Computer simulation study of nanoparticle interaction with a lipid membrane under mechanical stress
Kan
Lai
,
Biao
Wang
*,
Yong
Zhang
and
Yue
Zheng
School of Physics and Engineering, Sun Yat-Sen University, Guangzhou, China. E-mail: wangbiao@mail.sysu.edu.cn
First published on 23rd October 2012
Abstract
Pore formation of lipid bilayers under mechanical stress is critical to biological processes. A series of coarse grained molecular dynamics simulations of lipid bilayers with carbon nanoparticles different in size have been performed. Surface tension was applied to study the disruption of lipid bilayers by nanoparticles and the formation of pores inside the bilayers. The presence of small nanoparticles enhances the probability of water penetration thus decreasing the membrane rupture tension, while big nanoparticles have the opposite effect. Nanoparticle volume affects bilayer strength indirectly, and particle surface density can complicate the interaction. The structural, dynamic, elastic properties and lateral densities of lipid bilayers with nanoparticles under mechanical stress were analyzed. The results demonstrate the ability of nanoparticles to adjust the structural and dynamic properties of a lipid membrane, and to efficiently regulate the pore formation behavior and hydrophobicity of membranes.
1 Introduction
In recent years, the use of nanotechnology has been rapidly growing. As the most promising imaging materials and biomolecule carriers, nanoparticles are extensively used in medical and biological studies.1–5 The application of nanomaterial is underway.6Nanoparticles are materials with dimensions between 1 and 100 nm. The small size of nanoparticles facilitates the uptake of nanoparticle-based drugs in biological systems. However, it also brings considerable risk. As their toxicity has been reported,7–10 more attention is paid to the effect of nanoparticles on human health and the environment.11,12 For instance, nanoparticles were found to significantly enhance the rate of protein fibrillation involved in many human diseases such as Alzheimers, Creutzfeld–Jacob disease, and dialysis-related amyloidosis.13,14
Fullerenes are well studied nanoparticles with various biological applications.15 However, fullerene derivatives were proved to be quite toxic in cultured cells and showed hemolytic effects.16 Previous simulations on the interaction of fullerenes and lipid bilayers have focused on the interaction of C60 inside a lipid bilayer, translocation of C60 and thermodynamics properties of a tension free lipid bilayer.17 Recent molecular dynamics simulations showed that C60 would disperse in the membrane interior without inducing mechanical damage.17 While larger fullerenes are suggested to induce membrane distortion and affect bilayer stability.18
Biological membranes are fundamental elements of all living organisms. It is known that mechanical properties of biological membranes affect membrane protein configurations and thus cell functions. The influence of nanoparticles on the stability of biomembranes is of great concern. However, little is known about the effect of nanoparticles on the pore formation of biological membranes, though it has been shown to be important in various biological processes such as membrane fusion,19 the controlled transport of materials across cells. Moreover, irreversible pore formation may lead to cell death in the form of membrane rupture, e.g., hemolysis. Membrane surface tension plays an important role in pore formation of biomembranes and many other biological processes. Mechanical strength, which is determined by the rupture tension, is critical to biomembrane function. It is known that mechanical properties of a lipid membrane are determined by the membrane composition, e.g., the addition of 50% cholesterol was found to significantly strengthen the DOPC bilayer by increasing the rupture tension from 10 mN m−1 to 19 mN m−1.20 Therefore, we suggested that the addition of nanoparticles would affect the mechanical properties and stability of the lipid membrane which may be related to cytotoxicity.
However, the mechanism and the effect of nanoparticles on mechanical strength and structural properties of biomembranes remain uncertain. The interaction between nanoparticles and biomembranes is complicated, determined by the properties of nanoparticles such as surface coatings, size, shape and roughness.4,21 Geometry is considered seriously when designing nanoparticles as a drug carrier. Unsuitable geometric design would lead to more disruption to the bilayer, e.g., discs with larger radii have significant influence on the packing state of the bilayer.21 Silica nanoparticles were shown to increase biomembrane permeability22 and were observed to induce hemolytic activity in a concentration- and size-dependent manner.23,24 Moreover, surface tension is suggested to trigger the toxicity effect of nanoparticles. Recent molecular dynamics simulations showed that nanoparticles could induce pores in the lipid bilayer under surface tension but not in the tension free lipid bilayer.25
In this study, we have estimated rupture (lysis) behavior of lipid bilayers under mechanical stress in the presence of various carbon nanoparticles having different sizes using coarse grained molecular dynamic simulations (CG-MD, see Methods and ref. 26). The CG-MD simulations offer a powerful way of studying interaction of nanoparticles and lipid bilayers at a molecular level, and allow the use of larger system sizes and time scales for at least one order of magnitude than in the traditional all-atom molecular dynamic simulations. To determine the effect of nanoparticles on the membrane structure and mechanical properties under surface tension, lipid tail order parameter, membrane area-stretched modulus, diffusion coefficient and lateral density profiles are calculated.
2 Methods
We use the Martini coarse grained force field in the molecular dynamics simulation.27,28 A coarse grained model enables us to simulate larger systems and longer time. The Martini force field has been extensively studied and successfully shown to reproduce many crucial properties of lipid membranes, including phase behavior of lipid bilayers, the interaction with membrane proteins and nanoparticles. Based on the previous simulations,17 we developed six coarse grained carbon nanoparticles different in size using the 4 to 1 mapping scheme, four carbon atoms are represented by a single coarse grained particle. Three CG nanoparticles C45, C15 and C4 are models of real fullerenes C180, C60 and C20 with diameters 1.2 nm, 0.72 nm and 0.4 nm, respectively. The CG beads were initially placed on a spherical surface with diameters 1.2 nm, 0.72 nm and 0.4 nm, respectively. The final configurations were obtained using an energy minimization scheme in Gromacs simulation software.29,30 The CG beads were then connected by harmonic bonds with a force constant of 1250 kJ mol−1 nm−2. Nanoparticle C1 represented small hydrophobic nanoparticles, and nanoparticles C4L and C4LL which have the same shape as C4 (regular tetrahedron) but larger diameters (0.52 nm and 0.70 nm, respectively) represented nanoparticles with less density or rough surface. The CG beads were assigned to “SC4” interaction sites in MARTINI.The simulations under zero surface tension were started from fluid patches, which were obtained from standard lipid bilayer coordinate files from the website of Marrink's group (http://md.chem.rug.nl/∼marrink/coarsegrain.html). The particle free bilayers containing 128 lipids (with a box dimension of ∼6 nm) were fully hydrated with 4037 coarse grained water beads.
The nanoparticle–bilayer systems were prepared by mixing the nanoparticles in a fully hydrated lipid bilayer simulation box at nanoparticle–lipid molar ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 to 1
16 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The nanoparticle aggregates were initially placed ∼3 nm above the bilayer and they penetrate the bilayer spontaneously within microseconds. Three types of coarse grained lipids (dipalmitoylphosphatidylcholine (DPPC), dilaureoylphosphatidylcholine (DLPC) and distearoylphosphatidylcholine (DSPC)) different in lipid tail length were used in the simulations.
1. The nanoparticle aggregates were initially placed ∼3 nm above the bilayer and they penetrate the bilayer spontaneously within microseconds. Three types of coarse grained lipids (dipalmitoylphosphatidylcholine (DPPC), dilaureoylphosphatidylcholine (DLPC) and distearoylphosphatidylcholine (DSPC)) different in lipid tail length were used in the simulations.
In the experiments, stretch and rupture of lipid bilayers were usually induced by a micropipette with a suction pressure applied on vesicles.20,31,32 In our simulation, a semi-isotropic pressure coupling was therefore applied to the bilayer systems to realize the mechanical stress (see Fig. 1), allowing for changes in the normal pressure and lateral pressure independently. The fluid phase lipid bilayer systems were prepared by holding at high tension close to the threshold for rupture (∼5 mN m−1 below rupture tension) prestress by coupling to high lateral pressure (−60 bar to −120 bar, depending on the normal size of simulation box), and equilibrated at 325 K (well above the fluid to gel phase transition) during a multi-microsecond time. Then the bilayers were coupled to higher lateral pressure, which lead to the breakage of the lipid membrane. Five individual simulations up to 800 ns were performed at every set of lateral pressure in order to reduce the error induced by thermal fluctuation. Surface tension when membrane rupture occur was measured to estimate the lateral strength of the lipid membrane.
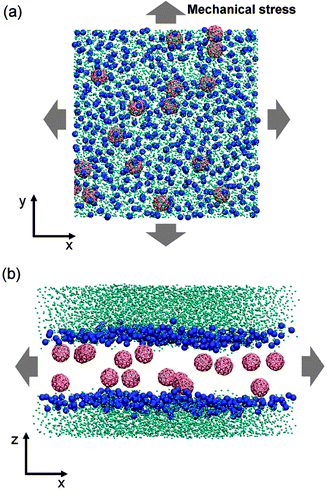 | ||
| Fig. 1 Schematic illustrations of the tense lipid bilayer system. (a) Top view, nanoparticles are dispersed in biomembranes. (b) Side view, mechanical stress is applied at the lateral direction. Phosphate groups are shown as blue spheres, nanoparticles are in red and water beads are in cyan. Lipid tails are not shown for clarity. | ||
The surface tension was calculated from the average surface tension given by the difference of the normal and lateral pressure in the box,
| γ = Lz(PN − PL) |
Simulations were performed with the Gromacs simulation software,29,30 version 4.0.3. Periodic boundary conditions were used, with constant temperature and pressure achieved using the Berendsen scheme.33 The coupling constant is 0.1 ps for the temperature, and 0.2 ps for the pressure. Short-range electrostatic and Lennard-Jones potentials were cut off at 1.2 nm, corresponding to the standard Martini force field. The time step of simulations was set to 40 fs. Visual images were prepared using the VMD (Visual Molecular Dynamics) software,34 version 1.8.7.
3 Results and discussion
The strength of the lipid bilayer was quantified by the value of critical tension at the instant of irreversible pore formation which has the maximum area stretch, the smaller the critical tension required, the easier the cell bursts.3.1 Effect of fullerenes on the rupture tension of biomembranes
To clarify the size-dependent effect, we performed a series of coarse grained molecular dynamics simulations of lipid bilayers containing coarse grained carbon nanoparticles different in size (see Fig. 2(a)). The rupture tensions of lipid bilayers in the presence of nanoparticles are shown in Fig. 2.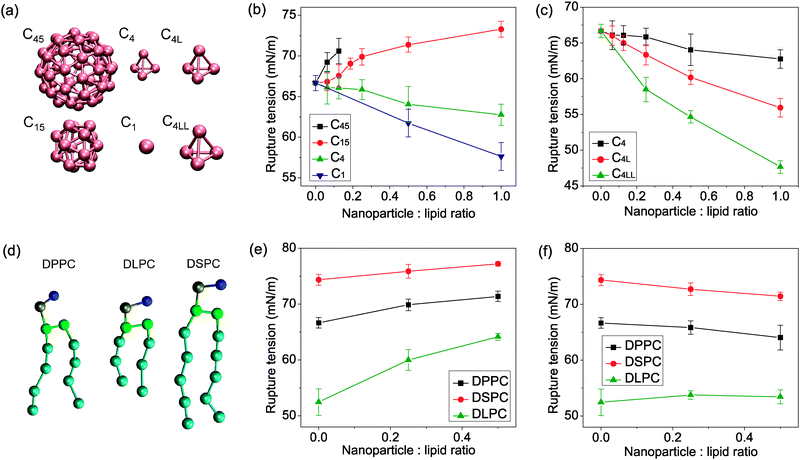 | ||
| Fig. 2 (a) Coarse grained model of C45, C15, C4, C1, C4L and C4LL nanoparticles. (b) Rupture tension of lipid bilayers containing C45, C15, C4 and C1 nanoparticles as a function of nanoparticle concentration. Larger nanoparticles C45 and C15 strengthen the bilayer, causing higher rupture tension than the pure lipid bilayer. Smaller nanoparticles C4 and C1 weaken the bilayer. (c) Rupture tension of lipid bilayers containing C4, C4L and C4LL nanoparticles as a function of nanoparticle concentration. The weakening effect of nanoparticles was enhanced with the increasing size of nanoparticles. (d) Coarse grained model of lipid DPPC, DSPC and DLPC different in lipid tail length. (e) Rupture tension of nanoparticle C15 embedded DPPC, DSPC and DLPC bilayer. The strengthening effect of the C15 nanoparticle increased in bilayer with shorter lipid tail length. (f) Rupture tension of nanoparticle C4 embedded DPPC, DSPC and DLPC bilayer. The C4 nanoparticle weaken the long tail length lipid bilayer, however, strengthen the short tail length lipid bilayer. The error bars indicate the spread of values obtained from multiple independent simulations. | ||
Firstly, we examined the effect of fullerene nanoparticles C45, C15, C4 and nanoparticle C1 on the strength of the most extensively studied DPPC bilayer. The strength of bilayer was found to depend on the size of the embedded nanoparticle. The strength of the nanoparticle embedded lipid bilayers decreased with increasing concentration of small nanoparticles C1 and C4 (e.g., decreasing by ∼14% at C1–DPPC molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). On the other hand, bilayers containing larger nanoparticles C15 and C45 were much stronger (e.g., increasing by ∼10% at C15–DPPC molar ratios of 1
1). On the other hand, bilayers containing larger nanoparticles C15 and C45 were much stronger (e.g., increasing by ∼10% at C15–DPPC molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) than pure lipid bilayers.
1) than pure lipid bilayers.
To confirm the size effect of nanoparticles on the membrane rupture tension, we performed additional simulations involving two kinds of nanoparticles with the same structure as C4 (the coarse grained model of fullerene C20) but with different diameters. The size effect of nanoparticles is completely different (see Fig. 2(c)). The weakening effect is more pronounced in the presence of larger nanoparticles C4L and C4LL.
Moreover, lipid tail length and degree of unsaturation have always been thought to be important in relation to pore formation. Here, additional lipids DLPC and DSPC different in tail length are taken into account. The results show that the effect of nanoparticles on the strength of lipid bilayers also depends on the lipid tail length. For lipid DSPC with longer tail length, the addition of 50% C15 nanoparticles increases the rupture tension for 4%, lower than that of DPPC (7%). However, for lipid DLPC with shorter tail length, the addition of 50% C15 nanoparticles increases the rupture tension for 22%. On the other hand, the presence of small nanoparticle C4 which weakens the DPPC and DSPC bilayers has a strengthening effect on the DSPC bilayer. The difference in weakening or strengthening effect of nanoparticles on lipids with different tail length suggested that the influence of nanoparticles on the membrane strength is strongly dependent on the nature of the lipid tails. The results indicated that the size effect of nanoparticle induced change in bilayer strength is lipid tail length sensitive and related to the ratio of the size of nanoparticles and the lipid tail length.
3.2 Pore formation behavior of biomembranes in the presence of nanoparticles
A cell membrane is a fluid membrane self-assembled by the hydrophobic effect of amphiphilic lipids with a bilayer structure. Therefore, the membrane can be considered as a two-dimensional liquid. When a small hydrophobic pore was induced manually in a membrane under zero surface tension, a rearrangement occurs with the polar head of lipids around the pore line in the rim of the pore and lipid tails buried inside the bilayer. After the transformation to a hydrophilic pore, lipids around the pore would move forward to the pore center to fill the space occupied by water, finally the pore resealed. During our simulation, the lifetime of transient pores is within nanoseconds, depending on the size of the pore. The rearrangement process is governed by forces that minimize exposure of hydrophobic chains to water. The driving force of self-healing is arised from the competition of the water/bilayer interfacial energy and energy cost of pore formation.However, the energy barrier is found to be lowered under mechanical stress.35 The cell membrane is expected to be unstable and an unhindered growth of a transient pore leading to the membrane rupture under high negative surface tension. When the mechanical stress applied on the lipid bilayer is higher, water penetration occurs more frequently than under low mechanical stress. As found in previous investigation, small hydrophobic pores are observed to form in the lipid head group due to the thermal and mechanical fluctuation.17,35–37 The hydrophobic pore was then filled by water. When the water forms a channel in the interior of bilayers, the formation of a hydrophobic pore is indicated, afterwards local lipids restructuring occurs. The heads of lipid around the pore reorganized and line in the rim of the pore, heading toward the pore center, making the pore hydrophilic.
When the membrane is under external mechanical stress, the water/bilayer interfacial area would increase, results in higher interfacial energy. The pore formation would effectively decrease the bilayer surface area thus interfacial energy, leading to a more stable system with lower free energy. The pore formation is irreversible. During the process of membrane rupture, no metastable pore was found, consistent with the former results that the rupture tension of pore free bilayer is higher than that of a bilayer containing preexisting pores.35
Interestingly, the location of the forming pore shows significant correlation with the presence of the nanoparticles. The carbon nanoparticles translocate in the membrane interior spontaneously by the hydrophobic effect and disperse in the lipid tail region, consistent with previous investigation.17 To investigate the effect of nanoparticles on the pore formation of the lipid membrane, we performed 24 unbiased simulations (400 ns each) of a large nanoparticle embedded DPPC lipid bilayers containing 512 DPPC lipids and 16 nanoparticles for nanoparticle–DPPC molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32. Two kinds of nanoparticles C4LL (12 simulations) and C45 (12 simulations) which showed a significant weakening or strengthening effect on biomembranes, respectively, were investigated in the trajectories analysis. In the pure lipid bilayer, a transient pore was formed in the bilayer surface due to the surface area fluctuation. In the presence of nanoparticles, the surface area fluctuation was affected by the nanoparticles. The small pore exist at the bilayer surface was never found near the larger nanoparticle C45 in all the 12 simulations. In contrast, the pore formation shows great tendency to occur near the small nanoparticle C4LL. In two simulations the rupture pore formed in the nanoparticle-free region, similar to the behavior of pore formation in pure lipid bilayers. In all other cases (10 simulations), a rupture pore appears to be induced by the presence of the C4LL nanoparticle. From Fig. 3, it can be seen that the water defects in the bilayer hydrophobic tail region form near the small nanoparticles, leading to the opening of a hydrophobic pore, implying that the presence of nanoparticles facilitates the pore formation. After the formation of a stable water line, nanoparticles move toward the hydrophobic region during the transformation from hydrophobic pores into hydrophilic pores.
32. Two kinds of nanoparticles C4LL (12 simulations) and C45 (12 simulations) which showed a significant weakening or strengthening effect on biomembranes, respectively, were investigated in the trajectories analysis. In the pure lipid bilayer, a transient pore was formed in the bilayer surface due to the surface area fluctuation. In the presence of nanoparticles, the surface area fluctuation was affected by the nanoparticles. The small pore exist at the bilayer surface was never found near the larger nanoparticle C45 in all the 12 simulations. In contrast, the pore formation shows great tendency to occur near the small nanoparticle C4LL. In two simulations the rupture pore formed in the nanoparticle-free region, similar to the behavior of pore formation in pure lipid bilayers. In all other cases (10 simulations), a rupture pore appears to be induced by the presence of the C4LL nanoparticle. From Fig. 3, it can be seen that the water defects in the bilayer hydrophobic tail region form near the small nanoparticles, leading to the opening of a hydrophobic pore, implying that the presence of nanoparticles facilitates the pore formation. After the formation of a stable water line, nanoparticles move toward the hydrophobic region during the transformation from hydrophobic pores into hydrophilic pores.
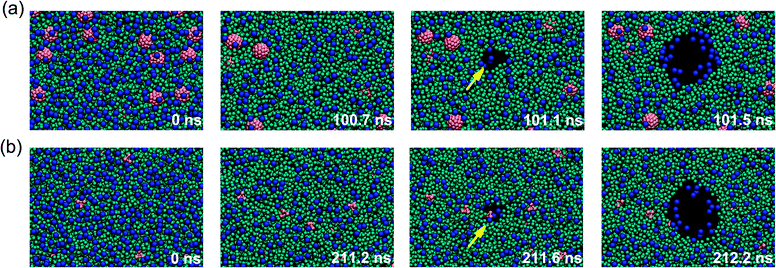 | ||
Fig. 3 Mechanism of pore formation in a lipid bilayer composed of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 nanoparticle–lipid under high mechanical stress. Mechanical stretch was applied to a tension free lipid bilayer (0 ns), then the bilayer is largely expanded and becomes unstable. Rupture pore formation occurs when the surface tension higher than the rupture tension. (a) A hydrophobic pore was formed in the particle-free region in the bilayer in the presence of large nanoparticles. (b) A hydrophobic pore was formed in the position of a small nanoparticle (211.6 ns). The hydrophobic pore rapidly transformed into a hydrophilic pore and irreversibly expanded. The nanoparticles were shown in red, the lipid head groups in blue, and lipid tails in cyan. The water is not shown for clarity. The simulation time is indicated in each snapshot. 32 nanoparticle–lipid under high mechanical stress. Mechanical stretch was applied to a tension free lipid bilayer (0 ns), then the bilayer is largely expanded and becomes unstable. Rupture pore formation occurs when the surface tension higher than the rupture tension. (a) A hydrophobic pore was formed in the particle-free region in the bilayer in the presence of large nanoparticles. (b) A hydrophobic pore was formed in the position of a small nanoparticle (211.6 ns). The hydrophobic pore rapidly transformed into a hydrophilic pore and irreversibly expanded. The nanoparticles were shown in red, the lipid head groups in blue, and lipid tails in cyan. The water is not shown for clarity. The simulation time is indicated in each snapshot. | ||
3.3 Lipid tail order parameter under tension
The nanoparticle is found between the bilayer center and water/bilayer interface. Therefore, the nanoparticle is believed to affect structural, dynamic and elastic properties of the membrane. Previous simulation studies of the C60 nanoparticle showed that the mechanical damage of a tension free membrane is not very large.17 However, it is known that bilayer thickness would decrease under high surface tension,38 the disruption of nanoparticles on lipid bilayers is expected to be more pronounced in that case. The nanoparticle induced deformation can be revealed by the order parameter, which can be used to express the packing orientation of lipids in the bilayer. The order parameter for C1B–C2B–C3B of lipid tails is shown in Fig. 4 as a function of particle concentration and applied surface tension.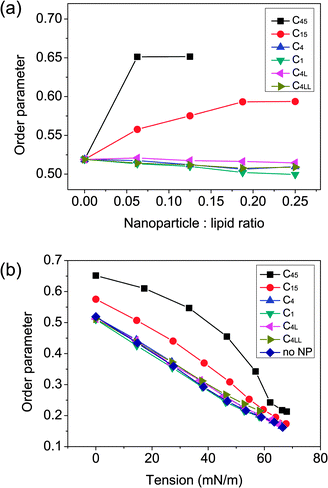 | ||
| Fig. 4 (a) Average order parameter of the lipid tails as a function of nanoparticle concentration. (b) Average order parameter of the lipid tails as a function of applied surface tension. The perturbation of membrane structural properties could not be ignored. | ||
When the surface tension is high, the lipids become clearly disordered (order parameter decreased), as was found in previous atomistic study of pure lipid bilayers.38 Analysis of the order parameter of lipid tails shows the different effect of smaller and larger nanoparticles on the lipid chain order. The inclusion of small nanoparticles slightly disrupts the lipid packing while the larger nanoparticles ordered the lipid packing significantly. The relationship of lipid tail ordering and bilayer strength is consistent with previous experimental finding.39
3.4 Structural and dynamical properties of nanoparticle rich lipid bilayers under tension
To characterize the effect of nanoparticles on bilayer structure, area-stretch modulus, diffusion coefficient and lateral density profiles were calculated.As shown in Table 1, before the rupture, the bilayers were much more resistant to change in area (e.g., ∼233 mN m−1 for pure DPPC bilayer) than following the rupture (e.g., ∼62 mN m−1 for pure DPPC bilayer). The elastic response is consistent with previous experiments.20 The changes in area-stretched moduli indicate a stiffening of the membrane in the presence of large nanoparticles, while the small nanoparticle has the slight softening effect on the membrane. The change in area-stretched moduli may due to the change in lipid packing (Fig. 4). Lipids are disordered under high surface tension, result in lower area-stretch modulus. Similarly, the significant ordering effect of larger nanoparticles C45 and C15 (Fig. 4(a)) increases the packing of lipid bilayers thus enhance the lateral strength. While the slightly disordering effect of small nanoparticles (Fig. 4(b)) leads to lower lateral strength.
| C45 | C15 | No NP | C4 | C1 | C4L | C4LL | |
|---|---|---|---|---|---|---|---|
| a Area-stretched modulus of the bilayer under low tension (7 mN m−1). b Area-stretched modulus of the bilayer under high tension (55 mN m−1). c Lateral diffusion coefficient of DPPC lipids. d Lateral diffusion coefficient of nanoparticles. | |||||||
| K ALow (×10−3 N m−1) | 312 | 286 | 233 | 225 | 224 | 218 | 198 |
| K AHigh (×10−3 N m−1) | 108 | 59 | 62 | 62 | 60 | 63 | 61 |
| D Lipid (×10−7 cm2 s−1) | 0.22 | 1.09 | 2.06 | 2.00 | 2.01 | 2.07 | 1.84 |
| D NP (×10−7 cm2 s−1) | 0.02 | 1.06 | — | 5.74 | 28.69 | 5.62 | 5.31 |
The diffusion of lipids and nanoparticles is also related to the stability of the lipid membrane. To characterize the dynamics of nanoparticle embedded in the bilayer under surface tension, we calculated the local lateral diffusion coefficients. The diffusion coefficients were obtained from the mean-square displacements. For DPPC lipids in the absence of nanoparticles, the diffusion coefficient (2.06 × 10−7 cm2 s−1) is in agreement with experiment.40 A C15 concentration of 12.5% reduces the lateral diffusion coefficients by ∼47%, close to values previously reported for DOPC bilayers 40%.17 The presence of large nanoparticles reduces the diffusion of lipid significantly. However, the small nanoparticle reduces the diffusion coefficients by less than 10%. The reduction of lateral diffusion coefficient of lipid by adding large nanoparticles can be ascribed to the increase in the ordering of the lipid acyl chains upon nanoparticle addition. The effect of large nanoparticles on the lateral diffusion coefficient is similar to the effect of cholesterol in previous experimental and simulation studies.41,42
Moreover, the diffusion of nanoparticles is also strongly depend on the size. The diffusion of small nanoparticles which weakens the bilayer is two to three orders of magnitude higher than in the case of larger nanoparticles with the strengthening effect.
To further characterize the structural changes induced by nanoparticles, lateral density profiles are developed (Fig. 5) for pure lipid bilayers and the bilayer containing nanoparticles C15 and C4LL which has the opposite effect on bilayer strength.
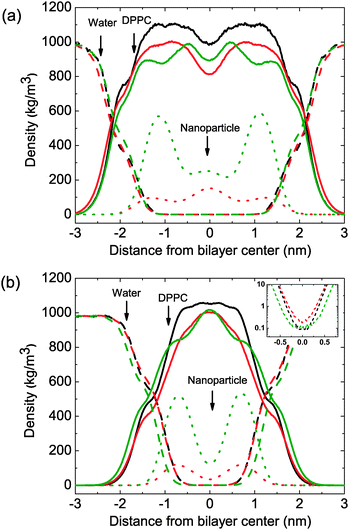 | ||
| Fig. 5 Lateral density profiles for lipid bilayer systems containing different nanoparticles. (A) Bilayer systems under zero surface tension. Bilayer thickness was not affected obviously by the inclusion of nanoparticles. (B) Bilayer systems under high surface tension (58 mN m−1). The solid line, dashed line and dotted line stand for lipids, water and nanoparticles, respectively. The black line, red line and green line correspond to nanoparticle free lipid bilayers, C4LL embedded bilayer and C15 embedded bilayer, in respective order. | ||
Under high mechanical stress, the lipid tails are strongly spread to cover the free volume induced by the surface tension. A significant increase in lipid occupied area was observed. However, the value in the center of density profiles which demonstrates the interdigitation of lipid tails from the two monolayers does not decrease as the other region of bilayer, indicated that the interdigitation of lipid tails increased with the increasing surface tension. The results show qualitative agreement with atomistic studies of pure lipid bilayers,38 although the coarse-grained model suggests a higher rupture tension. The deviations from atomistic results can be ascribed to differences in the details of the force fields.
We found a redistribution of small nanoparticles in the tense membrane. At low surface tension, small nanoparticles induce membrane thickening and reduce tail interdigitation, for the preference to stay in the center of the bilayer (Fig. 5(a)). When the surface tension increased, the bilayer spreads at a fast rate due to the low area-stretch modulus. Under higher surface tension, a redistribution occurs. As the high interdigitation of lipid tails, lower free volume is available to hold permeant nanoparticles, small nanoparticles tend to locate near the head group region of the two monolayer, and induce membrane thinning and instability. The small nanoparticles move closer to the lipid head group region. On the other hand, the distribution of larger nanoparticles would not be affected by the surface tension significantly. Moreover, the nanoparticles were somewhat exposed to the water phase, thus lead to high energy due to the unfavorable hydrophobic contact. Similar to the tensionless bilayer, translocation of nanoparticles outside is never observed, consistent with the energy barrier for transfer from the bilayer to water.
As shown in the inset of Fig. 5(b), density of water in the center of lipid bilayers is higher for the one containing small nanoparticles, indicated that water penetrated easier in the thinner bilayer (higher permeability) in the presence of high diffused small nanoparticles. Indeed, membrane thinning and permeability are crucial for the pore formation of lipid bilayers. Recently, experimental investigation of water permeability of a single-component bilayer and bilayers containing raft showed a prominent correlation between permeability and rupture tension.20,31 Water is less soluble in the hydrocarbon interior of lipid bilayers,43 the penetration of water strongly depends on the creation of “free volume” defects in the hydrocarbon region driven by thermal fluctuation.20
The lipid bilayer is thinner under tension, from ∼4.3 nm at zero surface tension to ∼2.5 nm at 58 mN m−1 in our simulations. Therefore, the small pore induced by the thermal fluctuation is large enough to reach the lipid tail region, would disrupt the hydrophobic barrier represented by the lipid tails. The decrease in bilayer thickness and the reduced tail order facilitates the water penetration of lipid bilayers, water molecules can pass the bilayer easier. The observation in the simulation is consistent with former experimental finding.31
Based on our simulation, the strengthening and weakening effect on membrane strength is mainly due to the nanoparticle induced thickening and thinning effect on bilayer thickness under high surface tension. The inclusion of large nanoparticles which have high interaction strength with lipids results in the ordering effect on lipid packing, thus increases the area-stretch modulus and reduces the thinning effect of mechanical stretch. In contrast, the inclusion of small nanoparticles which have low interaction strength with lipids increases the thinning effect of mechanical stretch.
On the other hand, the enhanced weakening effect for small nanoparticles C4L and C4LL points to a different underlying mechanism. Small nanoparticles with higher diffusibility are suggested to disturb lipid packing, induce larger local undulation and transient “free volume” in the interior of the lipid membrane. The “free volume” was filled by water afterward thus facilitates water penetration and increases the permeability. The highly diffused nanoparticle unstablized the lipid membrane and triggered the opening of a transient pore. While the low diffusible large nanoparticles with a large surface binding site tend to stabilize the surrounding lipids, thus reduced the possibility to produce “free volume” by thermal fluctuation near the particle. Namely, the effect of large nanoparticle addition to the bilayer is to occupy “free volume”.
The results reveal two competing mechanisms: disruption on a bilayer hydrophobic barrier depending on dynamics, distributions of embedded nanoparticles governed by the geometric of nanoparticles, and hydrophobic matching (binding) interaction of nanoparticles and lipid tails.
The hydrophobic nature of the carbon nanoparticles is crucial for this behavior since the nanoparticles only interact with the hydrophobic tails of lipid, thus affect the hydrophobic barrier. The increase of the size of nanoparticles leads to higher disruption to the bilayer structure. High diffusion of small nanoparticles is suggested to induce “free volume” in the bilayer interior. The small nanoparticle induced thinning effect on bilayer structure and increased permeability by disturbing the hydrophobic barrier of lipid bilayers, therefore decreased the bilayer strength (Fig. 6).
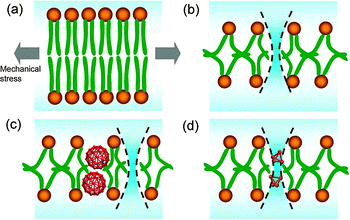 | ||
| Fig. 6 Mechanism of the fullerene strengthening or weakening effect on a lipid membrane. (a) Lipid bilayers under zero surface tension. (b) Water penetration of lipid bilayers under critical rupture tension. (c) Smaller fullerenes facilitate water penetration under lower critical rupture tension. (d) Larger fullerenes inhibit water penetration nearby under higher critical rupture tension. | ||
On the other hand, the diameter of large nanoparticles is comparable to the length of lipid tails (∼1.5 nm), indicated that the energy gained from hydrophobic binding of nanoparticles and lipid tails could not be ignored. When the nanoparticle is large enough to become a part of a hydrophobic barrier, the inclusion of nanoparticles would lead to an ordering effect and increase the strength of lipid bilayers. The simulation of lipid bilayers with different tail length is consistent with our expectation that the energy gained from the binding of nanoparticles and short tail lipid (DLPC, in Fig. 2(D) and (E)) is comparably higher thus influences the pore formation process.
The nanoparticle C4LL also represents a large nanoparticle with a rough surface, e.g., a nanoparticle with four surface bumps. The presence of surface bumps decreases the binding site (or contact surface) between nanoparticles and lipid tails and induces larger deformation of lipids nearby, thus increases the free energy of hydrophobic interaction. Therefore, we suggested that roughness of the nanoparticle surface is also a factor of the instability of biomembranes. However, for small nanoparticles, the disruption of lipid bilayers is due to the high diffusion of the particle, it is the size of nanoparticles that dominates the rupture behavior of lipid bilayers.
Somewhat similar behavior was observed in bilayers with different inclusions in recent experiments. The presence of certain peptides such as antimicrobial peptides, was found to weakened the biomembrane.44–46 In contrast, the addition of cholesterol and lipid rafts increases the strength of the membrane.20,39 Cholesterol is a hydrophobic molecule with a rigid planar backbone the length of which is comparable to that of the lipid tail. It is well know that addition of cholesterol reduces lipid mobility and permeability, and is accompanied by an increase in lipid packing, lateral compressibility and rupture strength. The same trend is observed in our simulation with large nanoparticles. It is suggested that the strength of biomembranes depends on the strength of the interaction between lipids and the surrounding membrane inclusions,47 consistent with our findings.
In summary, we showed that both the small particles and large particles would disturb the pore formation process by strengthening or weakening the biomembrane. The cytotoxicity of the tested nanoparticles is strongly correlated to their size, the smaller nanoparticles may be more harmful than larger particles, for the potential to kill the cell in the form of cell lysis under high surface tension (e.g., in osmotic swelling). The strengthening or weakening effect of lipid bilayers by nanoparticles would disturb the pore formation involving the process of cell, such as membrane fusion,19 the controlled transport of materials across cells. And the changes in the permeability may destroy the concentration balance of the ions between the inside and outside environments of cells, which related to cytotoxicity. Changes in cell membrane area-stretch properties may also alter cellular functions by modifying the properties and functioning of membrane proteins.48 Namely, the potential risk of exposures of human to nanoparticles is non-ignorable.
Furthermore, by controlling the size of carbon nanoparticles, it is possible to improve the strengthening or weakening effect on specific cells in certain medical purpose, e.g., kill specific cells by rupture the membrane. A typical example is the application to replace antibiotics. Antibiotics are used to kill or slow down the growth of bacteria. The overuse of antibiotics weakens their defensive capabilities (multidrug resistance) and cause the development of new antibiotics more difficult and expensive. The use of nanoparticles to mimic the behavior of antimicrobial peptides is underway.49 Importantly, our results also provide new insight of protein induced membrane strengthening. Recent simulation study suggests that the membrane protein MscL embedded membrane system is 22% more difficult to expand than the pure lipid membrane.50 Similar to the effect of large nanoparticles on the area stretch modulus in our simulation.
4 Conclusions
In the present study we described the mechanical properties, dynamics and mechanism of rupture behavior of lipid bilayers in the presence of nanoparticles under mechanical stress, based on coarse grained molecular dynamics simulations. We have shown the strengthening and weakening effect of large and small nanoparticles on the strength of lipid bilayers, respectively.The inclusion of small hydrophobic nanoparticles induced free volumes (defects) in the hydrophobic barrier of bilayer, thus causes disruption to bilayer structure and benefits water penetration and pore formation in a lipid membrane. On the other hand, the hydrophobic binding of larger nanoparticles and lipid tails increases the lipid tail packing thus lowering the free energy of the system, strengthening the bilayer by preventing the formation of hydrophobic pores nearby in bilayer interior.
Our study gives possible undesirable results that the interactions of nanoparticles and biological systems may be harmful, with the potential to disturb the pore formation process and generate toxicity by weakening the strength of cell in the form of decreasing the rupture tension of biomembranes.
This study helps in better understanding the role of length scales involved in the mechanisms of cytotoxicity of carbon nanoparticles upon pore formation of the nanoparticle rich lipid membrane under mechanical stress. It is also helpful in the selection of dimensions and shapes for drug delivery cargo, altering the geometry of nanoparticles can minimise hazard and bring about new applications. Furthermore, the size effect of nanoparticles may also help our understanding of the interactions between biomacromolecules and the cell membrane in the pore formation process, e.g., membrane fusion.
Acknowledgements
We thank Xiaoyue Zhang, Pengcheng Li and Yongwei Zhang for helpful suggestions. Financial support from the National Natural Science Foundation of China (10732100, 10972239, 11004255, 11072271) is gratefully acknowledged.References
- R. F. Service, Science, 2005, 310, 1132–1134 CrossRef.
- Y. Xia, Nat. Mater., 2008, 7, 758–760 CrossRef CAS.
- S. Mitragotri and J. Lahann, Nat. Mater., 2009, 8, 15–23 CrossRef CAS.
- A. E. Nel, L. Madler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS.
- P. Decuzzi and M. Ferrari, Biophys. J., 2008, 94, 3790–3797 CrossRef CAS.
- H. Murayama, S. Tomonoh, J. M. Alford and M. E. Karpuk, Fullerenes, Nanotubes, Carbone Nanostruct., 2005, 12, 1–9 CrossRef.
- T. Xia, M. Kovochich, J. Brant, M. Hotze, J. Sempf, T. Oberley, C. Sioutas, J. Yeh, M. Wiesner and A. Nel, Nano Lett., 2006, 6, 1794–1807 CrossRef CAS.
- C. A. Poland, R. Duffin, I. Kinloch, A. Maynard, W. A. H. Wallace, A. Seaton, V. Stone, S. Brown, W. MacNee and K. Donaldson, Nat. Nanotechnol., 2008, 3, 423–428 CrossRef CAS.
- A. Nel, T. Xia, L. Mdler and N. Li, Science, 2006, 311, 622–627 CrossRef CAS.
- G. Oberdörster, E. Oberdörster and J. Oberdörster, Environ. Health Perspect., 2005, 113, 823–839 CrossRef.
- R. Behra and H. Krug, Nat. Nanotechnol., 2008, 3, 253–254 CrossRef CAS.
- W. H. Suh, K. S. Suslick, G. D. Stucky and Y. H. Suh, Prog. Neurobiol., 2009, 87, 133–170 CrossRef CAS.
- S. Linse, C. Cabaleiro Lago, W. F. Xue, I. Lynch, S. Lindman, E. Thulin, S. E. Radford and K. A. Dawson, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8691–8696 CrossRef CAS.
- V. L. Colvin and K. M. Kulinowski, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8679–8680 CrossRef CAS.
- A. W. Jensen, S. R. Wilson and D. I. Schuster, Bioorg. Med. Chem., 1996, 4, 767–779 CrossRef CAS.
- S. Bosi, L. Feruglio, T. Da Ros, G. Spalluto, B. Gregoretti, M. Terdoslavich, G. Decorti, S. Passamonti, S. Moro and M. Prato, J. Med. Chem., 2004, 47, 6711–6715 CrossRef CAS.
- J. Wong Ekkabut, S. Baoukina, W. Triampo, I. M. Tang, D. P. Tieleman and L. Monticelli, Nat. Nanotechnol., 2008, 3, 363–368 CrossRef CAS.
- A. Jusufi, R. H. DeVane, W. Shinoda and M. L. Klein, Soft Matter, 2011, 7, 1139–1146 RSC.
- S. Martens, M. M. Kozlov and H. T. McMahon, Science, 2007, 316, 1205–1208 CrossRef CAS.
- W. Rawicz, B. Smith, T. McIntosh, S. Simon and E. Evans, Biophys. J., 2008, 94, 4725–4736 CrossRef CAS.
- K. Yang and Y. Q. Ma, Nat. Nanotechnol., 2010, 5, 579–583 CrossRef CAS.
- M. R. R. de Planque, S. Aghdaei, T. Roose and H. Morgan, ACS Nano, 2011, 5, 3599–3606 CrossRef CAS.
- T. Yu, A. Malugin and H. Ghandehari, ACS Nano, 2011, 5, 5717–5728 CrossRef CAS.
- Y. S. Lin and C. L. Haynes, J. Am. Chem. Soc., 2010, 132, 4834–4842 CrossRef CAS.
- J. Q. Lin, Y. G. Zheng, H. W. Zhang and Z. Chen, Langmuir, 2011, 27, 8323–8332 CrossRef CAS.
- S. J. Marrink, A. H. de Vries and D. P. Tieleman, Biochim. Biophys. Acta Biomembr., 2009, 1788, 149–168 CrossRef CAS.
- S. J. Marrink, H. J. Risselada, S. Yefimov, D. P. Tieleman and A. H. de Vries, J. Phys. Chem. B, 2007, 111, 7812–7824 CrossRef CAS.
- S. J. Marrink, A. H. de Vries and A. E. Mark, J. Phys. Chem. B, 2004, 108, 750–760 CrossRef CAS.
- H. J. C. Berendsen, D. van der Spoel and R. van Drunen, Comput. Phys. Commun., 1995, 91, 43–56 CrossRef CAS.
- E. Lindahl, B. Hess and D. van der Spoel, J. Mol. Model, 2001, 7, 306–317 CAS.
- K. Olbrich, W. Rawicz, D. Needham and E. Evans, Biophys. J., 2000, 79, 321–327 CrossRef CAS.
- E. Evans, V. Heinrich, F. Ludwig and W. Rawicz, Biophys. J., 2003, 85, 2342–2350 CrossRef CAS.
- H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren, A. DiNola and J. R. Haak, J. Chem. Phys., 1984, 81, 3684–3690 CrossRef CAS.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS.
- H. Leontiadou, A. E. Mark and S. J. Marrink, Biophys. J., 2004, 86, 2156–2164 CrossRef CAS.
- M. Jansen and A. Blume, Biophys. J., 1995, 68, 997–1008 CrossRef CAS.
- D. W. Deamer and J. Bramhall, Chem. Phys. Lipids, 1986, 40, 167–188 CrossRef CAS.
- H. S. Muddana, R. R. Gullapalli, E. Manias and P. J. Butler, Phys. Chem. Chem. Phys., 2011, 13, 1368–1378 RSC.
- Y. W. Hsueh, M. T. Chen, P. J. Patty, C. Code, J. Cheng, B. J. Frisken, M. Zuckermann and J. Thewalt, Biophys. J., 2007, 92, 1606–1615 CrossRef CAS.
- A. L. Kuo and C. G. Wade, Biochemistry, 1979, 18, 2300–2308 CrossRef CAS.
- A. Filippov, G. Oradd and G. Lindblom, Langmuir, 2003, 19, 6397–6400 CrossRef CAS.
- E. Falck, M. Patra, M. Karttunen, M. T. Hyvnen and I. Vattulainen, Biophys. J., 2004, 87, 1076–1091 CrossRef CAS.
- A. Finkelstein, J. Gen. Physiol., 1976, 68, 127–135 CrossRef CAS.
- B. Bechinger, Biochim. Biophys. Acta Biomembr., 1999, 1462, 157–183 CrossRef CAS.
- K. Hristova, C. E. Dempsey and S. H. White, Biophys. J., 2001, 80, 801–811 CrossRef CAS.
- H. W. Huang, F. Y. Chen and M. T. Lee, Phys. Rev. Lett., 2004, 92, 198304 CrossRef.
- S. C. W. Tan, T. Yang, Y. Gong and K. Liao, J. Biomech. Eng., 2011, 44, 1361–1366 Search PubMed.
- J. A. Lundbæk, J. Phys.: Condens Matter, 2006, 18, S1305 CrossRef.
- A. Alexeev, W. E. Uspal and A. C. Balazs, ACS Nano, 2008, 2, 1117–1122 CrossRef CAS.
- J. Jeon and G. A. Voth, Biophys. J., 2008, 94, 3497–3511 CrossRef CAS.
| This journal is © the Owner Societies 2013 |
