Supramolecular approaches to metal–organic gels using ‘Chevrel-type’ coordination clusters as building units†
Lei
Zhang
a,
Bartosz
Marzec
a,
Rodolphe
Clérac
bc,
Yanhui
Chen
d,
Hongzhou
Zhang
d and
Wolfgang
Schmitt
*a
aSchool of Chemistry & CRANN, University of Dublin, Trinity College, Dublin 2, Ireland. E-mail: schmittw@tcd.ie; Tel: +353-1-896-3495
bCNRS, CRPP, UPR 8641, F-33600 Pessac, France
cUniv. Bordeaux, CRPP, UPR 8641, F-33600 Pessac, France
dSchool of Physics & CRANN, University of Dublin, Trinity College, Dublin 2, Ireland
First published on 4th September 2012
Abstract
A novel hexanuclear {Mn6} coordination complex with octahedral topology has been prepared and was subsequently used as a building unit for the construction of coordination polymers and metal–organic gels; the latter exhibit thixotropic behavior and reversible sol–gel phase transitions.
Metal–organic frameworks (MOFs) have attracted significant scientific interest due to potential applications in diverse areas such as gas adsorption, catalysis, regenerative medicine, bio-engineering and drug delivery.1 Successful synthetic efforts to develop new types of MOFs highly depend on the availability of suitable organic and inorganic secondary building units (SBUs), which determine the topologies and physical properties of the materials.2 Similar to MOFs, metal–organic gels (MOGs) are a class of extended structures based on coordination complexes.3 Recently, there has been increasing interest in the studies of MOGs as these materials can be used in template synthesis, catalysis, and molecular electronics.4 The formation of MOGs involves the supramolecular self-assembly of coordination complexes through noncovalent interactions such as hydrogen-bonding, π–π stacking and van der Waals forces.5
To improve their performances in targeted applications and create multifunctional MOF and MOG materials, there is a high demand for new multinuclear building units which endow the materials with unique electronic, magnetic and structural attributes.6 Unfortunately, the discovery of new multinuclear SBUs with appropriate topologies remains challenging and often involves serendipitous synthetic approaches. We are interested in the rational synthetic concepts and topological aspects of coordination cages and clusters7a–c and according to our previous studies, polynuclear Mn-organophosphonate complexes can adopt highly symmetric geometries which are necessary for qualified SBU candidates.7d,e More importantly, Mn coordination clusters may contain kinetically active MnIII Jahn–Teller ions that can easily be functionalized by organic linkers and may facilitate the organisation of these SBUs into desired MOFs and MOGs.8
Herein we report a new symmetric type of hexanuclear Mn coordination complex in [Cl⊂MnIII6(tert-butyl-PO3)8(L1)6]+ (L1 = 4-picoline for 1; pyridine for 2) and [Cl⊂MnIII6(tert-butyl-PO3)8(L2)4(H2O)2l]+ (L2 = MeOH for 3; 4-pyridinecarboxaldehyde for 4) that may serve as octahedral SBUs for MOFs and MOGs. We exploit the kinetic lability of MnIII ions and stepwise exchange of terminal pyridine ligands by bifunctional 4,4′-bipyridine linkers results in the formation of a 1D coordination polymer [Cl⊂MnIII6(tert-butyl-PO3)8(pyridine)4(4,4′-bipyridine)]+ (5), and a supramolecular gel [Cl⊂Mn6(tert-butyl-PO3)8(4,4′-bipyridine)6]Cl·xH2O (6) with thixotropic properties. These materials were characterized by X-ray diffraction, scanning and transmission electron microscopy (SEM and TEM), mass spectrometry and elemental analysis.
The comproportionation reaction between MnCl2·4H2O and KMnO4 at a molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of tert-butyl-PO3H2, 4-picoline and CH3CN gives rise to red crystals of [Cl⊂MnIII6(tert-butyl-PO3)8(4-picoline)6]Cl·H2O ([1]Cl·H2O). The compound crystallizes in the cubic space group Pa
1 in the presence of tert-butyl-PO3H2, 4-picoline and CH3CN gives rise to red crystals of [Cl⊂MnIII6(tert-butyl-PO3)8(4-picoline)6]Cl·H2O ([1]Cl·H2O). The compound crystallizes in the cubic space group Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . As shown in Fig. 1a, the cationic {Mn6} cage contains six MnIII atoms in an octahedral arrangement, with a Cl− ion located in the centre at the position of an inversion centre. The coordination environments of these six MnIII can be regarded as tetragonally distorted octahedra with one N-donor originating from a 4-picoline ligand and a central Cl− ion occupying the apical positions. Four O atoms from four different tert-butylphosphonate ligands occupy the remaining equatorial coordination sites. The 8 triangular faces of the octahedral {Mn6} cage are capped by 8 fully deprotonated tert-butylphosphonate ligands with each of them bridging three MnIII atoms in a η1:η1:η1:μ3 binding mode. Accordingly, the 8 P atoms of the tert-butylphosphonate ligands arrange into a cube (Fig. 1b). The central Cl− ion weakly interacts with the six symmetry-related Mn centres resulting in Mn⋯Cl distances of 3.108(10) Å. The assigned +III oxidation states of the Mn centers are confirmed by bond valence sum analyses (BVSA).
. As shown in Fig. 1a, the cationic {Mn6} cage contains six MnIII atoms in an octahedral arrangement, with a Cl− ion located in the centre at the position of an inversion centre. The coordination environments of these six MnIII can be regarded as tetragonally distorted octahedra with one N-donor originating from a 4-picoline ligand and a central Cl− ion occupying the apical positions. Four O atoms from four different tert-butylphosphonate ligands occupy the remaining equatorial coordination sites. The 8 triangular faces of the octahedral {Mn6} cage are capped by 8 fully deprotonated tert-butylphosphonate ligands with each of them bridging three MnIII atoms in a η1:η1:η1:μ3 binding mode. Accordingly, the 8 P atoms of the tert-butylphosphonate ligands arrange into a cube (Fig. 1b). The central Cl− ion weakly interacts with the six symmetry-related Mn centres resulting in Mn⋯Cl distances of 3.108(10) Å. The assigned +III oxidation states of the Mn centers are confirmed by bond valence sum analyses (BVSA).
![(a) Crystal structure of 1. (b) Polyhedral representation of the {Mn6} octahedron in 1, with eight triangular faces each capped by a phosphonate group (only P atoms are shown for clarity). (c) The structure of a [Mo6Cl14]2− halide cluster, highlighting the {Mo6} octahedron (in purple) and the six labile Cl− ions (in light green). (d) The crystal structure of the 1D coordination polymer 5. [Mn light blue, P pink, Cl green, C black, N blue, Mo purple, H omitted for clarity].](/image/article/2013/CC/c2cc35486a/c2cc35486a-f1.gif) | ||
| Fig. 1 (a) Crystal structure of 1. (b) Polyhedral representation of the {Mn6} octahedron in 1, with eight triangular faces each capped by a phosphonate group (only P atoms are shown for clarity). (c) The structure of a [Mo6Cl14]2− halide cluster, highlighting the {Mo6} octahedron (in purple) and the six labile Cl− ions (in light green). (d) The crystal structure of the 1D coordination polymer 5. [Mn light blue, P pink, Cl green, C black, N blue, Mo purple, H omitted for clarity]. | ||
The structure of the hexanuclear Mn complex is indeed interesting in its own right as it can be regarded as a coordination chemistry analogue of the classical face-capped inorganic [Mo6X14]2− (X = Cl, Br, I) clusters in low-valent halides or as {Mo6Y8} (Y = chalcogen) in Chevrel phases (Fig. 1c).9 In halide and chalcogenide clusters terminal monodentate ligands in the apical octahedral positions are susceptible to ligand exchange reactions. This feature is also applicable to the {Mn6} complex.
In particular, the Jahn–Teller distorted coordination environments of these six MnIII centres in 1 provide kinetically labile active sites to the {Mn6} cage, supplying possible chemical functionality through replacing the 4-picoline by other ligands. Indeed the 4-picoline ligands in 1 can formally be replaced by pyridine, H2O, MeOH and 4-pyridinecarboxaldehyde ligands (2–4). The {Mn6} cluster cores in 1–4 only reveal slight geometrical differences (see ESI†). The complexes are stable in air. Analyses of the magnetic susceptibility of the {Mn6} complexes confirm moderate average intramolecular antiferromagnetic interactions (J/kBca. −2 K) between the S = 2 MnIII centres leading to S = 0 spin ground states (the following isotropic Heisenberg spin Hamiltonian was used: H = −2J ((S1 + S6)·(S2 + S3 + S4 + S5) + S2·S3 + S3·S4 + S4·S5 + S2·S5); see ESI†).
Considering the octahedral symmetry of the {Mn6} cages in 1–4 and the functionality with respect to ligand substitutions it appears plausible to use this coordination complex as a potential SBU for the preparation of extended structures (Fig. 1b). Indeed, when bifunctional 4,4′-bipyridine ligands (4,4′-bpy) were introduced into the reaction system, a 1D metal–organic polymer, [Cl⊂MnIII6(tert-butyl-PO3)8(pyridine)4(4,4′-bipyridine)]Cl ([5]Cl), was obtained. X-ray diffraction analysis confirms that 5 consists of {Mn6} cages similar to 1–4. Each of the {Mn6} cages is connected to two adjacent ones by two 4,4′-bipyridine linkers, with the remaining four Jahn–Teller sites occupied by pyridine ligands (Fig. 1d). The compound crystallizes in the monoclinic space group C2/m and the 1D chain structure extends in the direction of the crystallographic b-axis. Unfortunately, the use of increased aliquots of 4,4′-bipyridine did not result in other crystalline materials and phase-pure 3D MOFs could not yet be isolated. However, interestingly, only complete substitution of pyridine by 4,4′-bipyridine results in the formation of a stable supramolecular gel that exhibits thixotropic behavior and reveals reversible sol–gel phase transitions (Fig. 2e). These MOGs settle within a period of 24 h. On agitation (e.g. intensive shaking of the sample container), the gels liquify but they recover the gelatinous state again when allowed to stand at room temperature.
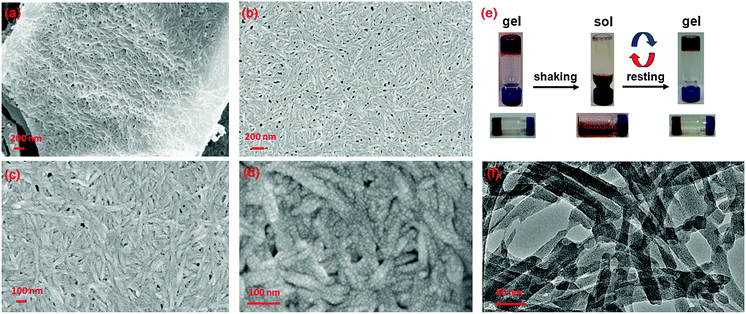 | ||
| Fig. 2 (a)–(d) SEM micrographs of dried gel 6 highlighting the fibrous, entangled morphology of the material that is composed of individual nanoparticles; (e) photographs showing the reversible sol–gel transformation of the gel material; (f) TEM image of the dried gel 6. | ||
The morphological properties of the obtained metal–organic gels were investigated by SEM and TEM, respectively. The corresponding images are shown in Fig. 2 and in the ESI.† The SEM images reveal the fibrous nature of the obtained MOGs; the morphology of the material is characterized by 3 dimensionally interwoven/entangled nanofibers that arrange into meshes. The nanofibers are relatively uniform and have cross-sectional diameters of ca. 20 nm and reach lengths of several micrometers. The TEM images are in agreement with the SEM images and confirm the fibrous morphology. Further structural details of the nanofibers are obtained by examining higher magnification images. As shown in Fig. 2d, the nanofibers are indeed 1D aggregates of nanoparticles with dimensions below 10 nm.
To confirm the composition of the MOGs, IR, ESI-MS, EDX, and element analyses were carried out on dried samples. FT-IR spectra of the MOGs are almost identical to those of the parent compounds 1–4. The ESI-MS spectrum of the dissolved MOG recorded in positive-mode contains two signals centered at m/z 2327.02 and at m/z 157.08 which can be assigned to [Cl⊂Mn6(tert-butyl-PO3)8(4,4′-dipyridine)5] and protonated 4,4′-bipyridine ligands. EDX spectra of the MOGs give an atomic ratio of P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 1.33
Mn = 1.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which corresponds to the expected P
1, which corresponds to the expected P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn ratio of the {Mn6} cluster. These analyses are very well in agreement with the elemental analysis of the material suggesting that the composition of the dried gel is represented by the formula [Cl⊂Mn6(tert-butyl-PO3)8(4,4′-bipyridine)6](Cl)·5H2O (6). Similar to 1–4, the {Mn6} cages of the MOGs are stabilized by six mono-coordinating 4,4′-bipyridine ligands and it is reasonable to assume that the SBUs are not linked by coordinative bonds. Aggregation into particles and 1D assemblies is most likely facilitated by π–π stacking interactions, H-bonds and van der Waals interactions (Fig. 3). Self-assembly into interwoven 1D structures may additionally involve electrostatic interactions of the positively charged molecular entities and counterions.
Mn ratio of the {Mn6} cluster. These analyses are very well in agreement with the elemental analysis of the material suggesting that the composition of the dried gel is represented by the formula [Cl⊂Mn6(tert-butyl-PO3)8(4,4′-bipyridine)6](Cl)·5H2O (6). Similar to 1–4, the {Mn6} cages of the MOGs are stabilized by six mono-coordinating 4,4′-bipyridine ligands and it is reasonable to assume that the SBUs are not linked by coordinative bonds. Aggregation into particles and 1D assemblies is most likely facilitated by π–π stacking interactions, H-bonds and van der Waals interactions (Fig. 3). Self-assembly into interwoven 1D structures may additionally involve electrostatic interactions of the positively charged molecular entities and counterions.
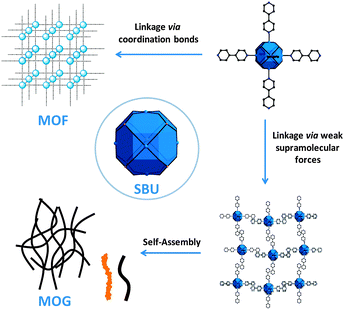 | ||
| Fig. 3 A formal approach to MOFs and MOGs using the octahedral {Mn6} coordination cluster as SBUs. | ||
The forces between the individual particulates are key for the gelation of the material. Agitated gels and gels that were treated with methanol liquify and loose their gel character. SEM images of dried samples that were previously treated with MeOH demonstrate that the formation of the liquid phase is accompanied with a disentanglement of the fibers reducing the viscosity of the material. As indicated by the structure of 3, it is feasible to assume that during this process 4,4′-bipyridine moieties are replaced by MeOH ligands. The analyses suggest that the transformation into a liquid phase results in the degradation of the 1D assemblies producing random aggregations of individual nanoparticles of the coordination complexes.
In summary, we report a remarkable hexanuclear MnIII complex that is structurally related to classical octahedral face-capped cluster compounds such as Chevrel clusters. The lability of apical ligands of the six MnIII Jahn–Teller sites provides functionality to the system and can be exploited for ligand substitutions allowing the preparation of a metal–organic polymer. Moreover, the synthetic approach can be exploited for the preparation of supramolecular gels with reversible thixotropic properties. Transformation between the liquid and gel phase is most likely facilitated by the aggregation of nanoparticles to form entangled 1D assemblies.
The authors thank the SFI (06/RFP/CHE173 and 08/IN.1/I2047), IRCSET, the Univ. of Bordeaux, the Région Aquitaine and the CNRS for financial support.
Notes and references
- (a) J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS; (b) M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef CAS; (c) L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248 RSC; (d) A. C. McKinlay, R. E. Morris, P. Horcajada, G. Férey, R. Gref, P. Couvreur and C. Serre, Angew. Chem., Int. Ed., 2010, 49, 6260 CAS; (e) Z. Y. Gu, Y. J. Chen, J. Q. Jiang and X. P. Yan, Chem. Commun., 2011, 47, 4787 RSC; (f) N. Zhu, G. Tobin and W. Schmitt, Chem. Commun., 2012, 48, 3638 RSC.
- (a) S. T. Zheng, J. J. Bu, T. Wu, C. Chou, P. Feng and X. Bu, Angew. Chem., Int. Ed., 2011, 50, 8858 CrossRef CAS; (b) G. Férey, Science, 2001, 291, 994 CrossRef CAS; (c) S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS; (d) J. J. Perry IV, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400 RSC.
- (a) M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke and J. W. Steed, Chem. Rev., 2010, 110, 1960 CrossRef CAS; (b) Y. Hu, Y. Fan, Z. Huang, C. Song and G. Li, Chem. Commun., 2012, 48, 3966 RSC; (c) Y. R. Liu, L. He, J. Zhang, X. Wang and C. Y. Su, Chem. Mater., 2009, 21, 557 CrossRef CAS.
- (a) S.-i. Kawano, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2004, 126, 8592 CrossRef CAS; (b) O. Roubeau, A. Colin, V. Schmitt and R. Clérac, Angew. Chem., Int. Ed., 2004, 43, 3283 CrossRef CAS; (c) P. Grondin, O. Roubeau, M. Castro, H. Saadaoui, A. Colin and R. Clérac, Langmuir, 2010, 26, 5184 CrossRef CAS; (d) J. Huang, L. S. He, J. Y. Zhang, L. P. Chen and C. Y. Su, J. Mol. Catal. A: Chem., 2010, 317, 97 CrossRef CAS; (e) B. Li, L. Tang, L. Qiang and K. Chen, Soft Matter, 2011, 7, 963 RSC.
- (a) G. O. Lloyd and J. W. Steed, Nat. Chem., 2009, 1, 437 CrossRef CAS; (b) S. Rieth, C. Baddeley and J. D. Badjic, Soft Matter, 2007, 3, 137 RSC; (c) J. W. Steed, Chem. Commun., 2011, 47, 1379 RSC.
- (a) B. Nohra, H. E. Moll, L. M. R. Albelo, P. Mialane, J. Marrot, C. Mellot-Draznieks, M. O'Keeffe, R. N. Biboum, J. Lemaire, B. Keita, L. Nadjo and A. Dolbecq, J. Am. Chem. Soc., 2011, 133, 13363 CrossRef CAS; (b) A. Schoedel, L. Wojtas, S. P. Kelley, R. D. Rogers, M. Eddaoudi and M. J. Zaworotko, Angew. Chem., Int. Ed., 2011, 50, 11421 CrossRef CAS; (c) Q. Gao, X. Wang and A. J. Jacobson, Chem. Commun., 2012, 48, 3990 RSC; (d) B. Liu, J. Yang, M. Yang, Y. Wang, N. Xia, Z. Zhang, P. Zheng, W. Wang, I. Lieberwirth and C. Kübel, Soft Matter, 2011, 7, 2317 RSC.
- (a) L. Zhang and W. Schmitt, J. Am. Chem. Soc., 2011, 133, 11240 CrossRef CAS; (b) J. M. Breen and W. Schmitt, Angew. Chem., Int. Ed., 2008, 47, 6904 CrossRef CAS; (c) J. M. Breen, R. Clérac, S. M. Cloonan, E. Kennedy, M. Feeney, T. McCabe, D. C. Williams and W. Schmitt, Dalton Trans., 2012, 41, 2918 RSC; (d) L. Zhang, R. Clérac, P. Heijboer and W. Schmitt, Angew. Chem., Int. Ed., 2012, 51, 3007 Search PubMed; (e) L. Zhang, R. Clérac, C. I. Onet, M. Venkatesan, P. Heijboer and W. Schmitt, Chem.–Eur. J., 2012 DOI:10.1002/chem.201202297.
- (a) D. Lieb, A. Zahl, T. E. Shubina and I. Ivanović-Burmazović, J. Am. Chem. Soc., 2010, 132, 7282 CrossRef CAS; (b) R. Bagai and G. Christou, Chem. Soc. Rev., 2009, 38, 1011 RSC.
- (a) R. H. Holm and S. C. Lee, Angew. Chem., Int. Ed., 1990, 29, 840 CrossRef; (b) C. Perrin, J. Alloys Compd., 1997, 10, 262 Search PubMed; (c) R. Chevrel, M. Sergent and J. Prigent, J. Solid State Chem., 1971, 3, 515 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details and characterization of 1−6; crystallographic data in CIF; additional figures and tables. CCDC 886125–886127, 886524 and 886525. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2cc35486a |
| This journal is © The Royal Society of Chemistry 2013 |
