Long perfluoroalkyl chains are not required for dynamically oleophobic surfaces†
Joonsik
Park
,
Chihiro
Urata
,
Benjamin
Masheder
,
Dalton F.
Cheng
and
Atsushi
Hozumi
*
National Institute of Advanced Industrial Science and Technology (AIST), 2266-98, Anagahora, Shimoshidami, Moriyama, Nagoya, Aichi 463-8560, Japan. E-mail: a.hozumi@aist.go.jp; Fax: +81-52-736-7406; Tel: +81-52-736-7388
First published on 12th November 2012
Abstract
Dynamically oleophobic surfaces for alkane liquids, such as n-decane, n-dodecane and n-hexadecane, were successfully prepared using a simple sol–gel mixture containing 3,3,3-trifluoropropyltrimethoxysilane (FAS3) and tetramethoxysilane (TMOS). Due to the enhancement of the motion of functional groups by addition of TMOS as a molecular spacer, small volume alkane droplets (3 μL) could be easily set in motion to move across and off our hybrid surfaces without pinning at low tilt angles (∼10°), and without relying on conventional surface roughening and long-chain perfluoroalkylsilanes.
Inspired by natural examples of materials with amazing surface dewetting/self-cleaning properties, for instance the lotus leaf and water strider's leg, various artificial superhydrophobic surfaces exhibiting a water contact angle (CA) larger than 150° have been developed.1 To realize such excellent water dewetting properties, the presence of surface micro/nanostructures and low surface energy components are commonly required. In contrast to the successful preparation of superhydrophobic surfaces, it is still very challenging to fabricate superoleophobic surfaces that strongly promote the dewetting of nonpolar alkane liquids with low surface tensions (in particular, lower than 27 dyn cm−1).2 In addition to extremely high CAs, such surfaces are required to allow small-volume alkane liquid droplets (∼3–10 μL) to easily roll across and off, at a tilt angle (TA) of less than 10°. Until now, only a handful of papers have described the successful formation of such superoleophobic surfaces (perfluorinated oxidized-silicone nanofilaments2d and re-entrant curvature surfaces2a–c). To accomplish this goal, besides surface roughening, surface energies should be decreased to as low as possible. Thus, most studies related to this research field have unsurprisingly employed perfluorinated compounds (PFCs), in particular, long-chain perfluoroalkylsilanes (FASs)2a–f or perfluorinated fluids2e to reduce interaction between the surface and the liquid, taking advantage of the extremely low surface energies of perfluoroalkyl groups (CF3(CF2)n–).3 Unfortunately, however, the chemical and physical effects of longer chain PFCs (in particular, n ≥ 7) on human health and environments have been lately viewed with suspicion.4 Thus, an alternative method not requiring long-chain perfluoroalkylsilanes has been strongly demanded. Furthermore, fabrication of the cited superoleophobic surfaces is only possible on certain appropriate substrates2 and it usually involves several individual processes, such as lithography, anodization and chemical etching, which consume significant energy and hinder their practical application.
To overcome these problems, our group and McCarthy's group have focused on the creation of flat surfaces showing negligible CA hysteresis (the difference between advancing (θA) and receding (θR) CAs) toward various probe liquids.5 By employing branched-5a–c or ring-shaped organosilane monolayers,5f ultrathin layers of low-molecular-weight PDMS,5e,g–i oligomeric layers from alkylmethyldichlorosilanes5a,d and alkylsilane-derived sol–gel hybrid films,5j we have successfully controlled dynamic dewetting behavior. Probe liquids have been shown to move very easily on these surfaces and roll off when only slightly tilted, regardless of the magnitude of CAs. A common feature among these surfaces is that the surface bound molecular arrays are flexible and liquid-like, and that droplets in contact with them experience very low energy barriers between metastable states, leading to the formation of low CA hysteresis surfaces. Among these approaches, the co-hydrolysis and co-condensation based sol–gel synthesis of functional organosilanes and tetraalkoxysilanes has provided encouraging results.6 It is a simple and promising approach, which allows arbitrary dewetting control by varying the composition of binary mixtures.5j Applying this principle to reduce the packing of functional groups of organosilanes at the outermost surface is expected to enhance the dewetting properties of the surfaces by increasing the ability of functional groups to move and become liquid-like. This approach is similar to that of more traditional surfaces on which packing density was reduced, either through shortening the reaction time5b or use of specific molecular structures.5a,c,f Our assumption is indirectly supported by Takahara's previous study,7 in which they successfully prepared super-liquid-repellent surfaces using a sol–gel solution consisting of FAS (CF3(CF2)7CH2CH2Si(OC2H5)3) and tetraethoxysilane (TEOS) with and without colloidal silica particles. They carefully studied the effects of surface roughness of the hybrid films on dynamic hydrophobicity/oleophobicity. Their results for n-dodecane implied that the achieved hybrid film surface without addition of silica nanoparticles would be liquid-like, because the smooth surface would exhibit significantly low CA hysteresis (∼5°), they did not however explain this phenomenon in detail or measure TAs. Consequently, we have paid attention to this unusual CA behavior and began to study various sol–gel hybrid films prepared by mixing tetramethoxysilane (TMOS: Si(OMe)4) and FAS with short or long perfluoroalkyl chains (CF3(CF2)n–, where n = 0 or 7) at controlled binary compositions based on Takahara's approach. It was quickly discovered that the dynamic dewettability of the FAS-derived hybrid films toward alkane liquids was less dependent on perfluoroalkyl chain lengths and surface fluorine concentrations than previously assumed. We report here a simple, reproducible and environmentally-friendly technique that enables the formation of flat and transparent hybrid surfaces exhibiting exceptional dynamic oleophobicity against various alkane liquids over a large area at ambient conditions, without relying on conventional surface roughening and long-chain PFCs. Such surfaces would be very appealing for practical applications including self-cleaning surfaces, window coatings, food packaging, stain-resistant/antifouling materials, touch panel displays, and so on.
Our hybrid films were prepared using a conventional co-hydrolysis and co-condensation method according to previous reports.5j,6,7 Briefly, precursor solutions were prepared by mixing FAS reagent (FAS3: CF3-CH2CH2Si(OMe)3 or FAS17: CF3(CF2)7–CH2CH2Si(OMe)3) and TMOS in an isopropanol (IPA)/hydrochloric acid (HCl) solution for 24 h at room temperature (25 °C ± 2). The molar ratio of precursor solution was as follows: Si-OR/IPA/H2O/HCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.3
6.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 × 10−4. In this study, the Si/C ratio (RSi/C) of the reaction mixture was in the range of 0.5 to 3. The precursor solutions were then spin-coated on glass or Si substrate. As a control experiment, silsesquioxane films of FAS3 (FAS3sil.) and FAS17 (FAS17sil.) were prepared in the same manner (see ESI† for detailed experimental conditions).
1.8 × 10−4. In this study, the Si/C ratio (RSi/C) of the reaction mixture was in the range of 0.5 to 3. The precursor solutions were then spin-coated on glass or Si substrate. As a control experiment, silsesquioxane films of FAS3 (FAS3sil.) and FAS17 (FAS17sil.) were prepared in the same manner (see ESI† for detailed experimental conditions).
The final precursor solutions of the hybrid (FAS–TMOS) films, after a reaction time of 24 hours, were clear and highly transparent over the full visible range of RSi/C ratios (Fig. S1; ESI†), and their transparency remained unchanged even after spin coating. The average film thicknesses of these samples were in the range of 320 to 520 nm. As shown in Fig. 1, the FAS3– and FAS17–TMOS hybrid films (RSi/C = 1) were highly transparent, with UV-vis spectroscopy showing over 90% transmission in the visible range. The average root-mean-square roughness (Rrms) of the FAS3– and FAS17–TMOS coatings, as measured by AFM, was estimated to be 0.41 and 0.82 nm, respectively, over a 3 × 3 μm area, which is similar to that of polished Si, the substrate for these measurements (Rrms = ∼0.2 nm) (Fig. S2a and b; ESI†). Hence, it could be concluded that the static/dynamic dewettability of these surfaces was not influenced by surface morphology.
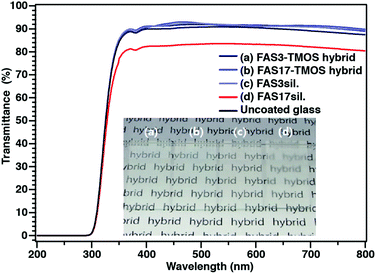 | ||
| Fig. 1 Transparency of FAS-derived hybrid films as characterized by UV-vis transmittance spectra and visual appearance of films (inset images). | ||
The θS, θA/θR, Δθcos (cos θR − cos θA), and TAs of water and three alkane liquids on FAS3– and FAS17–TMOS hybrid film (RSi/C = 1)-covered glass surfaces are summarized in Table 1. As a result of the decrease in probe liquid surface tension, the θS values on both hybrid surfaces decreased from water to n-decane. In all cases the θS values of FAS3–TMOS surfaces were smaller than those of FAS17–TMOS, results which can only be caused by the differences in surface chemical properties of the samples. As the RSi/C ratios were decreased it was noted that the fluorine concentrations (as estimated by XPS) and the θS values of all probe liquids on the samples also gradually increased (Fig. 2a), revealing an apparent relationship between θS values of the four probe liquids and the surface fluorine concentrations. Surprisingly, in contrast to such static situations, dynamic dewetting behavior of alkane liquids appeared less dependent on functional group chain lengths and surface fluorine concentrations (Table 1, Fig. 2b and 2c). The Δθcos values of water (Fig. 2b) were considerably larger than those of the alkane liquids and minimum substrate TAs were significantly high even for 10 μL water droplets (Fig. 2c). This was probably due to a strong mutual interaction between the fluorine and water,9 which led to greater resistance to the contact line motion of the water droplets (contact line pinning), resulting in the large Δθcos and TAs. Indeed, as shown in Fig. 2b, TAs for water also increased with increasing fluorine concentration. Such a phenomenon is easily observed on typical hydrophobic glass windows or car windshields, which even after treatment with conventional FASs (mainly FAS17) exhibit inadequate dynamic dewettability, as is obvious on rainy days. In contrast, minimum substrate TAs of very small (3 μL) alkane droplets on both samples were considerably lower than those for water, and further decreased with lower carbon number and surface tension. In comparison to water, the dynamic dewettability of alkane liquids on each sample surface appeared less dependent on the fluorine concentration. For example, in the case of the FAS3–TMOS hybrid film (RSi/C = 1), only a 5.1° angle of incline was sufficient to initiate movement for a 3 μL droplet of n-decane without pinning (Movie S1; ESI†). This value is smaller than that of the FAS17–TMOS hybrid film (TA = 6.9°), and almost identical to that reportedly needed to initiate movement of a 5 μL n-decane droplet on a superoleophobic surface with θS > 160° (TA = 5.3°).2d In addition to n-decane, excellent dynamic dewetting behavior was similarly observed for the other alkane probe liquids (n-dodecane and n-hexadecane) (Table 1).
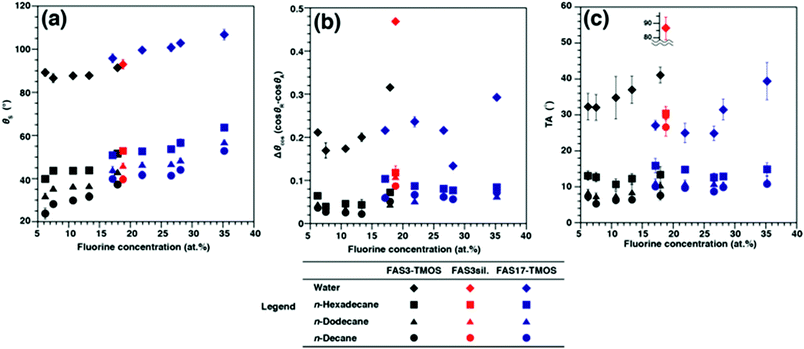 | ||
| Fig. 2 Changes in (a) θS values, (b) CA hysteresis, and (c) TAs with XPS-estimated fluorine-concentration of FAS3–TMOS, FAS3sil., and FAS17–TMOS films for three alkanes and water. | ||
| Probe liquida | θ S (°) | θ A/θR (°) | Δθcos (cos θR − cos θA) | TAb (°) | |
|---|---|---|---|---|---|
| a Surface tension (γ(dyn cm−1)) values of n-decane, n-dodecane, n-hexadecane, and water are 23.8, 25.4, 27.5, and 72.8, respectively, at 20 °C (from ref. 8). b 3 μL of three alkanes and 10 μL of water were set on each hybrid film. | |||||
| FAS3–TMOS hybrid (RSi/C = 1) | n-Decane | 31 | 32/29 | 0.022 | 5.1 |
| n-Dodecane | 37 | 38/34 | 0.042 | 7.0 | |
| n-Hexadecane | 42 | 43/38 | 0.050 | 9.4 | |
| Water | 91 | 98/81 | 0.299 | 34.6 | |
| FAS17–TMOS hybrid (RSi/C = 1) | n-Decane | 47 | 49/45 | 0.056 | 6.9 |
| n-Dodecane | 52 | 53/48 | 0.076 | 8.5 | |
| n-Hexadecane | 58 | 59/54 | 0.077 | 11.4 | |
| Water | 104 | 109/101 | 0.135 | 26.5 | |
| FAS3sil. | n-Decane | 40 | 41/32 | 0.087 | 25.4 |
| n-Dodecane | 46 | 47/38 | 0.108 | 29.3 | |
| n-Hexadecane | 53 | 54/45 | 0.118 | 30.0 | |
| Water | 93 | 104/77 | 0.469 | 86.4 | |
These excellent dynamic oleophobicities for alkane liquids on our hybrid film surfaces appeared only when TMOS molecules were added to the precursor solutions. As can be seen in Table 1 and Fig. 2c, when compared with FAS3–TMOS hybrid films, the FAS3sil. film surface required increased TAs to initiate alkane liquid droplet motion, although there were no marked differences in surface flatness (Fig. S2c; ESI†, Rrms = 0.52 nm) and chemical state (fluorine concentration was about 18.8 at.%) for all FAS3-derived samples.
Comparison of the Δθcos values of our FAS3–TMOS hybrid film surfaces (0.022 to 0.050), and those of the FAS3sil. film surface (0.087 to 0.118) reveals a large difference, which caused the alkane probe liquids to stick to the surface and require large TAs (Table 1 and Fig. 2c). This marked difference in the final dynamic oleophobicity observed between the FAS3–TMOS hybrid and FAS3sil. films was believed to be due to the differences in the physical nature (solid-like or liquid-like) of these surfaces. Reduction of packing density through the simple addition of TMOS into the film matrix was considered to impart a liquid-like nature to the surfaces by increasing the physical mobility of the surface-tethered functional moieties.5j On the other hand, judging from the dynamic CA data (large Δθcos and TAs) shown in Fig. 2b and 2c, the FAS3sil. film surface can be considered to be more solid-like, possessing negligible rotational freedom of the functional moieties. However, in the case of FAS17, we cannot compare the results directly, but after considering its excellent dynamic CA behavior, the motion of functional groups is expected to similarly increase by the addition of TMOS. However, it is well known that longer perfluoroalkyl chains are rigid and show strong mutual interactions with each other,10 thus the chain mobility of FAS17 is less than that of FAS3. Indeed, Δθcos values of alkane liquids on the FAS17–TMOS hybrid films are slightly larger than those of FAS3–TMOS, indicating that the shorter chain of FAS3 provided slightly less resistance to the contact line motion of alkane liquid drops. In addition, TMOS also worked effectively as a binder to aid the formation of continuous/homogeneous FAS17 transparent films, which were difficult to prepare in the absence of TMOS (Fig. 1).
FAS3–TMOS hybrid films have another advantage in that they showed greater thermal stability. Thermal gravimetric analysis (TGA) confirmed that they remained intact even after heating to 470 °C as demonstrated by the significant weight loss starting at this temperature. On the other hand, decomposition of FAS17–TMOS hybrids started at around 410 °C. Indeed, as confirmed by dynamic CA measurements, the latter were thermally damaged faster than the former. For example, a droplet of n-decane was completely stuck to the latter surfaces after thermal treatment at 450 °C for 10 min in air, while excellent dynamic dewettability of the former remained unchanged even after the same thermal treatment (Fig. S3; ESI†).
Conclusions
We have successfully prepared flat and transparent surfaces exhibiting excellent dynamic oleophobicity against various alkane liquids, using a simple sol–gel mixture of 3,3,3-trifluoropropyltrimethoxysilane (FAS3) and tetramethoxysilane (TMOS). It is common knowledge that the final dewetting performance of FAS-derived monolayers/films strongly depends on the perfluoroalkyl chain lengths. To obtain the best performance, the use of longer perfluoroalkyl-terminated silanes has been believed to be necessary, our results demonstrated here are completely opposite to this conventional understanding. These findings also offer clear evidence that the addition of TMOS plays key roles in both the formation of homogeneous/continuous films and the improvement of dynamic dewetting behavior against various alkane liquids. In contrast to the conventional oleophobic/superoleophobic treatments reported thus far, our one-pot process is simple, effective and environmentally friendly, since it does not require any long-chain perfluoroalkylsilanes, thermal treatment or physical/chemical treatments to roughen the surfaces. Thus, we expect this technique to provide widely applicable and convenient oleophobic coatings and surface treatments for various substrates.Acknowledgements
We would like to thank Prof. Kazuyuki Kuroda and Ms Saeko Hayase of Waseda University for the permission to use the stylus profilometer and their technical assistance. This work was partially supported by a Grant-in-Aid for Scientific Research on Innovative Areas (A.H. and C.U.; no. 24120005) and Research Activity Start-up (C.U.; no. 23850020) of The Ministry of Education, Culture, Sports, Science, and Technology, Japan and by the Public Foundation of Chubu Science and Technology Center “Greater Nagoya Project”.Notes and references
- (a) E. Ruiz-Hitzky, M. Darder, P. Aranda and K. Ariga, Adv. Mater., 2010, 22, 323 CrossRef CAS; (b) B. Bhushan, Philos. Trans. R. Soc. London, Ser. A, 2009, 367, 1445 CrossRef CAS; (c) K. Koch and W. Bartholott, Philos. Trans. R. Soc. London, Ser. A, 2009, 367, 1487 CrossRef CAS.
- (a) A. Tuteja, W. Choi, M. L. Ma, J. M. Mabry, S. A. Mazella, G. C. Rutledge, G. H. McKinley and R. E. Cohen, Science, 2007, 318, 1618 CrossRef CAS; (b) A. Tuteja, W. Choi, J. M. Mabry, G. H. McKinley and R. E. Cohen, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18200 CrossRef CAS; (c) A. Tuteja, W. Choi, G. McKinley, R. E. Cohen and M. F. Rubner, MRS Bull., 2008, 33, 752 CrossRef CAS; (d) J. Zhang and S. Seeger, Angew. Chem., Int. Ed., 2011, 50, 6652 CrossRef CAS; (e) T.-S. Wong, S.-H. Kang, S. K. Y. Tang, E. J. Smythe, B. D. Hatton, A. Grinthal and J. Aizenberg, Nature, 2011, 477, 443 CrossRef CAS; (f) X. Deng, L. Mammen, H.-J. Butt and D. Vollmer, Science, 2012, 335, 67 CrossRef CAS.
- (a) E. F. Hare, E. G. Sharfrin and W. A. Zisman, J. Phys. Chem., 1954, 58, 236 CrossRef CAS; (b) W. A. Zisman, Contact Angle, Wettability, and Adhesion, ed. F. M. Fowkes, American Chemical Society, Washington, DC, 1964, vol. 43 Search PubMed.
- (a) Y. Zushi, J. N. Hogarh and S. Masunaga, Clean Technol. Environ. Policy, 2011, 14, 9 CrossRef; (b) A. B. Lindstrom, M. J. Strynar and E. L. Libelo, Environ. Sci. Technol., 2011, 45, 7954 CrossRef CAS; (c) A. Zaggia and B. Ameduri, Curr. Opin. Colloid Interface Sci., 2012, 17, 188 CrossRef CAS; (d) http://www.epa.gov/oppt/pfoa/pubs/stewardship/index.html .
- (a) W. Chen, A. Y. Fadeev, M. C. Hsieh, D. Öner, J. Youngblood and T. J. McCarthy, Langmuir, 1999, 15, 3395 CrossRef CAS; (b) A. Y. Fadeev and T. J. McCarthy, Langmuir, 1999, 15, 3759 CrossRef CAS; (c) A. Y. Fadeev and T. J. McCarthy, Langmuir, 1999, 15, 7238 CrossRef CAS; (d) A. Y. Fadeev and T. J. McCarthy, Langmuir, 2000, 16, 7268 CrossRef CAS; (e) J. W. Krumpfer and T. J. McCarthy, Faraday Discuss., 2010, 146, 103 RSC; (f) A. Hozumi, D. F. Cheng and M. Yagihashi, J. Colloid Interface Sci., 2011, 353, 582 CrossRef CAS; (g) J. W. Krumpfer and T. J. McCarthy, Langmuir, 2011, 27, 11514 CrossRef CAS; (h) D. F. Cheng, C. Urata, M. Yagihashi and A. Hozumi, Angew. Chem., Int. Ed., 2012, 51, 2956 CrossRef CAS; (i) D. F. Cheng, C. Urata, B. Masheder and A. Hozumi, J. Am. Chem. Soc., 2012, 134, 10191 CrossRef CAS; (j) C. Urata, D. F. Cheng, B. Masheder and A. Hozumi, RSC Adv., 2012, 2, 9805 RSC.
- (a) A. Shimojima and K. Kuroda, Chem. Rec., 2006, 6, 53 CrossRef CAS; (b) M. Pagliaro, R. Ciriminna and G. Palmisano, J. Mater. Chem., 2009, 19, 3116 RSC; (c) A. Shimojima, Y. Sugahara and K. Kuroda, J. Am. Chem. Soc., 1998, 120, 4528 CrossRef CAS; (d) Y. Tang, L. A. Finlay, G. L. Kowalke, A. E. Meyer, F. V. Bright, M. E. Callow, J. A. Callow, D. E. Wendt and M. R. Detty, Biofouling, 2005, 21, 59 CrossRef CAS.
- M. Hikata, K. Tanaka, T. Nakamura, T. Kajiyama and A. Takahara, Langmuir, 2005, 21, 7299 CrossRef.
- (a) http://www.surface-tension.de ; (b) L. D. A. Chumpitaz, L. F. Coutinho and J. A. Meirelles, J. Am. Oil Chem. Soc., 1999, 76, 379 CrossRef CAS; (c) http://www.etc-cte.ec.gc.ca/databases/Oilproperties/ .
- L. Gao and T. J. McCarthy, Langmuir, 2008, 24, 9183 CrossRef CAS.
- K. Honda, M. Morita, H. Otsuka and A. Takahara, Macromolecules, 2005, 38, 5699 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, detailed experimental conditions, characterization methods, appearance of precursor solutions, AFM images, CA hysteresis data, and movie. See DOI: 10.1039/c2gc36415h |
| This journal is © The Royal Society of Chemistry 2013 |
