Shape-selective growth of silver nanoparticles under continuous flow photochemical conditions†
Simone
Silvestrini
,
Tommaso
Carofiglio
* and
Michele
Maggini
*
Department of Chemical Sciences, University of Padova, via Marzolo 1, 35131 Padova, Italy. E-mail: simone.silvestrini@unipd.it; tommaso.carofiglio@unipd.it; michele.maggini@unipd.it
First published on 9th November 2012
Abstract
A microfluidic setup for the photochemical nucleation and growth of silver nanoparticles with controlled morphologies is described. The combination of microstructured reactors and efficient LED illumination speeds the growth process up and enhances the shape-wise homogeneity of the produced nanostructures.
The interesting optical properties of silver and gold nanoparticles (NPs) have been appreciated since ancient times on masterpieces such as the Lycurgus cup (4th century AD). Since the first nanoparticles were synthesized, their applications have found their way into many different areas of science, such as catalysis, biomedicine or real-time optical sensing through surface-enhanced Raman scattering (SERS), just to name a few.1 It is now well established that the shape and size of metal NPs play a pivotal role in determining their properties.2 Therefore, the nanoscale control of the morphology of the particles is a fundamental prerequisite to develop new applications.3
This work focuses on the photochemical generation of small (<10 nm), citrate-stabilized AgNPs (seeds) and their light-induced growth into larger structures within microstructured photoreactors (MRs) operating in continuous mode. Microflow photochemical technologies represent a valuable alternative to conventional batch photoreactors processing,4,5 because in a photochemical MR a higher spatial illumination homogeneity and a better light penetration through the whole reactor depth can be achieved. Although the microfluidic approach to prepare metal NPs has shown promising results under both thermal6 or ambient conditions,7 to our knowledge a controlled NPs shape conversion in a continuous flow photochemical MR has not been reported yet.
Two different photo-MR modules were made; the former was employed to nucleate the AgNp seeds, the latter to carry out their shape-selective growth. AgNP seeds were prepared by irradiating a mixture of silver nitrate and a benzophenone-type photoinitiator, 4-(2-hydroxyethoxy)phenyl-(2-hydroxy-2-propyl)ketone (I2959), to reduce Ag+ to Ag0. An aqueous stock solution containing all starting materials (AgNO3 0.2 mM, I2959 0.2 mM, and sodium citrate 1 mM) was de-oxygenated and pumped through a PTFE tube (0.5 mm inner diameter) coiled around the external jacket of an immersion photoreactor equipped with a high pressure mercury lamp (Fig. S1 of ESI†). The residence time of the reagents inside the plastic tube was controlled by varying the flow rate and the yellow solutions, collected at the coil outlet, were characterized by UV-Vis spectroscopy. Fig. 1 illustrates the absorption spectra of AgNP seeds prepared at different flow rates, each showing a surface plasmon band centered at 402 nm, consistent with the data reported in the literature for the dipolar plasmon absorption of silver spheres of comparable size range.8 TEM images of the sample collected at 60 ml h−1 can also be found in the ESI,† Section S2, together with an analysis of the size distribution of the NPs (5.9 ± 2.9 nm). The effect of the flow rate on the seeding process is reported in the inset. An exceedingly long exposition to the intense irradiation of the mercury lamp induces extensive aggregation of the NPs, as witnessed by the color change of the colloid (from yellow to opalescent) and the decrease of the absorbance at 402 nm. On the basis of this optimization experiment, the flow rate was set to 60 ml h−1 to achieve a space-time-yield of 0.015 molAg h−1 L−1 which is six times higher than that calculated for a comparable, batch-wise seeds preparation.9
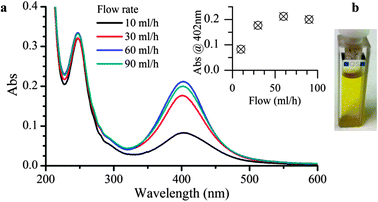 | ||
| Fig. 1 UV-Vis absorption spectra (a) and hue of the AgNP colloids after seeding (b). The dependency of the plasmon band at 402 nm is plotted versus the flow rate of the reactor in the inset. | ||
The (monochromatic) light-induced selective shape conversion of AgNP seeds into decahedra and platelets is a fascinating process associated to dramatic spectral changes of the AgNP suspensions. It is generally accepted that the reaction requires citrate, oxygen and light3 through the so-called “photovoltage mechanism” which has been previously discussed in literature.10 In summary, citrate adsorbed on the NPs is photooxidized upon excitation of localized surface plasmon resonance modes, yielding acetone-1,3-dicarboxylate and CO2. Upon decarboxylation, electrons are injected into the particle which, becoming negatively charged, induces the deposition and reduction of Ag+ ions.10 Triangles, disks, bipyramids, decahedra and other unconventional NPs shape11,12 can all be obtained by this method as we further discuss in Section S3 in the ESI.†
The amounts of citrate and oxygen in solution are the parameters that mostly influence the morphology of the NPs.13,14 Usually, photochemical NPs growth experiments are carried out with laser sources (intense and monochromatic) or high power lamps coupled with optical filters. Stamplecoskie and Scaiano have recently reported a versatile procedure which is based on the use of inexpensive, narrow bandwidth light emitting diodes (LEDs) as light sources.9 However, the amount of NP-seeds that can be processed is limited to the cuvette volume, thus making the production of larger amounts of AgNPs a discontinuous, time demanding process in which reproducibility could be an issue. We envisioned that working under continuous flow conditions could tackle both the productivity and reproducibility issues. Therefore a polymer MR, which is illustrated in Fig. 2 during testing with a dye solution, was made according to a procedure reported earlier15 and detailed in the ESI,† Section S4. The MR has two inlets for loading the AgNP-seeds and injecting air to oxygenate the mixture.14 The channels were patterned with a high aspect ratio (4 mm width, 300 μm height) to maximize the surface exposed to illumination, accomplished by an array of five high intensity LEDs (Fig. 2 and ESI,† Section S5). Air bubbles form at the junction between the two inlet channels and move along with the solution, ensuring its optimal oxygenation. The total flow was kept at 400 μl h−1 throughout this work, except where otherwise noted, with a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 flow rate ratio between the colloid and air (that is, 350 μl h−1 of colloid and 50 μl h−1 of air). The MR had an internal volume of 400 μl and under these conditions, a nominal illumination time of 60 min can be calculated as the ratio between the internal volume of the reactor and the flow rate.
1 flow rate ratio between the colloid and air (that is, 350 μl h−1 of colloid and 50 μl h−1 of air). The MR had an internal volume of 400 μl and under these conditions, a nominal illumination time of 60 min can be calculated as the ratio between the internal volume of the reactor and the flow rate.
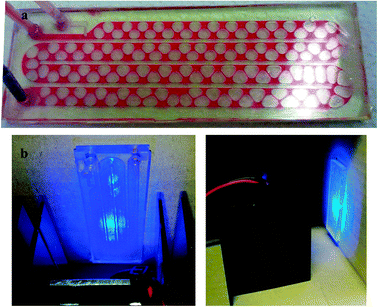 | ||
| Fig. 2 Microfluidic apparatus for the photochemical growth of silver NPs: (a) air bubbles forming and flowing in a stream of red tracer dye; (b) two images of the illuminator/microchip assembly during operation. | ||
In a first set of experiments, AgNP seed solutions were irradiated at either 455 or 505 nm to prompt the shape conversion from spheres to decahedra or platelets, respectively. The merit of the microfluidic setup was assayed by comparing the UV-Vis absorption spectra of the colloids obtained with the apparatus described above (referred to as “flow” samples; spectra reported as solid lines) with those obtained by irradiating a 1 cm quartz cuvette filled with a seed solution, under air exposure, for 5 hours (“bulk” samples; spectra reported as dash-dotted lines). We chose UV-Vis spectroscopy as our main analytical technique because plasmonic absorption bands are considered an unambiguous diagnostic tool to characterize AgNPs in this size range. Indeed, the positions of the bands are strongly correlated with the size and shape of the nanoparticles, while their intensity is proportional to the number of particles in solution.2
Fig. 3 compares the UV-Vis absorption spectra of AgNP seed colloids after illumination under flow or under bulk conditions, at 455 nm (top) and 505 nm (bottom). The change in the hue of the suspensions of the resulting NPs is reported in Fig. 3 as well. Irradiation at 455 nm led to the formation of silver decahedra, which present an absorption maximum at 483 nm due to an in-plane dipole.8 Small differences in the positions and intensities of the maxima in the absorption spectra of the bulk and flow samples can be explained by considering the presence of different amounts of unconverted AgNP seeds. In particular, the intensity of the in-plane dipole absorption is higher for the flow sample, indicating a better conversion of the starting seeds. This is remarkable, since the irradiation time, under continuous flow conditions, was only 20% of the light exposition time in bulk. In addition, the bulk sample displays increased absorbance around 650 nm (highlighted by a grey box), which is typically associated to the presence of larger, anisotropic particles.
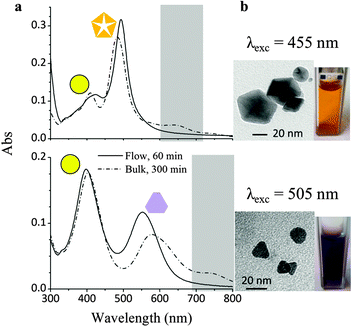 | ||
| Fig. 3 (a) UV-Vis absorption spectra of silver NP colloids irradiated with visible light at 455 nm and 505 nm under flow (solid line) and bulk (dash-dotted line) conditions. (b) Hue of the colloids after irradiation and TEM micrograph of anisotropic particles contributing to visible light absorption. | ||
When a 505 nm wavelength illumination was used to irradiate the seeds, the solution turned purple and an absorption band appeared at 542 nm, suggesting the formation of nanoplates.13 The presence of the 402 nm absorption band of the spherical seeds can still be observed in both flow and bulk samples, but the band at 542 looks broader and is shifted to higher wavelengths for the bulk one. As in the previous case, the lower background absorption for wavelengths exceeding 650 nm (also highlighted) indicates reduced formation of larger particles under flow conditions.
For both experiments presented above, the rate of the shape conversion process increased markedly, thanks to the MR that allowed a uniform and intense illumination of the mixture. The 300 μm light path in the MR channels was less than 1/30 of that of a 1 cm cuvette. Therefore, the light reaching the deepest liquid layer was much more intense, making the shape conversion process faster. In addition, the NPs produced under flow conditions were morphologically quite homogeneous for the efficient oxygenation induced by the air bubbles.
The benefits of flow processing were further confirmed by the experiments at 627 nm. The absorption spectrum of the seeds colloid, reported as a solid black line in Fig. 4, indicates a negligible absorbance at wavelengths higher than 590 nm. As a matter of fact, the colloid is stable for hours upon irradiation at 627 nm, showing no change in the absorption spectrum (circles overlapping the black line in Fig. 4). This is why the light-induced shape conversion of AgNPs is typically carried out after exposing the seeds colloid to ambient light for a few days, in order to grow larger, anisotropic particles that can act as growth centers.9
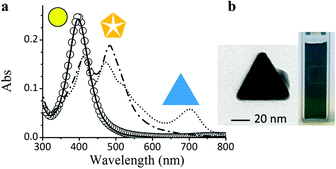 | ||
| Fig. 4 (a) Evolution of the UV-Vis spectrum of silver NP colloids following successive irradiation at different wavelengths: pristine sample (solid black line); after irradiation at 627 nm (circles); after irradiation at 455 nm (dash-dotted line); after irradiation at 627 nm (dotted line spectrum). (b) Hue of the colloid after the treatments and TEM micrograph of anisotropic particles contributing to visible light absorption. | ||
To circumvent this time-demanding pre-irradiation process, a multistep illumination of the colloid was carried out in the MR. The colloid was firstly exposed, under flow conditions, to LED irradiation at 455 nm for 30 minutes at a flow rate of 800 μl h−1 to convert only a fraction of the spherical seeds into decahedral NPs. This was done to better assess, in the subsequent step, the behavior of NPs with different shapes. The spectrum of the colloid after 30 min residence time is reported in Fig. 4 as a dash-dotted line, where the contribution from both spherical and decahedral NPs can be appreciated (absorption maxima at 402 and 483 nm). In the second step, the sample, showing a non-zero absorption in the red region, was again injected into the MR and irradiated at 627 nm to yield a colloid with a blue-green hue. Its UV-Vis spectrum (dotted line in Fig. 4) shows a new absorption band at 702 nm. Interestingly, only the intensity of the band at 483 nm decreased, indicating that exclusively the decahedral particles had been converted into large triangular nanoplates. Therefore, the days-long, ambient light illumination procedure, that is usually required to grow NPs at long wavelengths, can be substituted by a pre-irradiation step at 455 nm for a much shorter period of time. A careful planning of irradiation wavelengths and times has the potential to convert selectively part of one shape population into another towards the isolation of NP mixtures with tailored composition.
In conclusion, a microfluidic setup for the photochemical nucleation and shape conversion of spherical, decahedral and triangular silver nanoparticles with LED light has been presented. The small thickness of the colloidal layer flowing through a microreactor and its efficient oxygenation reduced the conversion time and enhanced the degree of morphological control of the final NP samples. A multistep approach has also been proposed for shape conversion at long wavelengths, which are hardly absorbed by the spherical seed NPs, but readily convert decahedral ones. We plan to exploit this microfluidic approach by using multiple wavelengths to produce morphologically homogeneous NPs samples and further increase our control over the growth process (i.e. favouring the growth along a given crystallographic direction),16 develop SERS applications and carry out fundamental studies alike.
Support for this work has been provided by MIUR (through PRIN 20085M27SS and FIRB NANOSOLAR RBAP11C58Y).
Notes and references
- K. M. Mayer and J. H. Hafner, Chem. Rev., 2011, 111, 3828–3857 CrossRef CAS.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2002, 107, 668–677 CrossRef.
- M. Rycenga, C. M. Cobley, J. Zeng, W. Li, C. H. Moran, Q. Zhang, D. Qin and Y. Xia, Chem. Rev., 2011, 111, 3669–3712 CrossRef CAS.
- C. Wiles, Micro reaction technology in organic synthesis, Taylor & Francis, Boca Raton, FL, 2011 Search PubMed.
- M. Oelgemöller and O. Shvydkiv, Molecules, 2011, 16, 7522–7550 CrossRef.
- X. Z. Lin, A. D. Terepka and H. Yang, Nano Lett., 2004, 4, 2227–2232 CrossRef CAS.
- V. Sebastian Cabeza, S. Kuhn, A. A. Kulkarni and K. F. Jensen, Langmuir, 2012, 28, 7007–7013 CrossRef CAS.
- X. Zheng, X. Zhao, D. Guo, B. Tang, S. Xu, B. Zhao, W. Xu and J. R. Lombardi, Langmuir, 2009, 25, 3802–3807 CrossRef CAS.
- K. G. Stamplecoskie and J. C. Scaiano, J. Am. Chem. Soc., 2010, 132, 1825–1827 CrossRef CAS.
- X. Wu, P. L. Redmond, H. Liu, Y. Chen, M. Steigerwald and L. Brus, J. Am. Chem. Soc., 2008, 130, 9500–9506 CrossRef CAS.
- S. Chen and D. L. Carroll, Nano Lett., 2002, 2, 1003–1007 CrossRef CAS.
- R. Jin, Y. Cao, C. A. Mirkin, K. L. Kelly, G. C. Schatz and J. G. Zheng, Science, 2001, 294, 1901–1903 CrossRef CAS.
- C. Xue, G. S. Métraux, J. E. Millstone and C. A. Mirkin, J. Am. Chem. Soc., 2008, 130, 8337–8344 CrossRef CAS.
- M. Maillard, P. Huang and L. Brus, Nano Lett., 2003, 3, 1611–1615 CrossRef CAS.
- Z. T. Cygan, J. T. Cabral, K. L. Beers and E. J. Amis, Langmuir, 2005, 21, 3629–3634 CrossRef CAS.
- R. Jin, Y. Charles Cao, E. Hao, G. S. Metraux, G. C. Schatz and C. A. Mirkin, Nature, 2003, 425, 487–490 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: S1, a flow photoreactor for the formation of silver seed nanoparticles; S2, transmission electron microscopy analysis of silver colloids; S3, silver NP growth according to the photovoltage model; S4, fabrication of the polymer microstructured reactor chip; S5, description of LED modules used for sample irradiation. See DOI: 10.1039/c2cc35652j |
| This journal is © The Royal Society of Chemistry 2013 |
