Enhancing CO2 adsorption of a Zn-phosphonocarboxylate framework by pore space partitions†
Yun
Ling
,
Mingli
Deng
,
Zhenxia
Chen
,
Bing
Xia
,
Xiaofeng
Liu
,
Yongtai
Yang
,
Yaming
Zhou
* and
Linhong
Weng
Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, P. R. China. E-mail: ymzhou@fudan.edu.cn; Fax: +86 21 65643925; Tel: +86 21 65642261
First published on 5th November 2012
Abstract
Using structure-directing agents, pore space partitions of a Zn-phosphonocarboxylate framework have been achieved. Selective adsorption of CO2 over N2 has been greatly improved from ca. 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 94
1 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Porous materials with selective adsorption of CO2 are highly desired.1 Metal–organic frameworks (MOFs) are considered to be ideal porous materials,2 because of their tunable host–guest interactions,3 adjustable pore size,4 and infinite structural possibilities.5
To enhance CO2 adsorption and separation, various methods have been proposed to modify MOFs, such as constructing MOFs with high surface areas,6 grafting polar groups (–OH, –NH2, etc.) or exposing metal sites,7 adjusting pore sizes for size-dependent separation,8 and synthesizing flexible frameworks for kinetic separation.9 Recently, it has been realized that a high surface area and large pore volume may not necessarily lead to a high CO2 adsorption under ambient conditions.10 How to efficiently utilize the pore space to improve CO2 adsorption has therefore become a hot topic. A feasible method is pore space partition (PSP), proposed recently.10a The PSP process can be viewed as building molecular-scale walls in a known structure to divide its original large space into segments. However, few examples are known to date10 due to the great challenge to locate components into MOFs during crystallization.
Multi-functional phosphonate-based MOFs are robust, porous materials.11 In the synthesis of these MOFs, structure-directing agents (SDAs) are widely used. Some SDAs can be incorporated into the framework during crystallization, while some of them can not,12 which provides us with a good platform to study PSP in MOFs. The purpose of this paper is to present such an example. The rutile-type Zn(II)-phosphonocarboxylate framework, {[Zn3(pbdc)2]·2H3O}n (H4pbdc = 5-phosphonobenzene-1,3-dicarboxylic acid, ZnPC-2), is a 3D porous structure synthesized by using triethylamine (TEA) as a SDA (Fig. 1).11c By using pyrrolidine (PYR) and piperidine (PIP) to replace TEA, two iso-reticular frameworks, {[Zn3(pbdc)2]·HPYR·H3O·4H2O}n (HPYR@ZnPC-2) and {[Zn3(pbdc)2]·HPIP·H3O·5H2O}n (HPIP@ZnPC-2), have been isolated respectively, in which the incorporated HPYP and HPIP serve as the molecular-scale walls, dividing the original channel structures into segments. Gas adsorption results show that the selective adsorption of CO2 over N2 has been improved from ca. 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for ZnPC-2 to 94
1 for ZnPC-2 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for Hpip@ZnPC-2.
1 for Hpip@ZnPC-2.
![A view of the 3D porous structure of ZnPC-2 constructed by 6-connected [Zn3(PO3)2(COO)4] (Zn3-SBU) and 3-connected pbdc ligands.](/image/article/2013/CC/c2cc37174j/c2cc37174j-f1.gif) | ||
| Fig. 1 A view of the 3D porous structure of ZnPC-2 constructed by 6-connected [Zn3(PO3)2(COO)4] (Zn3-SBU) and 3-connected pbdc ligands. | ||
3D porous iso-structures of ZnPC-2, HPYR@ZnPC-2 and HPIP@ZnPC-2 crystallize in the same tetragonal system, I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2d space group, which has a crystallographically imposed twofold symmetry (Table S1, Fig. S1, ESI†). Pure crystalline phase of ZnPC-2 was isolated when TEA was used as the SDA (Fig. S2 and S3, ESI†). The host framework is constructed by 6-connected Zn3-SBUs and tritopic pbdc ligands, showing a (4·62)2(42·610·83) topology (Fig. 1). Two types of channels (ca. 6 × 6 Å along the c axis and 6 × 4 Å along the a and b axes, Fig. 2) are interconnected with each other (Fig. 2a). When TEA was replaced by PYR and PIP in the synthetic procedure, HPYR@ZnPC-2 and HPIP@ZnPC-2 were successfully isolated as the pure crystalline phase respectively (Fig. 2, Fig. S2 and S3, ESI†). Because of the incorporation of SDAs, the cell volume is enlarged from 7785 Å3 for ZnPC-2 to 7898 and 7928 Å3 for HPYR@ZnPC-2 and HPIP@ZnPC-2, respectively (Table S1, ESI†). According to the single-crystal diffraction results, HPYR and HPIP are located in the small channels and are connected to the host framework by strong hydrogen bonding interactions. Their amino groups point towards the Zn3-SBUs and are completely covered by the host framework (Fig. S4, ESI†). Notably, their alkyl groups protrude into the large channels (Fig. 2). If considering the large channel as an idealized rectangular channel with small windows on each side, the located organic molecules, therefore, can be regarded as a kind of molecular-scale wall, which divides large channel into uniform segments with a length of ca. 12 Å and an entrance size of ca. 5.3 Å for HPYR@ZnPC-2 and ca. 4.8 Å for HPIP@ZnPC-2 (Fig. 3, Fig. S5, ESI†). From ZnPC-2 to HPYR@ZnPC-2 and HPIP@ZnPC-2, the PSP has been achieved in the rutile-type phosphonate-based MOF.
2d space group, which has a crystallographically imposed twofold symmetry (Table S1, Fig. S1, ESI†). Pure crystalline phase of ZnPC-2 was isolated when TEA was used as the SDA (Fig. S2 and S3, ESI†). The host framework is constructed by 6-connected Zn3-SBUs and tritopic pbdc ligands, showing a (4·62)2(42·610·83) topology (Fig. 1). Two types of channels (ca. 6 × 6 Å along the c axis and 6 × 4 Å along the a and b axes, Fig. 2) are interconnected with each other (Fig. 2a). When TEA was replaced by PYR and PIP in the synthetic procedure, HPYR@ZnPC-2 and HPIP@ZnPC-2 were successfully isolated as the pure crystalline phase respectively (Fig. 2, Fig. S2 and S3, ESI†). Because of the incorporation of SDAs, the cell volume is enlarged from 7785 Å3 for ZnPC-2 to 7898 and 7928 Å3 for HPYR@ZnPC-2 and HPIP@ZnPC-2, respectively (Table S1, ESI†). According to the single-crystal diffraction results, HPYR and HPIP are located in the small channels and are connected to the host framework by strong hydrogen bonding interactions. Their amino groups point towards the Zn3-SBUs and are completely covered by the host framework (Fig. S4, ESI†). Notably, their alkyl groups protrude into the large channels (Fig. 2). If considering the large channel as an idealized rectangular channel with small windows on each side, the located organic molecules, therefore, can be regarded as a kind of molecular-scale wall, which divides large channel into uniform segments with a length of ca. 12 Å and an entrance size of ca. 5.3 Å for HPYR@ZnPC-2 and ca. 4.8 Å for HPIP@ZnPC-2 (Fig. 3, Fig. S5, ESI†). From ZnPC-2 to HPYR@ZnPC-2 and HPIP@ZnPC-2, the PSP has been achieved in the rutile-type phosphonate-based MOF.
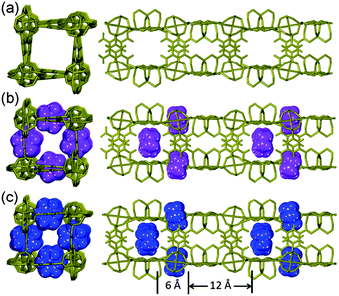 | ||
| Fig. 2 3D iso-recticular structures of ZnPC-2 (a), HPYR@ZnPC-2 (b) and HPIP@ZnPC-2 (c) (deep yellow: the 3D framework; violet: HPYR and blue: HPIP). | ||
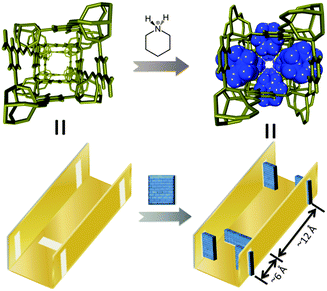 | ||
| Fig. 3 A view of the channel structure of the rutile-type ZnPC-2 before and after PSP. | ||
TG-MS analyses were then carried out to assess their thermal stability (Fig. S6, ESI†). The results show that in the temperature range 30–300 °C, about 8.4% and 13.8% weight loss was observed for HPYR@ZnPC-2 and HPIP@ZnPC-2 respectively, which can be attributed to the release of water molecules. The mass signals of pyrrolidine (b.p. 89 °C, F.W. 71.1) and piperidine (b.p. 106 °C, F.W. 85.1) were detected at ca. 460 °C after decomposition of the host framework, respectively. These results agree well with the above structural analyses that the guests of HPYR and HPIP are strongly bonded to the host framework by hydrogen bonding interactions.
The framework integrity of the three samples was confirmed by the PXRD patterns. Before the gas adsorption studies, all three samples were activated at 150 °C under vacuum for 8 h. Like most phosphonate-based MOFs,11a there was no significant adsorption of N2 at 77 K. This could be related to the presence of kinetic barriers in the phosphonate-based MOFs for N2 at low temperature.13 The surface area was then calculated14 by a probe atom with a radius of 1.84 Å (Table S2, ESI†). The theoretically accessible surface area for ZnPC-2 is ca. 1429.28 Å2 per cell (1497 m2 g−1), which is much higher than that of HPYR@ZnPC-2 (909.17 Å2 per cell, 822 m2 g−1) and HPIP@ZnPC-2 (811.91 Å2 per cell, 709 m2 g−1). The decrease in the surface area would be mainly related to the blocking of the small channels along the a and b axes.
CO2 adsorption was then carried out. As illustrated in Fig. 4a, with the increase of pressure, the CO2 adsorption in HPYR@ZnPC-2 and HPIP@ZnPC-2 was significantly higher than that of ZnPC-2. At 0.15 bar, the uptake amount of CO2 is 31.1 mg g−1 and 49.6 mg g−1 for HPYR@ZnPC-2 and HPIP@ZnPC-2 respectively, much higher than that of ZnPC-2 (11.5 mg g−1). The selectivity is then calculated based on the adsorption data of CO2 at 0.15 bar and N2 at 0.75 bar.2e The selectivity is ca. 9 for ZnPC-2 and ca. 27 and 94 for HPYR@ZnPC-2 and HPIP@ZnPC-2, respectively (Table S3, ESI†). The uptake amount and selectivity for HPIP@ZnPC-2 are comparable to amino-functionalized MOFs by the post-exchange method15 and are the highest in phosphonate-based MOFs as far as we know. At 1 bar, the uptake amount of CO2 for HPYR@ZnPC-2 and HPIP@ZnPC-2 is three times more than that of ZnPC-2 (24.3 mg g−1). Furthermore, the desorption curve almost coincides with the adsorption curve (Fig. S7, ESI†); no marked hysteresis was observed. This suggests that there is no strong interaction between CO2 and the framework, which is consistent with the characteristic of the structure that the amino groups are completely covered by the host framework. High pressure adsorption results show that even at 20 bar, the uptake amount of CO2 on ZnPC-2 can only reach 42.7 mg g−1 (Fig. 4c). In contrast, the uptake amount reaches 149.2 mg g−1 on HPIP@ZnPC-2. Slight substeps in the adsorption isotherms for HPYR@ZnPC-2 and HPIP@ZnPC-2 are observed around 8 bar and 7 bar, respectively. These phenomena may be ascribed to further adsorption of CO2 at the corners formed by the molecular-scale wall with the host framework. All these results illustrate that the adsorption amount and the selective adsorption ability of CO2 have been greatly improved after the PSP.
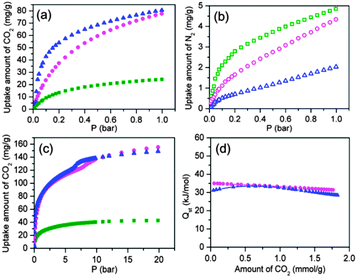 | ||
| Fig. 4 Adsorption isotherms of CO2 (a) and N2 (b) at 298 K (olive square: ZnPC-2, magneta sphere: HPYR@ZnPC-2, blue triangle: HPIP@ZnPC-2); (c) high pressure CO2 adsorption isotherms of ZnPC-2, HPYR@ZnPC-2 and HPIP@ZnPC-2; (d) the CO2 adsorption enthalpy at zero coverage of HPYR@ZnPC-2 and HPIP@ZnPC-2. | ||
The isosteric heat of CO2 adsorption was then calculated by the Virial method based on their adsorption isothermals at 298 K and 288 K (Fig. S8, ESI†). The enthalpies at zero coverage are ca. 36 and 32 kJ mol−1 for HPYR@ZnPC-2 and HPIP@ZnPC-2, respectively (Fig. 4d), which are lower than some amino-functionalized MOFs (Table S3, ESI†). These results illustrate that this kind of MOF can be easily regenerated after adsorption of CO2. To illustrate the reversible CO2 adsorption from the gas mixture, selective adsorption was further carried out using HPIP@ZnPC-2 as the adsorbent on a TG apparatus at 305 K and atmospheric pressure (Fig. S9, ESI†). The gas cycling experiment was carried out using a flow of CO2 in N2 (V(CO2)/V(N2): 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), and followed by a flow of pure N2 gas. A weight increase of ca. 0.45 wt% was quickly achieved after switching to mixed gases, and a quick weight loss was observed after switching to pure N2 gas. The process can be cycled several times.
4), and followed by a flow of pure N2 gas. A weight increase of ca. 0.45 wt% was quickly achieved after switching to mixed gases, and a quick weight loss was observed after switching to pure N2 gas. The process can be cycled several times.
In summary, PSP has been realized in a Zn-phosphonocarboxylate framework by using appropriate SDAs for the first time. Gas adsorption results confirm that the uptake amount of CO2 was greatly enhanced. Selective adsorption of CO2 over N2 on HPIP@ZnPC-2 is comparable to most reported carboxylate-based MOFs2e and is the highest in phosphonate-based MOFs up to now.
This work was financially supported by NSFC (No. 21171042, No. 91027044, No. 21101031) and Shanghai Leading Academic Discipline Project (Project No. B108). The authors acknowledge helpful discussion from Prof. Qiwei Li in the Dept. Chem., Fudan University.
Notes and references
- (a) R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173–1177 RSC; (b) D. M. D'Alessandro and T. McDonald, Pure Appl. Chem., 2011, 83, 57–66 CrossRef CAS; (c) H. Kim, Y. Kim, M. Yoon, S. Linn, S. M. Park, G. Seo and K. Kim, J. Am. Chem. Soc., 2010, 132, 12200–12202 CrossRef CAS; (d) D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef CAS.
- (a) H. L. Jiang and Q. Xu, Chem. Commun., 2011, 47, 3351–3370 RSC; (b) A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58–67 CrossRef CAS; (c) D. Britt, H. Furukawa, B. Wang, T. G. Glover and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20637–20640 CrossRef CAS; (d) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS; (e) S. C. Xiang, Y. B. He, Z. J. Zhang, H. Wu, W. Zhou, R. Krishna and B. L. Chen, Nat. Commun., 2012, 3, 954 CrossRef; (f) Y. S. Bae and R. Q. Snurr, Angew. Chem., Int. Ed., 2011, 50, 11586–11596 CrossRef CAS.
- (a) Z. Q. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC; (b) S. J. Garibay, Z. Q. Wang, K. K. Tanabe and S. M. Cohen, Inorg. Chem., 2009, 48, 7341–7349 CrossRef CAS; (c) Y. G. Zhao, H. H. Wu, T. J. Emge, Q. H. Gong, N. Nijem, Y. J. Chabal, L. Z. Kong, D. C. Langreth, H. Liu, H. P. Zeng and J. Li, Chem.–Eur. J., 2011, 17, 5100–5108 Search PubMed; (d) A. Torrisi, R. G. Bell and C. Mellot-Draznieks, Cryst. Growth Des., 2010, 10, 2839–2841 CrossRef CAS.
- (a) D. Zhao, D. J. Timmons, D. Yuan and H.-C. Zhou, Acc. Chem. Res., 2011, 44, 123–133 CrossRef CAS; (b) S. Kitagawa and R. Matsuda, Coord. Chem. Rev., 2007, 251, 2490–2509 CrossRef CAS.
- (a) D. J. Tranchemontagne, J. L. Mendoza-Cortes, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257–1283 RSC; (b) J. J. Perry, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC; (c) M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675–702 CrossRef CAS.
- A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998–17999 CrossRef CAS.
- (a) R. Babarao and J. W. Jiang, Ind. Eng. Chem. Res., 2011, 50, 62–68 CrossRef CAS; (b) W. Morris, B. Leung, H. Furukawa, O. K. Yaghi, N. He, H. Hayashi, Y. Houndonougbo, M. Asta, B. B. Laird and O. M. Yaghi, J. Am. Chem. Soc., 2010, 132, 11006–11008 CrossRef CAS; (c) R. Vaidhyanathan, S. S. Iremonger, K. W. Dawson and G. K. H. Shimizu, Chem. Commun., 2009, 5230–5232 RSC; (d) J. P. Zhang, A. X. Zhu, R. B. Lin, X. L. Qi and X. M. Chen, Adv. Mater., 2011, 23, 1268–1269 CrossRef CAS; (e) Y. S. Bae, O. K. Farha, A. M. Spokoyny, C. A. Mirkin, J. T. Hupp and R. Q. Snurr, Chem. Commun., 2008, 4135–4137 RSC; (f) Z. Q. Wang, K. K. Tanabe and S. M. Cohen, Inorg. Chem., 2009, 48, 296–306 CrossRef CAS.
- (a) J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC; (b) R. Banerjee, H. Furukawa, D. Britt, C. Knobler, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 3875–3876 CrossRef CAS.
- (a) F. Salles, H. Jobic, A. Ghoufi, P. L. Llewellyn, C. Serre, S. Bourrelly, G. Ferey and G. Maurin, Angew. Chem., Int. Ed., 2009, 48, 8335–8339 CrossRef CAS; (b) S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef CAS; (c) H. S. Choi and M. P. Suh, Angew. Chem., Int. Ed., 2009, 48, 6865–6869 CrossRef CAS; (d) J. P. Zhang and X. M. Chen, J. Am. Chem. Soc., 2008, 130, 6010–6017 CrossRef CAS.
- (a) S.-T. Zheng, J. T. Bu, Y. Li, T. Wu, F. Zuo, P. Y. Feng and X. H. Bu, J. Am. Chem. Soc., 2010, 132, 17062–17064 CrossRef CAS; (b) S. Chen, J. Zhang, T. Wu, P. Y. Feng and X. H. Bu, J. Am. Chem. Soc., 2009, 131, 16027–16029 CrossRef CAS.
- (a) G. K. H. Shimizu, R. Vaidhyanathan and J. M. Taylor, Chem. Soc. Rev., 2009, 38, 1430–1449 RSC; (b) K. J. Gagnon, H. P. Perry and A. Clearfield, Chem. Rev., 2012, 112, 1034–1054 CrossRef CAS; (c) T. B. Liao, Y. Ling, Z. X. Chen, Y. M. Zhou and L. H. Weng, Chem. Commun., 2010, 46, 1100–1102 RSC.
- R. Xu, W. Pang, J. Yu and J. Cheng, Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure, John Wiley & Sons, 2007 Search PubMed.
- S. S. Iremonger, J. Liang, R. Vaidhyanathan and G. K. H. Shimizu, Chem. Commun., 2011, 47, 4430–4432 RSC.
- T. Dueren, F. Millange, G. Ferey, K. S. Walton and R. Q. Snurr, J. Phys. Chem. C, 2007, 111, 15350–15356 CAS.
- (a) J. An and N. L. Rosi, J. Am. Chem. Soc., 2010, 132, 5578–5579 CrossRef CAS; (b) A. Demessence, D. M. D'Alessandro, M. L. Foo and J. R. Long, J. Am. Chem. Soc., 2009, 131, 8784–8786 CrossRef CAS; (c) T. M. McDonald, D. M. D'Alessandro, R. Krishna and J. R. Long, Chem. Sci., 2011, 2, 2022–2028 RSC; (d) J. An and N. L. Rosi, J. Am. Chem. Soc., 2010, 132, 38–39 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of measurements, synthesis, figures and tables are available. CCDC reference numbers 746203, 776925 and 894708. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2cc37174j |
| This journal is © The Royal Society of Chemistry 2013 |
