton. The findings showed that lignin interfered with the cellulases. The filter paper and cotton did not contain lignin. The amount of reducing sugar reached 3.838 mg mL−1 when cotton was used as a substrate; the cotton contained less hemicellulose than the filter paper. The results showed that hemicellulose also hindered the saccharification of cellulose.26![Cellulase-catalyzed hydrolysis of native biomass in the presence of 15% [C2OHmim][OAc] (v/v). The error bars indicate the standard deviation.](/image/article/2012/RA/c2ra22128d/c2ra22128d-f8.gif) | ||
| Fig. 8 Cellulase-catalyzed hydrolysis of native biomass in the presence of 15% [C2OHmim][OAc] (v/v). The error bars indicate the standard deviation. | ||
Using the method of affine extraction, [C2OHmim][OAc] was effectively concentrated from a dilute reducing sugar aqueous solution, and then almost completely recovered; the content of reducing sugar in the IL was less than 5%. The regenerated [C2OHmim][OAc] was used to dissolve MCC. The solubility of MCC in the IL reached 13% after 3 h at 50 °C. The regenerated IL was used in the hydrolysis of MCC, and approximately 77% of the MCC was hydrolyzed during the first 3 h; the performances of the regenerated IL were almost the same as the fresh IL in terms of solubilizing ability and compatibility with the enzyme. It is clear that this advantage will promote the industrial application of ILs.18
4. Conclusion
In conclusion, [C2OHmim][OAc] showed excellent solubilizing ability for lignocellulosic biomass, and excellent compatibility with cellulase. In the process of dissolution, the inter- or intramolecular hydrogen bonds were broken, and the accessiblity between the cellulase and the lignocellulosic biomass was enhanced; the enzymatic hydrolysis conversion of lignocellulosic biomass was therefore increased in the [C2OHmim][OAc] system. Furthermore, we believe that pH was the main factor affecting the cellulase activity in the IL (in addition to the viscosity and the type of IL). In particular, [C2OHmim][OAc] provides the possibility of producing biofuels using native biomass as materials. Further study of the interaction mechanism between [C2OHmim][OAc] and enzyme is in progress.Acknowledgements
This study was supported by the National Natural Science Foundation of China (31070520), Shandong Province Office of Education (J09LG16), Open Foundation of Chemical Engineering Subject (Qingdao University of Science and Technology).References
- H. Zhang, J. Wu, J. Zhang and J. He, Macromolecules, 2005, 38, 8272–8277 CrossRef CAS.
- (a) C. Roosen, P. Muller and L. Greiner, Appl. Microbiol. Biotechnol., 2008, 81, 607–614 CrossRef CAS; (b) M. Sureshkumar and C. K. Lee, J. Mol. Catal. B: Enzym., 2009, 60, 1–12 CrossRef CAS; (c) M. Moniruzzaman, N. Kamiya and M. Goto, Org. Biomol. Chem., 2010, 8, 2887–2899 RSC; (d) P. Lozano, Green Chem., 2010, 12, 555–569 RSC.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS.
- (a) S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC; (b) D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- (a) L. Kline, D. Hayes, A. R. Womac and N. Labbe, Bioresource, 2010, 5, 1366–1383 CAS; (b) B. Li, J. Asikkala, I. Filpponen and D. S. Argyropoulos, Ind. Eng. Chem. Res., 2010, 49, 2477–2484 CrossRef CAS; (c) C. Li, B. Knierim, C. Manisseri, R. Arora, H. V. Scheller, M. Auer, K. P. Vogel, B. A. Simmons and S. Singh, Bioresour. Technol., 2010, 101, 4900–4906 CrossRef CAS; (d) Q. Li, X. L. Jiang, Y. C. He, L. Z. Li, M. Xian and J. M. Yang, Appl. Microbiol. Biotechnol., 2010, 87, 117–126 CrossRef CAS; (e) L. Y. Liu and H. Z. Chen, Chin. Sci. Bull., 2006, 51, 2432–2436 CrossRef CAS; (f) I. P. Samayam and C. A. Schall, Bioresour. Technol., 2010, 101, 3561–3566 CrossRef CAS; (g) K. Ohira, Y. Abe, M. Kawatsura and K. Suzuki, ChemSusChem, 2012, 5, 388–391 CrossRef CAS.
- P. Engel, R. Mladenov, H. Wulfhorst, G. Jager and A. C. Spiess, Green Chem., 2010, 12, 1959–1966 RSC.
- M. B. Turner, S. K. Spear, J. G. Huddleston, J. D. Holbrey and R. D. Rogers, Green Chem., 2003, 5, 443–447 RSC.
- J. Woodward, N. E. Lee, J. S. Carmichael, S. L. McNair and J. M. Wichert, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1990, 1037, 81–85 CrossRef CAS.
- R. Rinaldi, R. Palkovits and F. Schuth, Angew. Chem., Int. Ed., 2008, 47, 8047–8050 CrossRef CAS.
- B. Sayantan, A. B. Charles and W. J. Petrich, Biotechnol. Bioeng., 2012, 109, 434–443 CrossRef.
- (a) Y. Wang, M. Radoseveich, D. Hayes and N. Labbe, Biotechnol. Bioeng., 2011, 108, 1042–1048 CrossRef CAS; (b) S. Datta, B. Holmes, J. I. Park and Z. Chen, Green Chem., 2010, 12, 338–345 RSC; (c) J. Vitz, T. Erdmenger, C. Haensch and U. S. Schubert, Green Chem., 2009, 11, 417–424 RSC; (d) Ö. P. Çetinkol, D. C. Dibble, G. Cheng, M. S. Kent, B. Knierim, M. Auer, D. E. Wemmer, J. G. Pelton, Y. B. Melnichenko, J. Ralph, B. A. Simmons and B. M. Holmes, Biofuels, 2010, 1, 33–46 CrossRef; (e) W. Y. Li, N. Sun, B. Stoner, X. Y. Jiang, X. M. Lu and R. D. Rogers, Green Chem., 2011, 13, 2038–2047 RSC.
- L. Feng and Z. L. Chen, J. Mol. Liq., 2008, 142, 1–5 CrossRef.
- H. Zhao, G. A. Baker, Z. Song, O. Olubajo, T. Crittle and D. Peter, Green Chem., 2008, 10, 696–705 RSC.
- (a) A. R. Xu, J. J. Wang and H. Y. Wang, Green Chem., 2010, 12, 268–275 RSC; (b) Y. Fukaya, A. Sugimoto and H. Ohno, Biomacromolecules, 2006, 7, 3295–3297 CrossRef CAS; (c) H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- C. F. Liu, R. C.Sun and A. P. Zhang, J. Agric. Food Chem., 2007, 55(6), 2399–2406 CrossRef CAS.
- (a) A. Mitsuru, F. Yukinobu and O. Hiroyuki, Chem. Commun., 2012, 48, 1808–1810 RSC; (b) S. H. Lee, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2009, 102, 1368–1376 CrossRef CAS.
- G. J. Griffin and L. Shu, J. Chem. Technol. Biotechnol., 2004, 79(5), 505–511 CrossRef CAS.
- T. C. R. Brenman, S. Datta, H. W. Blanch, B. A. Simmons and B. M. Holmes, BioEnergy Res., 2010, 3, 123–133 CrossRef.
- B. Ruxanda, T. A. Carmen and S. Iuliana, Monatsh. Chem., 2010, 141, 1043–1048 CrossRef.
- M. L. Nelson and R. T. O'Connor, J. Appl. Polym. Sci., 1964, 8, 1325–1341 CrossRef CAS.
- R. Bodirlau, C. A. Teaca and I. Spiridon, Bioresources, 2008, 3, 789–800 CAS.
- H. Zhang, J. Wu, J. Zhang and J. He, Macromolecules, 2005, 38, 8272–8277 CrossRef CAS.
- S. M. Hudson and J. A. Cuculo, J. Macromol. Sci., Part C, 1980, 18, 1–82 CrossRef.
- A. Berlin, M. Balakshin and N. Gilkes, J. Biotechnol., 2006, 125, 198–209 CrossRef CAS.
- R. Kumar and C. E. Wyman, Biotechnol. Prog., 2009, 25, 807–819 CrossRef CAS.
- W. Liao, Z. Wen and S. Hurley, Appl. Biochem. Biotechnol., 2005, 124, 1017–1030 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |