Solid-state photodimerization reactions of racemic and homochiral phenylalanine sulfonamidecinnamic acids†
Zhiqing
Yan
,
Andrew J.
Bolokowicz
,
Teage K.
Collett
,
Sarah A.
Reeb
,
Joshua D.
Wiseman
and
Kraig A.
Wheeler
*
Department of Chemistry, Eastern Illinois University, Charleston, Illinois 61920, USA. E-mail: kawheeler@eiu.edu; Tel: +(1) 217 581 3119
First published on 3rd September 2012
Abstract
Racemic and enantiopure phenylalanine sulfonamides preferentially crystallize to give supramolecular dimers with favorable olefin⋯olefin spacing (3.77–4.01 Å) for programmed solid-state [2 + 2] photodimerizations.
Recent advances with multi-molecular reactions in crystals underscore the practical use of programmed supramolecular assemblies for generating reactive phases with interesting outcomes.1 Developments with engineered structural motifs continue to facilitate the transition of the discipline from studies based on reactions of happenstance to a mature science with design strategies consistently directed at components with prescribed alignment.2 Inspired by these advances, our group's recent attention to organic solid-state reactivity developed a sulfonamidecinnamic acid framework that promotes [2 + 2] photodimerizations.3 These studies showed that conformationally flexible components engineered with the structural features of ‘fish hook’ shaped topologies and complementary intermolecular non-bonded contacts provide a unique synthon that, when crystallized, form robust supramolecular dimers (Scheme 1, motif A). Crystallographic assessment showed such dimeric motifs persist despite variations in configurational (racemic, quasiracemic, and single-component homochiral forms) and spatial (Me, Et, i-Pr) attributes of the attached R groups. Though this approach offers an effective tool for organizing supramolecular motifs, the greater utility of these materials rests with the alignment of C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups for chemical transformations. Our strategy exploits the propensity of these materials to form inversion (or near inversion) related motifs with pairs of neighboring olefinic groups oriented mutually co-planar. Given this design element and the engineered 3.6–4.0 Å olefin⋯olefin spacings, these systems are consistent with Schmidt and Cohen's topochemical criteria for reactivity and, thus, well suited for UV initiated pericyclic dimerization.4 In each case, photochemical illumination of crystalline samples resulted in the expected cyclobutane photoproducts. Other notable results from these investigations include desymmetrized crystal environments, quantitative yields, enantiocontrolled reaction outcomes, and single-crystal-to-single-crystal transformations.
C groups for chemical transformations. Our strategy exploits the propensity of these materials to form inversion (or near inversion) related motifs with pairs of neighboring olefinic groups oriented mutually co-planar. Given this design element and the engineered 3.6–4.0 Å olefin⋯olefin spacings, these systems are consistent with Schmidt and Cohen's topochemical criteria for reactivity and, thus, well suited for UV initiated pericyclic dimerization.4 In each case, photochemical illumination of crystalline samples resulted in the expected cyclobutane photoproducts. Other notable results from these investigations include desymmetrized crystal environments, quantitative yields, enantiocontrolled reaction outcomes, and single-crystal-to-single-crystal transformations.
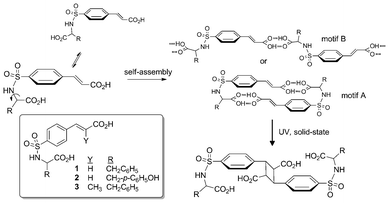 | ||
| Scheme 1 Design strategy and target compounds 1–3 for the construction of crystalline photoactive supramolecular homodimers. | ||
In general, the family of sulfonamidecinnamic acids provides a rich chemical framework for investigating programmed reactivity in molecular crystals. While our recent reports highlight the benefit of this method for photodimerization reactions in crystals, extending these studies to include a more diverse set of frameworks and R substituents is essential to gain understanding of the supramolecular landscape of this design strategy. Herein, we present the crystal structures of phenylalanine 1, tyrosine 2, and α-methylcinnamyl 3 and the photochemical outcomes of several of these systems (ESI†). By examining (rac)-1, (R)-1, (rac)-2, and (rac)-3, this study presents an interesting departure from our previous examples by including R groups of greater steric bulk and, in the case 2 and 3, additional functional groups (phenolic OH and CH3) capable of disrupting the motifs responsible for reactive crystalline assemblies. Additionally, by introducing an amino acid with a second aryl group to the sulfonamide framework another conformer that utilizes intramolecular π-stacking could compete with the desired ‘fish hook’ molecular topology. How this collection of structural features impacts molecular shape and the formation of the preferred dimeric architectures, and ultimately reactivity, is critical to understanding the breadth of application of this approach.
Initial crystal growth studies of (rac)-1 and (R)-1 from slow evaporation of acetone solutions resulted in colorless lathes and plates, respectively. The equivalent crystalline phase for the racemic phase, identified as (rac)-1-I, was also retrieved from 2-butanone and MeOH; however, on several occasions use of MeOH gave a second polymorph, (rac)-1-II, as colorless plates. Inspection of the crystal structures of these three phases revealed asymmetric units with Z′ = 1 [(rac)-1-I and -II] and 2 [(R)-1]; each with components adopting the expected molecular topology as shown in Fig. 1a and 2. In the case of (rac)-1-II, this conformer persists despite the observed two-part disorder (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio) of the sulfonamide group. Each of these crystalline phases contain molecules assembled into hydrogen-bonded dimers directed by the complementary features of carboxyl⋯carboxyl contacts and molecular topology. As described in our previous investigations of comparable sulfonamidecinnamic acids, the construction of these supramolecular dimers provides a powerful design strategy to accessing reactivity. In the case of 1 where the benzyl group of the phenyl alanine fragment is substantially larger, this supramolecular dimer persists in the structures of these three phases and directs the favorable orientation of pairs of juxtaposed olefins [(rac)-1-I, 3.88 Å; (rac)-1-II, 4.01 Å; and (R)-1, 3.77 Å].
5 ratio) of the sulfonamide group. Each of these crystalline phases contain molecules assembled into hydrogen-bonded dimers directed by the complementary features of carboxyl⋯carboxyl contacts and molecular topology. As described in our previous investigations of comparable sulfonamidecinnamic acids, the construction of these supramolecular dimers provides a powerful design strategy to accessing reactivity. In the case of 1 where the benzyl group of the phenyl alanine fragment is substantially larger, this supramolecular dimer persists in the structures of these three phases and directs the favorable orientation of pairs of juxtaposed olefins [(rac)-1-I, 3.88 Å; (rac)-1-II, 4.01 Å; and (R)-1, 3.77 Å].
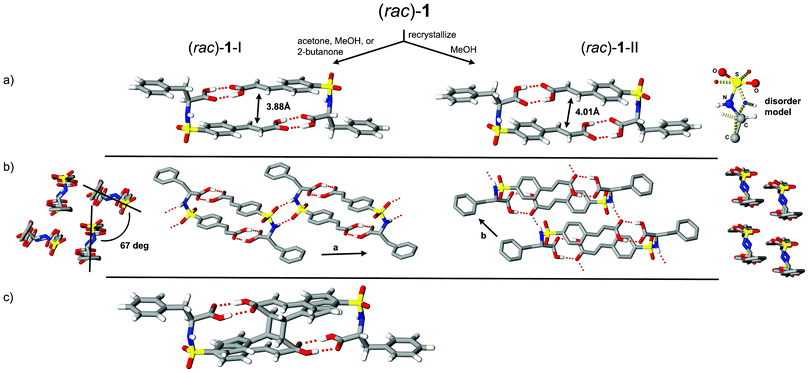 | ||
| Fig. 1 Crystal structures of (rac)-1-I and -II showing a) supramolecular dimers and sulfonamide disorder for form II, b) crystal packing motifs, and c) UV-irradiated form I indicating reactant and cyclobutane product phases (8 hours, 32% conversion). | ||
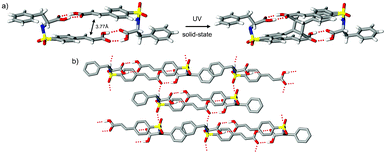 | ||
| Fig. 2 Crystal structure of (R)-1 showing a) hydrogen-bond dimer and outcome of SCSC UV irradiation (1 hour, 19% conversion) and b) crystal packing pattern. | ||
Inspection of the crystal structures of (rac)-1 reveals molecules assembled in space groups P21/n [(rac)-1-I] and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) [(rac)-1-II]. In both forms pairwise alignment of left and right handed molecules produced hydrogen-bonded dimers related by inversion symmetry. These patterns further link to adjacent motifs to give 1D architectures (Fig. 1b) with structural differences related to the alignment of dimers and mode of non-bonded contacts. As shown in Fig. 1b, the supramolecular dimers for (rac)-1-I form R22(8) patterns5via N–H⋯O
[(rac)-1-II]. In both forms pairwise alignment of left and right handed molecules produced hydrogen-bonded dimers related by inversion symmetry. These patterns further link to adjacent motifs to give 1D architectures (Fig. 1b) with structural differences related to the alignment of dimers and mode of non-bonded contacts. As shown in Fig. 1b, the supramolecular dimers for (rac)-1-I form R22(8) patterns5via N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bonds that connect to translationally related neighbors. The resulting 1D pattern extends along the a axis with adjacent parallel chains related by n-glide symmetry and skewed by ∼67°. In contrast, the linear motifs in (rac)-1-II organize via N–H⋯O
S hydrogen bonds that connect to translationally related neighbors. The resulting 1D pattern extends along the a axis with adjacent parallel chains related by n-glide symmetry and skewed by ∼67°. In contrast, the linear motifs in (rac)-1-II organize via N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interactions and extend along the b axis. This crystal arrangement produces molecular stacks with both aryl groups taking part in π–π interactions (∼3.4 Å).
C interactions and extend along the b axis. This crystal arrangement produces molecular stacks with both aryl groups taking part in π–π interactions (∼3.4 Å).
Compound (R)-1 also forms hydrogen-bonded dimers (Fig. 2a). This motif, constructed from two identical components, gives rise to a desymmetrized pattern in space group P21. Interestingly, though the imposed chiral induction from the R-phenylalanine groups requires this pattern to be rigorously noncentrosymmetric, the overall motif topology mimics the inversion related patterns that exist in the two forms of (rac)-1. This high degree of mimicry is directed by the near symmetric alignment of the cinnamyl and benzyl fragments with the sulfonamide groups acting as the principal violator of this approximate symmetry. The subtle interplay of conformational differences and hydrogen-bond interactions offers a unique entry point to asymmetric crystalline environments via pairwise molecular assemblies. This approach was previously applied to quasiracemic3a and single-component homochiral3b sulfonamidecinnamic acids and is likely directed by the propensity of achiral and racemic organic compounds6 to crystallize with inversion symmetry. Inspection of Fig. 2b reveals molecules of (R)-1 assembled by use of N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N–H⋯S
C and N–H⋯S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interactions to give 2D motifs that extend in the bc plane.
C interactions to give 2D motifs that extend in the bc plane.
UV illumination studies of single crystals of (rac)-1-I and (R)-1 gave cyclobutane products from [2 + 2] photodimerization processes. These transformations were initially studied as single-crystal-to-single-crystal (SCSC) reactions using the “tail irradiation technique” with a 200 W Xe(Hg) arc lamp equipped with a 360 nm cut-on optical filter.7 Though periodic collection of full sets of X-ray data provided an important view of the reaction progress, sufficient crystal degradation (as apparent from distinct cracking and diffuse–split reflections) at 32% [(rac)-1-I] and 19% [(R)-1] conversion prevented further assessment using this method (Fig. 1c and 2a). Stress accumulation in these crystals likely follows from the i) change in hybridization and ∼1 Å movement of olefin C atoms required to form the cyclobutane product and ii) insufficient cooperative movement of the reactant–product components in the crystal. The dynamic nature of these photodimerizations illustrates the mechanochemical effects of reactions in crystals and the strain that can result from imposed molecular movement. Due to crystal degradation of (rac)-1-I and (R)-1, these reactions were then pursued using polycrystalline samples and unfiltered UV radiation. Periodic grinding and 1H NMR assessment resulted in converged product yields of 74% [(rac)-1-I, 27.25 hours] and 50% [(R)-1, 21.5 hours].
From a supramolecular viewpoint the inclusion of racemic tyrosine 2 and the α-methyl derivative 3 offer an interesting departure from the frameworks of 1. The addition of pendant OH and CH3 groups for these compounds provide iterative modifications to phenylalanine 1 that could ultimately disrupt (or enhance) the recognition profile responsible for hydrogen bond dimer formation. In the crystal, (rac)-3 exists in the ‘fish hook’ conformer with sulfonamide group disorder (86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14) similar to that observed for (rac)-1-II. The crystal structure of 2 contains tetramers consisting of hydrogen-bonded dimers of 2 with the phenolic OH connected to acetone molecules via O–H⋯O
14) similar to that observed for (rac)-1-II. The crystal structure of 2 contains tetramers consisting of hydrogen-bonded dimers of 2 with the phenolic OH connected to acetone molecules via O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C contacts (Fig. 3a). Though the solid-state reactivity of this phase was not pursued due to complications associated with solvates, the observed supramolecular pattern highlights the persistence of dimer formation and favorable olefin alignment at 3.90 Å. In the case of (rac)-3, introducing a methyl substituent to the α position of the cinnamyl fragment should conceptually offer easy access to a new photoactive crystalline framework. Even so, molecules of 3 lack the desired fishhook shape and supramolecular dimers (Fig. 3b). Furthermore, this system exhibits a loss of conjugation by adopting 42° twist about the phenyl–olefin groups and aligns to form undulating photostable chains (Scheme 1, motif B) with nearest C
C contacts (Fig. 3a). Though the solid-state reactivity of this phase was not pursued due to complications associated with solvates, the observed supramolecular pattern highlights the persistence of dimer formation and favorable olefin alignment at 3.90 Å. In the case of (rac)-3, introducing a methyl substituent to the α position of the cinnamyl fragment should conceptually offer easy access to a new photoactive crystalline framework. Even so, molecules of 3 lack the desired fishhook shape and supramolecular dimers (Fig. 3b). Furthermore, this system exhibits a loss of conjugation by adopting 42° twist about the phenyl–olefin groups and aligns to form undulating photostable chains (Scheme 1, motif B) with nearest C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C⋯C
C⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C contacts > 6 Å.
C contacts > 6 Å.
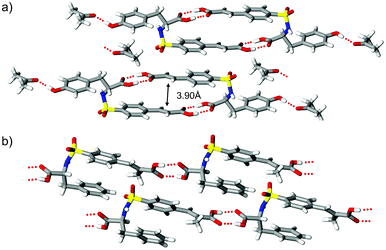 | ||
| Fig. 3 Crystal packing diagrams showing a) solvated supramolecular tetramers of (rac)-2 and b) carboxyl⋯carboxyl linked chains of (rac)-3. | ||
In summary, the supramolecular patterns described by compounds 1–3 reveal the supramolecular tendencies of a family of sulfonamidecinnamic acids. All but (rac)-3 form hydrogen-bonded dimers that persist despite the structural features of disorder, polymorphism, and in the case of (rac)-2 the use of an additional hydrogen bond group (phenolic OH). The structures described for these materials, and the overarching theme of this supramolecular strategy, show considerable potential for solid-state photodimerization behavior. UV illumination studies of compounds (rac)-1 and (R)-1 were initially processed as SCSC transformations. Only modest product conversions could be determined using this method; the result of sample degradation due to mechanical strain imposed on the crystals by the photodimerization processes. Reacting these compounds as polycrystalline samples with unfiltered radiation gave converged yields of 74% [(rac)-1] and 50% [(R)-1]. It should be also emphasized that because (R)-1 generates supramolecular dimers with reduced symmetry, the subsequent [2 + 2] photodimerization results in enantiopure photoproducts.
Acknowledgements
This work was supported by the National Science Foundation (CHE-0957391 and CHE-0722547).References
- (a) Supramolecular Chemistry: From Molecules to Nanomaterials, ed. J. W. Steed and P. A. Gale, Wiley, New York, vol. 4, 2012, pp. 1381–1612, vol. 6, pp. 3153–3165 Search PubMed; (b) I. Weissbuch and M. Lahav, Chem. Rev., 2011, 3208–3235 Search PubMed; (c) L. R. MacGillivray, G. S. Papaefstathiou, T. Friscic, T. D. Hamilton, D.-K. Bucar, Q. Chu, D. B. Varshney and I. G. Georgiev, Acc. Chem. Res., 2008, 41, 280 CrossRef CAS; (d) J. W. Lauher, F. W. Fowler and N. S. Gorgoff, Acc. Chem. Res., 2008, 41, 1215 CrossRef CAS; (e) F. Toda, Top. Curr. Chem., 2005, 254, 1 CAS.
- (a) M. E. van der Boom, Angew. Chem., Int. Ed., 2011, 50, 11846 CrossRef CAS; (b) D. K. Bukar, A. Sen, S. V. Mariappan and L. R. MacGillivray, Chem. Commun., 2012, 48, 1790 RSC; (c) S. Dawn, M. B. Dewal, D. Sobransingh, M. C. Paderes, A. C. Wibowo, M. D. Smith, J. A. Krause, P. J. Pellechia and L. S. Shimizu, J. Am. Chem. Soc., 2011, 133, 7025 CrossRef CAS; (d) M. H. Mir, J. X. Ong, G. K. Kole, G. K. Tan, M. J. McGlinchey, Y. Wu and J. J. Vittal, Chem. Commun., 2011, 47, 11633 RSC; (e) K. Tsaggeos, N. Masiera, A. Niwicka, V. Dokorou, M. G. Siskos, S. Skoulika and A. Michaelides, Cryst. Growth Des., 2012, 12, 2187 CrossRef CAS; (f) S. Yamada and C. Kawamura, Org. Lett., 2012, 14, 1572 CrossRef CAS; (g) M. Li, A. D. Schlüter and J. Sakamoto, J. Am. Chem. Soc., 2012, 134, 11721 CrossRef CAS.
- (a) R. C. Grove, S. H. Malehorn, M. E. Breen and K. A. Wheeler, Chem. Commun., 2010, 46, 7322 RSC; (b) K. A. Wheeler, J. D. Wiseman and R. C. Grove, CrystEngComm, 2011, 13, 3134 RSC; (c) K. A. Wheeler, S. H. Malehorn and A. E. Egan, Chem. Commun., 2012, 48, 519 RSC.
- (a) M. D. Cohen and G. M. J. Schmidt, J. Chem. Soc., 1964, 2000 RSC; (b) G. M. J. Schmidt, Pure Appl. Chem., 1971, 27, 647–678 CrossRef CAS.
- J. Bernstein, R. E. Davis, L. Shimoni and N.-L. Chang, Angew. Chem., Int. Ed. Engl., 1995, 34, 1555 CrossRef CAS.
- (a) B. Dalhus and C. H. Gorbitz, Acta Crystallogr., Sect. B: Struct. Sci., 2000, 56, 715 CAS; (b) M. Hendi, P. Hooter, V. Lynch, R. E. Davis and K. A. Wheeler, Cryst. Growth Des., 2004, 4, 95 CrossRef CAS; (c) J. Jacques, A. Collet, S. H. Wilen, Enantiomers, Racemates and Resolutions, Wiley-Interscience, New York, 1981 Search PubMed.
- (a) M. Kahn, V. Enkelmann and G. Brunklaus, Cryst. Growth Des., 2009, 9, 2354 CrossRef; (b) I. Abdelmoty, V. Buchholz, L. Di, V. Enkelmann, G. Wegner and B. M. Foxman, Cryst. Growth Des., 2005, 5, 2210 CrossRef CAS; (c) J. B. Benedict and P. Coppens, J. Phys. Chem. A, 2009, 113, 3116 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, photochemical studies, and full crystal structure details and tables for (rac)-1, (R)-1, (rac)-2, and (rac)-3. CCDC references numbers 895728–895734. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ce26307f |
| This journal is © The Royal Society of Chemistry 2013 |
