Lab-on-graphene: graphene oxide as a triple-channel sensing device for protein discrimination†
Yuexiang
Lu
,
Hao
Kong
,
Fang
Wen
,
Sichun
Zhang
* and
Xinrong
Zhang
*
Department of Chemistry, Beijing Key Laboratory of Microanalytical Methods and Instrumentation, Tsinghua University, Beijing, 100084, P.R. China. E-mail: sczhang@mail.tsinghua.edu.cn; xrzhang@mail.tsinghua.edu.cn; Fax: +86 010 62787678; Tel: +86 010 62787678
First published on 6th November 2012
Abstract
The fluorescence, catalytic activity and assembly behavior of GO could be simultaneously changed after interaction with proteins, leading to distinct response patterns related to each specific protein. Based on the phenomenon, a triple-channel optical sensor has been proposed in the present communication for protein discrimination with GO as a single sensing element.
A chemical sensor array, which is able to respond differentially to a variety of analytes, has been used for the discrimination and recognition of a wide range of analytes.1 The two key requirements of sensor array application are adding more information to improve the sensing performance and miniaturizing the sensing device. However, adding more information often requires adding more sensing elements to the array, which is conflicted with miniaturization. An alternative way to solve this problem is to extract more than one signal from single sensing material, by recording signals from fundamentally different transduction principles (electric, optic, and colorimetric).2 In the past few years, various materials, such as organic compounds,3 nanoparticles4 and quantum dots,5 have been used for multi-channel sensing and known as ‘lab-on-a-molecule’ or ‘lab-on-a-nanoparticle’. However, as materials with multidimensional information are rare or complicated for synthesis, it is still a challenge to develop new materials with different transduction principles for high-order sensing.
Graphene Oxide (GO), as a two-dimensional platform, has received much attention in medical diagnosis and biosensing because of its unique characteristics such as facile surface modification, biocompatibility, high mechanical strength, and good water dispersibility.6 However, in most cases, the role of GO in biosensing has been limited to a quenching substrate or a signal transducer.7 Recently, GO with near-infrared (NIR), visible and ultraviolet fluorescence has been reported, and the intrinsic and tuneable fluorescence could be quenched by fluorescence resonance energy transfer (FRET) or photoinduced charge transfer (PCT) between GO sheets and AuNPs/bio targets.8 It has also been found that carboxyl-modified graphene oxide (GO–COOH) has peroxidase-like activity that can catalyze the reaction of the peroxidase substrate in the presence of H2O2 to produce a color reaction, which has been utilized for colorimetric sensing of H2O2, glucose, DNA and cancer biomarker.9 Furthermore, GO exhibited different 2D or even 3D assembly behavior when interacting with proteins and DNA under different conditions.10 These target-induced aggregations are also attractive for sensing, but have not been utilized.
In our preliminary experiments, we found that the intrinsic fluorescence intensities of GO varied with the modification of different proteins on the GO surface. At the same time, the change of catalytic activity of GO was protein dependent. Moreover, the addition of proteins caused the assembly of GO, the assembly manner was closely related to the properties of proteins. Based on the phenomenon, we applied GO as a triple-channel sensing device (fluorescence, colorimetric and turbidity) for protein discrimination (Scheme 1). The triple-channel sensing device can be considered as a virtual sensor array giving a unique response pattern for each target.
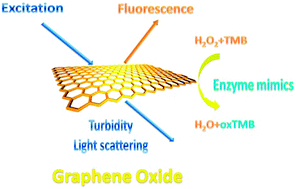 | ||
| Scheme 1 Schematic illustration of triple-channel properties of GO. | ||
GO with multiple properties was synthesized by a modified Hummers method (Fig. S1, ESI†).8e,11 The intrinsic fluorescence of GO shows a broad emission peak at around 600 nm when excited by 450 nm (Fig. 1).8a–c,e The colorimetric signals could be observed by recording the absorbance at 652 nm after adding 3,3′,5,5′-tetramethylbenzidine (TMB) and H2O2 into the solution (Fig. 1).9 The assembly behaviour results in changes of the size and shape characteristic of GO sheets and can be monitored by light scattering or turbidity signals (Fig. 1).12
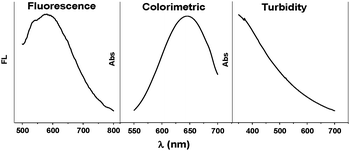 | ||
| Fig. 1 Fluorescence (Ex = 450 nm), colorimetric and turbidity signal of GO. | ||
As a proof-of-concept system, we chose six proteins that have different molecular weight (MW), isoelectric point (pI) and metal/non-metal containing properties as the sensing targets (Table 1). Upon adding different proteins into GO solution, GO assembled into different GO–protein hybrids and the turbidity signal was obtained by recording the absorbance at 405 nm (Fig. 2). Hem and Myo are observed to cause significant aggregation and increase the turbidity signal by 30% and 8% respectively. While Lys and TRF decreased the signal by 8% and 4%. Pep and Pap gave less impact under this condition, the signal was increased by about 3%.
| Protein | M W (kDa) | pI | Metal |
|---|---|---|---|
| Hemoglobin (Hem) | 64.5 | 6.8 | Yes |
| Pepsin (Pep) | 35 | 1–2.5 | No |
| Papain (Pap) | 23.0 | 9.6 | No |
| Lysozyme (Lys) | 14.4 | 9.6–11 | No |
| Myoglobin (Myo) | 17.0 | 7.2 | Yes |
| Transferrin (TRF) | ∼75 | 5.6 | Yes |
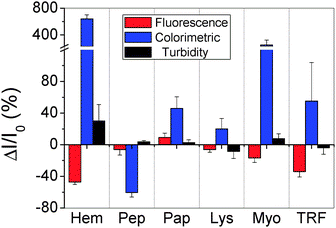 | ||
| Fig. 2 Fingerprints of six selected proteins based on the patterns for the corresponding values of ΔI/I0 obtained from the FL, colorimetric and turbidity signal. | ||
We then investigated the FL behavior of GO after interacting with different proteins by monitoring the FL intensity at 600 nm (Fig. 2). We found that Pap slightly increased the FL by 0.9%, while all the other proteins quenched the FL with different degrees. Pep and Lys quenched the FL by about 6%. Hem, Myo and TRF, the metal-containing proteins, decreased the FL intensity more efficiently, as they quenched the FL by 47%, 17% and 34% respectively.
We further studied the catalytic activity of different GO–protein hybrids, by recording the absorbance at 652 nm 10 min after adding TMB and H2O2 into the solution (Fig. 2). Pep was found to decrease the colorimetric signal by 60%, while TRF, Pap and Lys increased signals by 56%, 46% and 20% respectively. Hem and Myo increased colorimetric signals much more efficiently, as they increased the signals by 640% and 252%.
The mechanism for generating distinct response patterns can be explained as follows. GO could interact with proteins by various supramolecular interactions including hydrogen bonding, coordination, electrostatic interaction and π–π stacking.13 Proteins can either be attached on the GO surface or act as physical cross linkers of GO sheets, which result in different 2D or 3D hybrid structures and different turbidity signals.10a,b Since the non-covalent attraction between proteins and GO is a weak intermolecular interaction, the structure of both proteins and GO has not been changed significantly. All the GO–protein hybrids retained the fluorescence and catalytic activity although with different degrees. For the metal-containing proteins, they quenched the FL and increased the catalytic activity as all these proteins contain the Fe heme, which is an effective electron-transfer quencher for FL as well as a peroxidase mimic.8f,14 For other GO–protein hybrids, there was a balance between more stable structure for catalysis10a and more inhibited active sites, which resulted in an increase or decrease in the catalytic activity.15
Linear discriminant analysis (LDA), a statistical technique, was used to differentiate quantitatively the three-channel response patterns of the GO with target proteins.16 Three canonical factors were generated (79.7%, 19.5% and 0.8%) that represent linear combinations of the response matrices obtained from the three-channel response patterns (three channels × six proteins × four replicates). The 24 training cases were separated into six respective groups, with 100% accuracy according to the classification matrix derived from the analysis of subsets of the datasets, and the two factors that were most significant are plotted in 2D (Fig. 3). The detection efficiency was tested by discriminating unknown samples. The discrimination accuracy of the unknown samples was found to be 100% (Tables S2 and S3, ESI†). At 500 nM, the 24 training cases were separated into six respective groups with 95.8% accuracy (Fig. S2, ESI†) and the discrimination accuracy of the unknown samples was 93.3% (Tables S4 and S5, ESI†). At 250 nM, the six proteins could not be all discriminated (data not shown). It was demonstrated that the sensitivity and differentiability of our technique was high enough to detect and identify proteins at 500 nM, which is comparable to recently proposed methods based on the “chemical tongue” strategy (0.2 μM–0.5 μM).1e,5,17 The sensor could also discriminate proteins with close structures (Fig. S3, ESI†).
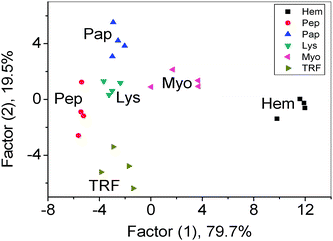 | ||
| Fig. 3 Canonical score plot for the three-channel patterns as obtained from LDA for six proteins at 1 μM. | ||
For a sensor array, it is a challenge to discriminate different proteins at various concentration levels.1e To illustrate the application potential of our system, we performed experiments with Hem and Pep at two different concentrations (500 nM and 1 μM). We found that the LDA plot for various concentrations followed certain patterns and can be differentiated from each other. Additionally, the response from different concentrations of one protein forms patterns around a centre and so each protein can be discriminated (Fig. 4). After training of the sensor array, the discrimination accuracy of 11 unknown samples was found to be 100% (Tables S6 and S7, ESI†).
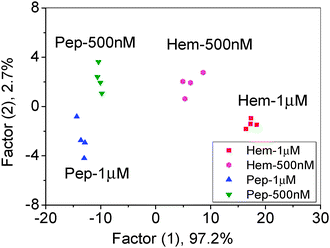 | ||
| Fig. 4 Canonical score plot for the three-channel patterns as obtained from LDA for Hem and Pep at different concentrations. | ||
In summary, we found that analyte proteins can interact with graphene oxide and influence the fluorescence, catalytic activity and assembly behavior of GO simultaneously. As different proteins gave distinct influence patterns, protein discrimination could be achieved on the triple-channel device in a ‘lab-on-graphene‘ approach. Given the good biocompatibility and diversity of graphene-based materials, this “proof-of-principle” study will broaden the application field of graphene-based materials in bio application. In our ongoing studies, by chemical modification and interaction with a wide range of organic and inorganic materials, GOs with unusual properties are obtained to add more signals to each sensing element and add more sensing elements to the array. Then, we can improve the sensing performance and miniaturize the sensing device simultaneously.
We acknowledge the National Natural Foundation of China (Grant No. 21027013 and No. 21125525), and the Tsinghua University Initiative Scientific Research Program.
Notes and references
- (a) K. J. Albert, N. S. Lewis, C. L. Schauer, G. A. Sotzing, S. E. Stitzel, T. P. Vaid and D. R. Walt, Chem. Rev., 2000, 100, 2595–2626 CrossRef CAS; (b) N. A. Rakow and K. S. Suslick, Nature, 2000, 406, 710–713 CrossRef CAS; (c) S. H. Lim, L. Feng, J. W. Kemling, C. J. Musto and K. S. Suslick, Nat. Chem., 2009, 1, 562–567 CrossRef CAS; (d) C.-C. You, O. R. Miranda, B. Gider, P. S. Ghosh, I.-B. Kim, B. Erdogan, S. A. Krovi, U. H. F. Bunz and V. M. Rotello, Nat. Nanotechnol., 2007, 2, 318–323 CrossRef CAS; (e) M. De, S. Rana, H. Akpinar, O. R. Miranda, R. R. Arvizo, U. H. F. Bunz and V. M. Rotello, Nat. Chem., 2009, 1, 461–465 CrossRef CAS.
- A. Hierlemann and R. Gutierrez-Osuna, Chem. Rev., 2008, 108, 563–613 CrossRef CAS.
- M. Schmittel and H. W. Lin, Angew. Chem., Int. Ed., 2007, 46, 893–896 CrossRef CAS.
- D. Liu, M. Liu, G. Liu, S. Zhang, Y. Wu and X. Zhang, Anal. Chem., 2010, 82, 66–68 CrossRef CAS.
- P. Wu, L. N. Miao, H. F. Wang, X. G. Shao and X. P. Yan, Angew. Chem., Int. Ed., 2011, 50, 8118–8121 CrossRef CAS.
- (a) K. P. Loh, Q. L. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS; (b) N. Mohanty and V. Berry, Nano Lett., 2008, 8, 4469–4476 CrossRef CAS; (c) Y. Ohno, K. Maehashi, Y. Yamashiro and K. Matsumoto, Nano Lett., 2009, 9, 3318–3322 CrossRef CAS; (d) C.-H. Lu, H.-H. Yang, C.-L. Zhu, X. Chen and G.-N. Chen, Angew. Chem., Int. Ed., 2009, 48, 4785–4787 CrossRef CAS; (e) S. He, B. Song, D. Li, C. Zhu, W. Qi, Y. Wen, L. Wang, S. Song, H. Fang and C. Fan, Adv. Funct. Mater., 2010, 20, 453–459 CrossRef CAS; (f) D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC; (g) S. J. Guo and S. J. Dong, Chem. Soc. Rev., 2011, 40, 2644–2672 RSC.
- S. S. Chou, M. De, J. Luo, V. M. Rotello, J. Huang and V. P. Dravid, J. Am. Chem. Soc., 2012, 134, 16725–16733 CrossRef CAS.
- (a) G. Eda, Y. Y. Lin, C. Mattevi, H. Yamaguchi, H. A. Chen, I. S. Chen, C. W. Chen and M. Chhowalla, Adv. Mater., 2010, 22, 505–509 CrossRef CAS; (b) X. M. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. J. Dai, Nano Res., 2008, 1, 203–212 CrossRef CAS; (c) Q. S. Mei, K. Zhang, G. J. Guan, B. H. Liu, S. H. Wang and Z. P. Zhang, Chem. Commun., 2010, 46, 7319–7321 RSC; (d) J. L. Chen and X. P. Yan, Chem. Commun., 2011, 47, 3135–3137 RSC; (e) J. H. Jung, D. S. Cheon, F. Liu, K. B. Lee and T. S. Seo, Angew. Chem., Int. Ed., 2010, 49, 5708–5711 CrossRef CAS; (f) J. L. Chen, X. P. Yan, K. Meng and S. F. Wang, Anal. Chem., 2011, 83, 8787–8793 CrossRef CAS.
- Y. Song, K. Qu, C. Zhao, J. Ren and X. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS.
- (a) C. Huang, H. Bai, C. Li and G. Shi, Chem. Commun., 2011, 47, 4962–4964 RSC; (b) Y. Xu, Q. Wu, Y. Sun, H. Bai and G. Shi, ACS Nano, 2010, 4, 7358–7362 CrossRef CAS; (c) S. H. Lee, H. W. Kim, J. O. Hwang, W. J. Lee, J. Kwon, C. W. Bielawski, R. S. Ruoff and S. O. Kim, Angew. Chem., Int. Ed., 2010, 49, 10084–10088 CrossRef CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- (a) K. Matsuzaki, O. Murase, K. Sugishita, S. Yoneyama, K. Akada, M. Ueha, A. Nakamura and S. Kobayashi, Biochim. Biophys. Acta Biomembr., 2000, 1467, 219–226 CrossRef CAS; (b) M. M. A. Elsayed and G. Cevc, Pharm. Res., 2011, 28, 2204–2222 CrossRef CAS.
- (a) Y. Sun, C. Li, Y. Xu, H. Bai, Z. Yao and G. Shi, Chem. Commun., 2010, 46, 4740–4742 RSC; (b) Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef CAS.
- Y. Guo, L. Deng, J. Li, S. Guo, E. Wang and S. Dong, ACS Nano, 2011, 5, 1282–1290 CrossRef CAS.
- Y. Song, W. Wei and X. Qu, Adv. Mater., 2011, 23, 4215–4236 CrossRef CAS.
- P. C. Jurs, G. A. Bakken and H. E. McClelland, Chem. Rev., 2000, 100, 2649–2678 CrossRef CAS.
- H. Kong, Y. Lu, H. Wang, F. Wen, S. Zhang and X. Zhang, Anal. Chem., 2012, 84, 4258–4261 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthesis, characterization, methods employed for protein discrimination and data tables. See DOI: 10.1039/c2cc37293b |
| This journal is © The Royal Society of Chemistry 2013 |
