Simultaneously sensitive detection of multiple miRNAs based on a strand displacement amplification†
Tian
Tian‡
a,
Heng
Xiao‡
a,
Xiaolian
Zhang
b,
Shuang
Peng
a,
Xiaoe
Zhang
a,
Shan
Guo
a,
Shaoru
Wang
*a,
Songmei
Liu
c,
Xin
Zhou
c,
Craig
Meyers
d and
Xiang
Zhou
*ae
aCollege of Chemistry and Molecular Sciences, Key Laboratory of Biomedical Polymers of Ministry of Education, Wuhan University, Wuhan, Hubei, 430072, P. R. China. E-mail: xzhou@whu.edu.cn; srwang@whu.edu.cn; Fax: +86-27-68756663
bState Key Laboratory of Virology, Department of Immunology, School of Medicine, Wuhan University, P. R. China
cZhongnan Hosptial, Wuhan University, P. R. China
dDepartment of Microbiology and Immunology H107, The Penn State College of Medicine, Hershey, PA 17033, U. S. A.
eState Key Laboratory of Natural and Biomimetic Drugs, Peking University, Beijing, P. R. China
First published on 5th November 2012
Abstract
We reported an efficient strategy based on a strand displacement amplification for serum miRNA detection. In such a system, a multiplexed, sensitive and quick detection of miRNAs could be achieved through a combination of fluorescence labeled probes, a common primer and a polymerase. This could be potentially used in the clinical field to achieve early disease diagnosis and prognosis.
MicroRNA (miRNA) is an important type of small functional RNA, whose length ranges from 18–25 nt in length.1,2 MiRNAs play important roles in gene regulation, which results from their degrading or blocking effects towards target mRNAs by complete or incomplete pairing at 3′ UTRs (un-translated regions). Recent reports have also demonstrated a close relationship between miRNAs and tumours.3 Thus, miRNA detection is significant for both fundamental research and disease diagnosis.
Traditional northern blot analyses have been widely used.4–7 To avoid the disadvantages associated with northern blotting, such as time-consuming procedures and limited sensitivity, RT-PCR,8–10 microarray based assays,11,12 nanoparticle-based detection,13 rolling cycle amplification14–16 and isothermal exponential amplification17,18 techniques were developed. Each of these methods have their own advantages, including high sensitivity19,20 and/or high throughput.21 To the best of our knowledge, the majority of these methods require expensive equipment and/or materials, such as microarrays and readers, electrochemical equipment or expensive imaging instruments.22 Therefore, the rapid, economical and sensitive detection of miRNA remains imperative.
Due to the low intracellular content and instability of miRNAs, rapid and efficient signal amplification is critical for the sensitive detection of miRNAs. PCR and/or isothermal amplification are commonly used signal-enhancing methods that are highly efficient. Recently, strand displacement amplification has been reported to be efficient for nucleic acid detection.23,24 Herein, we designed a fluorescent hairpin probe that can undergo a conformational change triggered by the hybridization of target miRNA and subsequent primer extension.
The probe was designed to have a hairpin configuration, which was labelled with a donor fluorophore at the 5′ end and a quencher at the 3′ end (Scheme 1). Black hole quencher (BHQ) was chosen as the fluorescence quenching group to label the 3′ end of our probes with a length of around 50 nucleotides, and we found that it could efficiently quench the fluorescence of carboxyfluorescein (FAM), Texas Red, Cy3 and Cy5 at the 5′ end. The loop region was fully complementary to the target miRNA. When the target miRNA was absent, the fluorescence was quenched due to the close proximity between the fluorophore and the quencher. A 9 bp primer contained a sequence that was complementary to the stem region at the 3′ end. With the addition of the target miRNA, the hairpin structure was opened upon hybridization between the miRNA and the probe at the loop region. Subsequently, the free primer annealed to the end of the probe, and a primer extension reaction happened in the presence of dNTPs and Bst polymerase (without exonuclease activity). Simultaneously, the target miRNA was displaced by the polymerization, and a duplex with full length was synthesized. The displaced miRNA subsequently bound to a new hairpin probe, and a new extension cycle then occurred. Taking advantage of the cycled primer extension, the newly synthesized duplex permanently separated the stems of the original hairpin probe, and an obvious enhancement of the fluorescence signal could be observed. Therefore, detection with a low background and high sensitivity could be achieved.
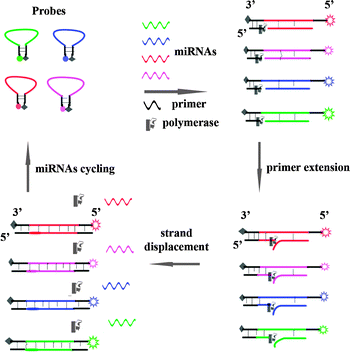 | ||
| Scheme 1 Schematic illustration of miRNA detection based on a strand displacement amplification triggered by target miRNA hybridization. | ||
To evaluate the efficiency of our strategy, we chose miR199a as the initial target for analysis.25 We conducted the analysis of increasing amounts of miR199a. As shown in Fig. 1, a clear trend of increasing fluorescence was identified upon analysis of increasing miR199a concentrations ranging from 0–50 nM. The detection limit of our method was 500 fM, which was similar to the limit for RT-PCR10 and the newly reported DSNSA methods.26 The extent of fluorescence enhancement showed a linear relationship with the logarithm (log) of the miR199a concentration within the range 500 fM–50 nM (fitting curve and equation in Fig. S1, ESI†), with a correlation equation of Fluorescence = 1316.55 + 106.36 log(miRNA) (R2 = 0.9657).
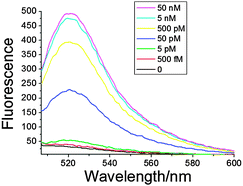 | ||
| Fig. 1 Concentration-dependent fluorescence response with different amounts of miRNA. The concentrations for Probe-199a1 and short primer are 50 nM and 500 nM, respectively, and 20 U of Bst polymerase was used in a 100 μL system. | ||
Gel electrophoresis was also performed to further clarify the target responsible for the polymerization triggered by the annealing of the primer to the probe. In the design of the hairpin probe (Table S1, ESI†), the recognition site of SpeI (5′-ACTAGT-3′) was introduced such that after polymerization via the recognition site, the formed duplex could be cut for separation and monitoring using gel electrophoresis. The digestion product of Probe-199a1 upon polymerization was analysed, and two expected bands were evident in the gel. In the absence of the target miR199a or Bst polymerase, the hairpin conformation would almost entirely quench the fluorescence signal, and thus, no bands could be observed on the gel. The intensity of the cleaved fragment increased in response to increasing concentrations of miR199a (Fig. S2, ESI†), which further demonstrated that our method was correctly designed.
To assess the sequence specificity of our probe, RNA199m with a high homology to miR199a and two members of the miR200 family (miR141 and miR429) were analysed using Probe-199a1.27,28 We used Probe-199a1 to analyze RNA199m, which possess a two-nucleotide difference from miR199a. Consistent with our expected results, an 8-fold decrease in the signal was observed when RNA199m was analysed using Probe-199a1 (Fig. S3, ESI†). Furthermore, there was almost no fluorescence enhancement in the presence of miR141 and miR429.
In many pathogenic processes, some miRNAs are typically upregulated, while some others are always downregulated.29,30 Thus, simultaneous detection of multiple miRNAs under a uniform condition is vital for the illustration of the miRNA expression profile in disease diagnosis and new drug development. To illustrate the multiplexing ability of our strategy, we used Cy3, Cy5, FAM and Texas Red to label Probe-21, Probe-155, Probe-199a and Probe-141, which were designed to individually target miR21, miR155, miR199a and miR141, respectively. The initial experiment illustrated a good detection of the different miRNAs at the four corresponding channels (Fig. S4, ESI†). Next, we prepared 15 samples with different combinations of the four miRNAs (Table S2, ESI†). As shown in Fig. 2, when only one type of miRNA was added, the solution only emitted the corresponding fluorescence of the respective probe. When all four target miRNAs were used, we obtained a correspondingly strong enhancement of the fluorescence in all four detection channels. When only two miRNAs were added to the system, two corresponding fluorescence signals were identified without any interference. We could directly identify the components and concentrations of miRNAs based on the fluorescence signals of the different channels. The presence of other miRNAs did not interfere with the signal response to specific miRNA. Therefore, the multiplexed detection of miRNAs could be easily achieved by our method.
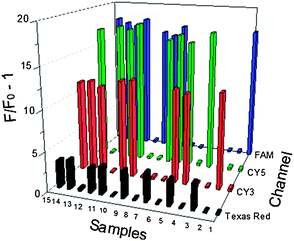 | ||
| Fig. 2 Multiplexed miRNAs detection. Four hairpin probes with their perfectly complementary miRNAs are labeled with different fluorophores. miR199 (FAM, blue emission at 518 nm); miR21 (CY3, orange emission at 580 nm); miR141 (Texas Red, red emission at 613 nm); miR155 (Cy5, red emission at 663 nm). | ||
Cell-free miRNAs (cf miRNAs) are known to be enriched in the small RNA portion fractionated from human body fluids.31,32 Unlike synthetic miRNAs, cf miRNAs in human sera are stable under a variety of conditions, such as high temperature, extreme pH, long-term storage and multiple thaw–freeze cycles.33 For a specific disease, several miRNAs have consistently had representative modes of expression level.34 Thus, cf miRNAs are potentially involved in the development of certain severe diseases.35 It was reported that human microRNA21 (miR21) and miR155 are involved in a variety of human cancers.36 Herein, we used Probe-21 and Probe-155 for the detection of human serum miRNAs (Table S1, ESI†). We used a Trizol extraction to obtain the total RNA from the human sera of four healthy people and four patients with breast tumours. After the extension step, the fluorescence of each reaction was measured. Consistent with previously published results,36 persons with breast cancer appeared to have more miR21 and miR155 in their sera than did the healthy peers (Fig. 3). Based on our results, the newly developed method could be used for further applications in future clinical diagnostics.
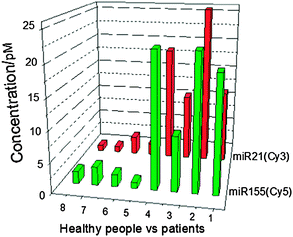 | ||
| Fig. 3 The multiplexed quantitation of extracted miRNAs from human serum via the Trizol method. Expression levels of miR21 and miR155 from four healthy people and four patients with breast cancer were analyzed. 1–4, patients with breast cancer; 5–8, healthy people. Bars represent the concentrations of the different miRNAs from our method. | ||
In summary, we have developed an efficient strategy based on a strand displacement amplification for the detection of miRNAs. Furthermore, a multiplexed detection system was implemented, and we achieved the simultaneous detection of four miRNAs. This method was successfully used in the analysis of practical samples. The performance of the detection process was simple and quick, and a high sensitivity could be achieved.
The authors thank the National Basic Research Program of China (973 Program) (2012CB720600, 2012CB720603, 2012CB720605), the National Science of Foundation of China (No. 90813031, 30973605, 21102108), the National Grand Program on Key Infectious Disease (2012ZX10003002-014), Open funding of the State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, the Chinese Academy of Sciences. Supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT1030).
Notes and references
- R. C. Lee, R. L. Feinbaum and V. Ambros, Cell, 1993, 75, 843–854 CrossRef CAS.
- L. He and G. J. Hannon, Nat. Rev. Genet., 2004, 5, 522–531 CrossRef CAS.
- J. Lu, G. Getz, E. A. Miska, E. Alvarez-Saavedra, J. Lamb, D. Peck, A. Sweet-Cordero, B. L. Ebert, R. H. Mak, A. A. Ferrando, J. R. Downing, T. Jacks, H. R. Horvitz and T. R. Golub, Nature, 2005, 435, 834–838 CrossRef CAS.
- A. Valoczi, C. Hornyik, N. Varga, J. Burgyan, S. Kauppinen and Z. Havelda, Nucleic Acids Res., 2004, 32, e175 CrossRef.
- A. G. Torres, M. M. Fabani, E. Vigorito and M. J. Gait, RNA, 2011, 17, 933–943 CrossRef CAS.
- E. Varallyay, J. Burgyan and Z. Havelda, Nat. Protoc., 2008, 3, 190–196 CrossRef CAS.
- J. Winter and S. Diederichs, Methods Mol. Biol., 2011, 676, 85–100 CAS.
- X. Luo, J. Zhang, H. Wang, Y. Du, L. Yang, F. Zheng and D. Ma, Biotechnol. Lett., 2012, 34, 627–633 CrossRef CAS.
- A. Markou, E. G. Tsaroucha, L. Kaklamanis, M. Fotinou, V. Georgoulias and E. S. Lianidou, Clin. Chem., 2008, 54, 1696–1704 CAS.
- S. D. Fiedler, M. Z. Carletti and L. K. Christenson, Methods Mol. Biol., 2010, 630, 49–64 CAS.
- Y. Saito, H. Suzuki, T. Taya, M. Nishizawa, H. Tsugawa, J. Matsuzaki, K. Hirata, H. Saito and T. Hibi, Biochem. Biophys. Res. Commun., 2012, 426, 33–37 CrossRef CAS.
- B. Wang, P. Howel, S. Bruheim, J. Ju, L. B. Owen, O. Fodstad and Y. Xi, PLoS One, 2011, 6, e17167 CAS.
- Y. Peng and Z. Gao, Anal. Chem., 2011, 83, 820–827 CrossRef CAS.
- Y. Wen, Y. Xu, X. Mao, Y. Wei, H. Song, N. Chen, Q. Huang, C. Fan and D. Li, Anal. Chem., 2012, 84, 7664–7669 CrossRef CAS.
- S. C. Chapin and P. S. Doyle, Anal. Chem., 2011, 83, 7179–7185 CrossRef CAS.
- Y. Zhou, Q. Huang, J. Gao, J. Lu, X. Shen and C. Fan, Nucleic Acids Res., 2010, 38, e156 CrossRef.
- Y. Zhang and C. Y. Zhang, Anal. Chem., 2012, 84, 224–231 CrossRef CAS.
- Y. Q. Liu, M. Zhang, B. C. Yin and B. C. Ye, Anal. Chem., 2012, 84, 5165–5169 CrossRef CAS.
- H. Jia, Z. Li, C. Liu and Y. Cheng, Angew. Chem., Int. Ed., 2010, 49, 5498–5501 CrossRef CAS.
- A. Ogawa, Bioorg. Med. Chem. Lett., 20, 6056–6060 CrossRef CAS.
- D. Duan, K. X. Zheng, Y. Shen, R. Cao, L. Jiang, Z. Lu, X. Yan and J. Li, Nucleic Acids Res., 2011, 39, e154 CrossRef CAS.
- W. Li and K. Ruan, Anal. Bioanal. Chem., 2009, 394, 1117–1124 CrossRef CAS.
- H. Dong, J. Zhang, H. Ju, H. Lu, S. Wang, S. Jin, K. Hao, H. Du and X. Zhang, Anal. Chem., 2012, 84, 4587–4593 CrossRef CAS.
- Q. Guo, X. Yang, K. Wang, W. Tan, W. Li, H. Tang and H. Li, Nucleic Acids Res., 2009, 37, e20 CrossRef.
- R. Chen, A. B. Alvero, D. A. Silasi, M. G. Kelly, S. Fest, I. Visintin, A. Leiser, P. E. Schwartz, T. Rutherford and G. Mor, Oncogene, 2008, 27, 4712–4723 CrossRef CAS.
- B. C. Yin, Y. Q. Liu and B. C. Ye, J. Am. Chem. Soc., 2012, 134, 5064–5067 CrossRef CAS.
- S. A. Georges, M. C. Biery, S. Y. Kim, J. M. Schelter, J. Guo, A. N. Chang, A. L. Jackson, M. O. Carleton, P. S. Linsley, M. A. Cleary and B. N. Chau, Cancer Res., 2008, 68, 10105–10112 CrossRef CAS.
- C. Nakada, K. Matsuura, Y. Tsukamoto, M. Tanigawa, T. Yoshimoto, T. Narimatsu, L. T. Nguyen, N. Hijiya, T. Uchida, F. Sato, H. Mimata, M. Seto and M. Moriyama, Am. J. Pathol., 2008, 216, 418–427 CAS.
- L. Cui, X. Lin, N. Lin, Y. Song, Z. Zhu, X. Chen and C. J. Yang, Chem. Commun., 2012, 48, 194–196 RSC.
- F. Moltzahn, A. B. Olshen, L. Baehner, A. Peek, L. Fong, H. Stoppler, J. Simko, J. F. Hilton, P. Carroll and R. Blelloch, Cancer Res., 2011, 71, 550–560 CrossRef CAS.
- T. Wang, M. Lv, S. Shen, S. Zhou, P. Wang, Y. Chen, B. Liu, L. Yu and Y. Hou, PLoS One, 2012, 7, e43268 CAS.
- H. Li, S. Huang, C. Guo, H. Guan and C. Xiong, PLoS One, 2012, 7, e34566 CAS.
- B. Mostert, A. M. Sieuwerts, J. W. Martens and S. Sleijfer, Expert Rev. Mol. Diagn., 2011, 11, 259–275 CAS.
- J. A. Tynan, V. Angkachatchai, M. Ehrich, T. Paladino, D. van den Boom and P. Oeth, Am. J. Obstet. Gynecol., 2011, 204, e251–e256 CrossRef.
- V. Swarup and M. R. Rajeswari, FEBS Lett., 2007, 581, 795–799 CrossRef CAS.
- M. V. Iorio, M. Ferracin, C. G. Liu, A. Veronese, R. Spizzo, S. Sabbioni, P. Musiani, S. Volinia, I. Nenci, G. A. Calin, P. Querzoli, M. Negrini and C. M. Croce, Cancer Res., 2005, 65, 7065–7070 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c2cc36728a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2013 |
