Sugar-based aromatic copolyesters: a comparative study regarding isosorbide and diacetalized alditols as sustainable comonomers†
Cristina
Lavilla
and
Sebastián
Muñoz-Guerra
*
Departament d'Enginyeria Química, Universitat Politècnica de Catalunya, ETSEIB, Diagonal 647, 08028, Barcelona. E-mail: sebastian.munoz@upc.edu
First published on 18th October 2012
Abstract
Three carbohydrate-based bicyclic diols, 1,4:3,6-dianhydro-D-glucitol (isosorbide, Is), 2,3:4,5-di-O-methylene-galactitol (Galx) and 2,4:3,5-di-O-methylene-D-mannitol (Manx), were made to react in the melt and under the same conditions with dimethyl terephthalate and 1,4-butanediol to produce respectively three series of PBxIsyT, PBxGalxyT and PBxManxyT random copolyesters. Five different copolymerizations, with molar feed ratios of 1,4-butanediol to sugar-based diols of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 70
20, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 60
30, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 50
40 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, were carried out for each bicyclic diol in order to make a comparative study on their reactivity and properties of the resulting copolyesters. Molecular weights and compositions data of the copolyesters revealed the greater facility of diacetalized diols to react under these conditions compared to isosorbide. The three bicyclic diols contributed to increase the thermal stability and also the glass-transition temperature of PBT. The replacement of 40% of 1,4-butanediol by sugar-based diols increased the glass transition temperature of PBT from 31 °C to ∼60 °C in the case of Galx and up to ∼90 °C regarding Is and Manx. All PBxGalxyT copolyesters as well as PBxIsyT and PBxManxyT ones with contents of Is and Manx of up to 32% and 41%, respectively, were semicrystalline.
50, were carried out for each bicyclic diol in order to make a comparative study on their reactivity and properties of the resulting copolyesters. Molecular weights and compositions data of the copolyesters revealed the greater facility of diacetalized diols to react under these conditions compared to isosorbide. The three bicyclic diols contributed to increase the thermal stability and also the glass-transition temperature of PBT. The replacement of 40% of 1,4-butanediol by sugar-based diols increased the glass transition temperature of PBT from 31 °C to ∼60 °C in the case of Galx and up to ∼90 °C regarding Is and Manx. All PBxGalxyT copolyesters as well as PBxIsyT and PBxManxyT ones with contents of Is and Manx of up to 32% and 41%, respectively, were semicrystalline.
1. Introduction
The urgent need to reduce the amount of petroleum consumed in the industry, as well as to minimize the impact of the use of plastics in the environment, is attracting great attention in research by following different approaches. Thus, significant increases in the recycling rates of polymers are taking place with facilities and changes in recent legislation that now permits even recycling of food contact polymers.1–3 On the other side substitution of petroleum-based monomers by those coming from renewable sources has become one of the most important challenges in current polymer research.4,5 Among the renewable naturally occurring sources, carbohydrates stand out because they are hugely abundant, readily available and able to provide great functional diversity; an increasing activity in the research of carbohydrate-based polymers is reflected in the recently reported literature.6–8Aromatic polyesters, poly(ethylene terephthalate) (PET) and poly(butylene terephthalate) (PBT) in particular, are high performance thermoplastic materials that are massively used in a wide diversity of applications due to their excellent mechanical and thermal properties in addition to other more specific ones. The high resistance to atmospheric action and biological agents exhibited by these materials is an added value when they are intended to be used in long-term applications. In contrast, they are considered unfriendly compounds when used in short-term applications because of their non-renewable origin and great resistance to degradation.9,10 In this regard, the development of aromatic polyesters from natural sources is currently drawing enormous interest. To this purpose a really attractive approach is to unfold sustainable processes able to produce the traditional monomers implied in their synthesis. Thus ethylene glycol can be produced via oxidation and subsequent hydrolysis of bio-based ethylene,11 and 1,4-butanediol by hydrogenation of succinic acid obtained from corn;12 also the production of terephthalic acid from limonene has been recently patented.13 However the industrial application of these methods is still far from realization and monomers used today for the synthesis of industrial aromatic polyesters are almost entirely produced from petrochemical feedstocks. Another possibility of creating sustainable copolymers of PET and PBT is replacing either the diol or the diacid by other bio-based monomers. This is an extremely appealing option provided that the properties of the original polymers are improved, or at least not significantly impoverished.
The high glass transition temperature (Tg) is a greatly appreciated property in those polyesters addressed to packaging under heating or to the manufacture of containers for pressurized gaseous beverages; copolymerization with cyclic diols such as 1,4-cyclohexanedimethanol14,15 or 1,3-cyclobutanediol16 has been a common strategy to obtain aromatic polyesters with enhanced Tg. Carbohydrate-derived monomers with a cyclic structure have achieved a privileged position because, in addition to their natural origin, they are able to provide polyesters with higher glass transition temperatures while essentially retaining their original pattern of behavior. Thus, the olden known 2,5-furandicarboxylic acid is receiving in the last few years a great deal of attention as an important alternative to replace terephthalic acid in polyterephthalates.17,18 A decade ago, the bicyclic anhydride 1,4:3,6-dianhydro-D-glucitol, known as isosorbide, emerged with great pushing force as an adequate monomer for replacing aliphatic diols in PET and PBT.19,20 Very recently we have shown that internally diacetalized alditols with a bicyclic structure are also carbohydrate derivatives very suitable to prepare aromatic copolyesters displaying satisfactory general properties and enhanced Tg.21–24
Given the structural proximity between isosorbide and diacetalized alditols, as well as their common potential use as polycondensation monomers, it is encouraging to make a comparative evaluation of their suitability for the synthesis of aromatic polyesters. This paper has been designed with such an aim and it reports a comparative study of PBT polyesters and copolyesters obtained by replacing 1,4-butanediol by 1,4:3,6-dianhydro-D-glucitol (Is), 2,3:4,5-di-O-methylene-galactitol (Galx), and 2,4:3,5-di-O-methylene-D-mannitol (Manx). They will be called PBxIsyT, PBxGalxyT and PBxManxyT, where x and y stand for the mole percentages (mol%) of 1,4-butanediol and sugar-based bicyclic diols, respectively, in the resulting copolyester. The study examines the reactivity of the three bicyclic diols in the polycondensation with dimethyl terephthalate (DMT) under the same polymerization conditions and makes a comparative evaluation of the influence that each monomer exerts on the glass-transition temperature and crystallinity of the polyesters.
Comparison of structure and properties of Is, Galx and Manx
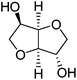
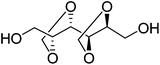
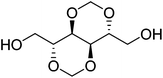
1,4:3,6-Dianhydro-D-glucitol (Is) is the dianhydride generated in the dehydration of D-glucitol (sorbitol). Sorbitol is prepared by hydrogenation of D-glucose coming from cereal starch. Is is the only bicyclic carbohydrate-based monomer commercially available today at an industrial level.25 It is composed of two cis-fused nearly planar tetrahydrofuran rings with a dihedral angle of 120° and the 2- and 5-hydroxyl groups in endo and exo positions, respectively. Since the two hydroxyl groups of Is are secondary, their accessibility to reagents is rather restricted, and due to their different spatial positions in the molecule they will display different reactivity; in fact, a deficiency of the reactivity of the endo hydroxyl group has been detected in several polymerization studies recently reported.25–272,3:4,5-Di-O-methylene-galactitol (Galx) is obtained from galactaric acid by acetalization of the four secondary hydroxyl groups with paraformaldehyde and subsequent reduction. The structure of Galx consists of two non-fused 1,3-dioxolane rings with the two primary hydroxyl groups in exo positions. Contrary to isosorbide, Galx is centrosymmetric so its two free hydroxyl groups display the same reactivity since they are spatially undistinguishable. 2,4:3,5-Di-O-methylene-D-mannitol (Manx) is obtained by acetalization of 1,6-di-O-benzoyl-D-mannitol followed by hydrolysis of the benzoxy groups. Manx consists of two fused 1,3-dioxane rings and possesses a twofold axis of symmetry and, similarly to Galx, the two primary hydroxyl groups are in exo positions and display the same reactivity.
A comparative summary of the main features of Is, Galx and Manx of relevance to this study is given in Table 1.
| Compound | Is | Galx | Manx |
|---|---|---|---|
| Chemical name | 1,4:3,6-Dianhydro-D-glucitol | 2,3:4,5-Di-O-methylene-galactitol | 2,4:3,5-Di-O-methylene-D-mannitol |
| Chemical structure |
|
|
|
| Protecting group | Dianhydride | Diacetal | Diacetal |
| OH groups | Secondary | Primary | Primary |
| Cycle structure | Tetrahydrofuran | 1,3-Dioxolane | 1,3-Dioxane |
| Symmetry | 1 | I | C 2 |
| OH stereo positions | Exo/endo | Exo/exo | Exo/exo |
| Melting point | 60–63 °C | 100–102 °C | 139–140 °C |
| Origin | D-Glucose | D-Galactose | D-Fructose |
2. Experimental
The three series of polyesters, PBxIsyT, PBxGalxyT and PBxManxyT, subjected here to study were obtained according to the synthesis scheme depicted in Scheme 1. Five different copolymerizations, with molar feed ratios of 1,4-butanediol to sugar-based diols of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 70
20, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 60
30, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 50
40 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, were carried out for each bicyclic diol. Exactly the same reaction conditions were used to prepare copolyesters of different series with the same molar feed ratio (1,4-butanediol to sugar-based diols) for the three series. Specific conditions were used for each molar feed ratio, as they are detailed in the ESI† file linked to this paper. The synthesis and characterization of PBxGalxyT and PBxManxyT copolyesters and their parent homopolyesters has been reported previously in detail.21,22 The synthesis of the PBxIsyT family has been carried out now with the purpose of providing the data necessary for the comparative study aimed at in this work. The common synthetic procedure was the following: the polymerization feed was a mixture of dimethyl terephthalate, 1,4-butanediol and the sugar-based diol in the selected compositions. A molar excess of the diol mixture to dimethyl terephthalate was used, and dibutyl tin oxide (DBTO) catalyst in 0.6% molar with respect to monomers as well as antioxidants Irganox 1010 (0.1% w/w) and Irgafos 126 (0.3% w/w) were added. The reaction was performed in the melt along two steps in a three-necked, cylindrical-bottom flask equipped with a mechanical stirrer, a nitrogen inlet and a vacuum distillation outlet. Firstly the transesterification reaction was carried out under a low nitrogen flow at temperatures in the 160–200 °C range depending on composition. The polycondensation reaction was left to proceed at a temperature between 210 and 240 °C under a 0.03–0.06 mbar vacuum. Then, the reaction mixture was cooled to room temperature, and the atmospheric pressure was recovered with nitrogen to prevent degradation. The resulting polymers were dissolved in chloroform or in a mixture of chloroform and trifluoroacetic acid (9
50, were carried out for each bicyclic diol. Exactly the same reaction conditions were used to prepare copolyesters of different series with the same molar feed ratio (1,4-butanediol to sugar-based diols) for the three series. Specific conditions were used for each molar feed ratio, as they are detailed in the ESI† file linked to this paper. The synthesis and characterization of PBxGalxyT and PBxManxyT copolyesters and their parent homopolyesters has been reported previously in detail.21,22 The synthesis of the PBxIsyT family has been carried out now with the purpose of providing the data necessary for the comparative study aimed at in this work. The common synthetic procedure was the following: the polymerization feed was a mixture of dimethyl terephthalate, 1,4-butanediol and the sugar-based diol in the selected compositions. A molar excess of the diol mixture to dimethyl terephthalate was used, and dibutyl tin oxide (DBTO) catalyst in 0.6% molar with respect to monomers as well as antioxidants Irganox 1010 (0.1% w/w) and Irgafos 126 (0.3% w/w) were added. The reaction was performed in the melt along two steps in a three-necked, cylindrical-bottom flask equipped with a mechanical stirrer, a nitrogen inlet and a vacuum distillation outlet. Firstly the transesterification reaction was carried out under a low nitrogen flow at temperatures in the 160–200 °C range depending on composition. The polycondensation reaction was left to proceed at a temperature between 210 and 240 °C under a 0.03–0.06 mbar vacuum. Then, the reaction mixture was cooled to room temperature, and the atmospheric pressure was recovered with nitrogen to prevent degradation. The resulting polymers were dissolved in chloroform or in a mixture of chloroform and trifluoroacetic acid (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and precipitated in excess of methanol in order to remove unreacted monomers and formed oligomers. Finally, the polymer was collected by filtration, extensively washed with methanol, and dried under vacuum.
1), and precipitated in excess of methanol in order to remove unreacted monomers and formed oligomers. Finally, the polymer was collected by filtration, extensively washed with methanol, and dried under vacuum.
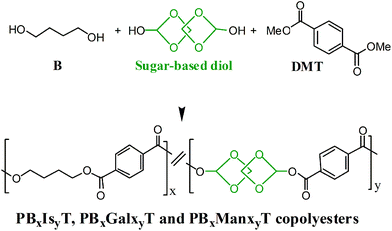 | ||
| Scheme 1 Polymerization reactions leading to PBxIsyT, PBxGalxyT and PBxManxyT copolyesters. | ||
The resulting polymers were extensively characterized and their thermal properties accurately evaluated in parallel. The chemical constitution, composition and microstructure of polyesters and copolyesters were determined by NMR and their thermal stability measured by thermogravimetry under an inert atmosphere. Their melting and glass transition behavior were examined by DSC and temperatures and enthalpies measured upon heating using exactly the same recording conditions.
The specific reaction conditions and results of the synthesis of the three series of polyesters as well as the technical and methodological details used in their characterization and properties evaluation are provided in the ESI† file linked to this paper.
3. Results and discussion
3.1. Synthesis: molecular weight and composition
The polymerization conditions used in the synthesis of homopolyesters and copolyesters were selected to imitate as far as possible those usually applied in the industrial practice; reactions were performed in the melt with the total absence of solvents, and they were left to proceed through a two-step sequence of increasing temperature and vacuum. Transesterification reactions were started at 160 °C in order to prevent volatilization of diols and left to proceed for 2–3 hours period along which, temperature was progressively increased to avoid crystallization of earlier formed oligomers. Polycondensation reactions were carried out for 2–4 hours at temperatures in the 210–240 °C range and under vacuum to facilitate the removal of volatile by-products; lower temperatures and longer reaction times were used for copolyesters with higher contents in sugar-based monomers. Antioxidant additives were added in the synthesis of PBxIsyT copolyesters to avoid discoloration, and dibutyl tin oxide (DBTO) was the catalyst of choice for the three series instead of the commonly used titanium(IV) tetrabutoxide (TBT). Our earlier results in the synthesis of aliphatic homopolyesters from the bicyclic diester dimethyl 2,3:4,5-di-O-methylene-galactarate and linear alkanediols demonstrated the higher activity of the DBTO catalyst compared to TBT which allowed to proceed at lower reaction temperatures without increasing reaction times.28 Under softer reaction conditions, decomposition of the thermally-sensitive sugar compounds was minimized and higher molecular weights could be attained. The synthesis results obtained for PBT, the three copolyester series, PBxGalxyT, PBxManxyT and PBxIsyT, and their respective parent homopolyesters PGalxT, PManxT and PIsT are compared in Table 2.| Copolyester | Yield (%) | Molar composition XB/Xsugar | Molecular weights | Thermal properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feed | Copolyestera | [η]b |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
M
w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
D |
T
5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
T
d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
T
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
T
m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g g |
ΔHm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g g |
||
| a Molar composition determined by integration of the 1H NMR spectra. b Intrinsic viscosity in dL g−1 measured in dichloroacetic acid at 25 °C. c Number-average and weight-average molecular weights in g mol−1 and dispersities measured by GPC in HFIP against PMMA standards. d Temperature at which 5% weight loss was observed. e Temperature for the maximum degradation rate. f Glass-transition temperature taken as the inflection point of the heating DSC traces of melt-quenched samples recorded at 20 °C min−1. g Melting temperature (Tm) and enthalpy (ΔHm) measured by DSC at a heating rate of 10 °C min−1. | ||||||||||||
| PBT | 90 | 100/0 | 100/0 | 0.93 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.4 | 371 | 408 | 31 | 223 | 56.2 |
| PB94Is6T | 85 | 90/10 | 93.9/6.1 | 0.31 | 6800 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.3 | 367 | 406 | 34 | 206 | 43.9 |
| PB85Is15T | 84 | 80/20 | 85.0/15.0 | 0.30 | 6500 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.3 | 367 | 407 | 54 | 189 | 31.7 |
| PB75Is25T | 82 | 70/30 | 74.6/25.4 | 0.35 | 7200 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.4 | 367 | 408 | 67 | 174 | 21.6 |
| PB68Is32T | 83 | 60/40 | 67.7/32.3 | 0.37 | 7300 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.5 | 368 | 409 | 78 | 158 | 11.7 |
| PB56Is44T | 85 | 50/50 | 55.9/44.1 | 0.42 | 8400 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.4 | 372 | 413 | 98 | — | — |
| PIsT | 80 | 0/100 | 0/100 | 0.20 | 2300 | 5400 | 2.3 | 374 | 424 | 148 | — | — |
| PB89Galx11T | 87 | 90/10 | 88.7/11.3 | 0.91 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.5 | 372 | 409 | 46 | 197 | 37.2 |
| PB79Galx21T | 89 | 80/20 | 78.9/21.1 | 0.90 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.4 | 373 | 411 | 53 | 179 | 26.1 |
| PB69Galx31T | 87 | 70/30 | 69.2/30.8 | 0.70 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.4 | 375 | 414 | 57 | 151 | 21.9 |
| PB61Galx39T | 88 | 60/40 | 61.3/38.7 | 0.65 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.5 | 377 | 417 | 62 | 138 | 14.8 |
| PB51Galx49T | 85 | 50/50 | 50.9/49.1 | 0.69 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
2.4 | 379 | 418 | 70 | 119 | 8.7 |
| PGalxT | 86 | 0/100 | 0/100 | 0.50 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.5 | 382 | 436 | 87 | — | — |
| PB91Manx9T | 86 | 90/10 | 91.0/9.0 | 1.18 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.5 | 371 | 408 | 55 | 197 | 34.1 |
| PB80Manx20T | 87 | 80/20 | 80.3/19.7 | 0.84 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.5 | 372 | 410 | 66 | 184 | 26.0 |
| PB69Manx31T | 86 | 70/30 | 69.2/30.8 | 0.75 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.3 | 374 | 411 | 77 | 162 | 5.5 |
| PB59Manx41T | 85 | 60/40 | 59.2/40.8 | 0.83 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.4 | 376 | 411 | 88 | 122 | 5.0 |
| PB49Manx51T | 88 | 50/50 | 49.0/51.0 | 0.77 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
2.4 | 377 | 412 | 100 | — | — |
| PManxT | 85 | 0/100 | 0/100 | 0.51 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.3 | 378 | 421 | 137 | — | — |
PBxGalxyT copolyesters were obtained from 1,4-butanediol, Galx and DMT with intrinsic viscosities of 0.7–0.9 dL g−1 and weight-average molecular weights between 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 41
000 and 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.21 Correspondingly, PBxManxyT copolyesters were obtained when Manx was used as sugar-based comonomers instead.22 In this case, relatively high molecular weight copolymers were also achieved, with intrinsic viscosities between 0.8 and 1.2 dL g−1 and weight-average molecular weights confined in the 38
000 g mol−1.21 Correspondingly, PBxManxyT copolyesters were obtained when Manx was used as sugar-based comonomers instead.22 In this case, relatively high molecular weight copolymers were also achieved, with intrinsic viscosities between 0.8 and 1.2 dL g−1 and weight-average molecular weights confined in the 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–52
000–52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 interval. In contrast, the results obtained in the copolymerizations based on Is were not as satisfactory as those based on Galx and Manx; intrinsic viscosities and weight-average molecular weights of PBxIsyT copolyesters were in the 0.3–0.4 dL g−1 and 15
000 g mol−1 interval. In contrast, the results obtained in the copolymerizations based on Is were not as satisfactory as those based on Galx and Manx; intrinsic viscosities and weight-average molecular weights of PBxIsyT copolyesters were in the 0.3–0.4 dL g−1 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–20
000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 intervals, respectively. Although polycondensations in bulk involving Is have been usually carried out with TBT as the catalyst;26,27,29,30 it was here replaced by DBTO by the reasons indicated above. The molecular weights reported for Is containing copolyesters obtained by using the TBT catalyst are even lower than those obtained here with DBTO. It is worthy to note that molecular weight values are very similar within each series (with the only exception of the homopolyester entirely made of sugar-based diols), and that essentially the same molecular weight dispersity is obtained for all the prepared polyesters regardless of which series is concerned. This satisfactory agreement adds confidence to the comparative analysis of thermal properties that is carried out below.
000 g mol−1 intervals, respectively. Although polycondensations in bulk involving Is have been usually carried out with TBT as the catalyst;26,27,29,30 it was here replaced by DBTO by the reasons indicated above. The molecular weights reported for Is containing copolyesters obtained by using the TBT catalyst are even lower than those obtained here with DBTO. It is worthy to note that molecular weight values are very similar within each series (with the only exception of the homopolyester entirely made of sugar-based diols), and that essentially the same molecular weight dispersity is obtained for all the prepared polyesters regardless of which series is concerned. This satisfactory agreement adds confidence to the comparative analysis of thermal properties that is carried out below.
A detailed comparison of the Mw attained for the three types of copolyesters for similar contents in the sugar-based comonomer is represented in Fig. 1. The lower molecular weights attained for PBxIsyT compared to PBxGalxyT and PBxManxyT when polymerized under the same polymerization conditions must be attributed to the lower reactivity of the secondary hydroxyl groups of Is compared to that of the primary ones present in both Galx and Manx. The limited reactivity of Is in melt polycondensation has been previously reported.25–27,29 Isosorbide copolyesters with higher molecular weights are achievable via polycondensation in solution,29 but due to the excess use of solvents, this method is not appropriate for industrial application.31 Recently, Sablong et al. investigated the incorporation of isosorbide into PBT via solid-state polymerization and reported the synthesis of higher molecular weight polymers by this method.32
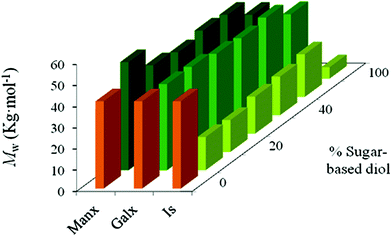 | ||
| Fig. 1 Weight-average molecular weight versus composition plot for PBT copolyesters containing isosorbide (Is), 2,3:4,5-di-O-methylene-galactitol (Galx) and 2,4:3,5-di-O-methylene-D-mannitol (Manx). | ||
The chemical constitution and composition of the copolyesters were ascertained by NMR. A selection of spectra is provided in the ESI† file for illustration. The 1H NMR spectra corroborated the chemical structure of the copolyesters with all signals being properly assigned to the different protons contained in their repeating units. Integration of the proton signals arising from 1,4-butylene and sugar-based units led to quantify the composition of the copolyesters in such units. Five different experiments, with molar feed ratios of 1,4-butanediol and sugar-based diols of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 70
20, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 60
30, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 50
40 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, were carried out within each series. The compositions of the resulting PBxIsyT, PBxGalxyT and PBxManxyT copolyesters are shown in Table 2. The correspondence between the comonomeric composition used in the feed and that present in the resulting copolyester depended on which sugar-based comonomer was involved. The fraction (%) of sugar-based diols (Is, Galx or Manx) that is incorporated in the PBT copolyester for the different feed compositions that have been studied in this work is depicted in Fig. 2. The content of the copolyesters in Is units was found to be in all cases significantly lower than in their corresponding feeds, with losses of up to 40%; such discrepancy should be attributed to the higher reactivity of the hydroxyl groups of 1,4-butanediol compared to those of isosorbide. Conversely, no noteworthy differences in the relative incorporation of bicyclic acetalized diols and 1,4-butanediol were detected, suggesting that the primary hydroxyl groups of both Galx and Manx are able to react at a rate similar to those of 1,4-butanediol. Furthermore, the reactivity of the primary hydroxyl groups of Galx and Manx seems to be not affected by the presence of the neighboring acetalic cyclic structure, neither in the 2,3:4,5 nor in the 2,4:3,5 arrangement, since both PBxGalxyT and PBxManxyT copolyesters have compositions very close to those of their corresponding feeds.
50, were carried out within each series. The compositions of the resulting PBxIsyT, PBxGalxyT and PBxManxyT copolyesters are shown in Table 2. The correspondence between the comonomeric composition used in the feed and that present in the resulting copolyester depended on which sugar-based comonomer was involved. The fraction (%) of sugar-based diols (Is, Galx or Manx) that is incorporated in the PBT copolyester for the different feed compositions that have been studied in this work is depicted in Fig. 2. The content of the copolyesters in Is units was found to be in all cases significantly lower than in their corresponding feeds, with losses of up to 40%; such discrepancy should be attributed to the higher reactivity of the hydroxyl groups of 1,4-butanediol compared to those of isosorbide. Conversely, no noteworthy differences in the relative incorporation of bicyclic acetalized diols and 1,4-butanediol were detected, suggesting that the primary hydroxyl groups of both Galx and Manx are able to react at a rate similar to those of 1,4-butanediol. Furthermore, the reactivity of the primary hydroxyl groups of Galx and Manx seems to be not affected by the presence of the neighboring acetalic cyclic structure, neither in the 2,3:4,5 nor in the 2,4:3,5 arrangement, since both PBxGalxyT and PBxManxyT copolyesters have compositions very close to those of their corresponding feeds.
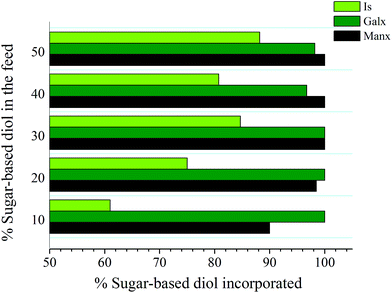 | ||
| Fig. 2 Fraction (%) of sugar-based diols (Is, Galx or Manx) that is incorporated into the PBT copolyester for the different feed compositions studied in this work. | ||
The 13C NMR spectra of PBxIsyT, PBxGalxyT and PBxManxyT copolyesters show the non-protonated aromatic carbon signal at 133–135 ppm with resolution enough (Fig. 3) to estimate their degree of randomness,33 leading to the conclusion that the microstructure was at random in the three series. Regarding PBxIsyT copolyesters, each type of dyad consists of multiple peaks as a consequence of the two orientations possible for the non-symmetrical Is unit. Conversely, a single signal is detected for every dyad in PBxGalxyT and PBxManxyT copolyesters. Due to the symmetry of Galx and Manx units, only one orientation is feasible for these units when incorporated in the polymer chain. Consequently, copolyesters made from Galx and Manx should be expected to be stereoregular as it has been observed.
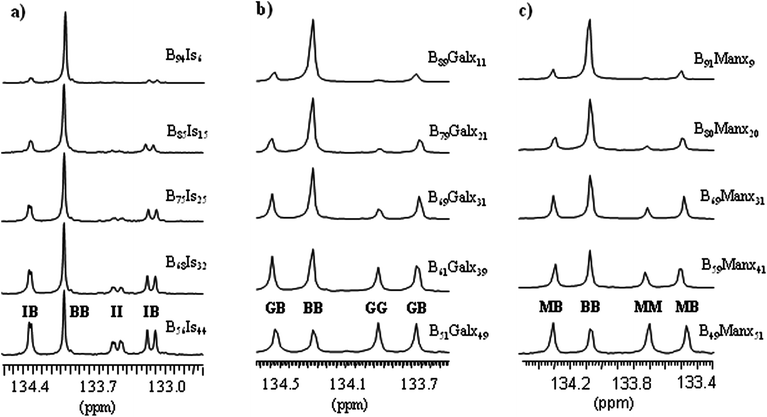 | ||
| Fig. 3 13C NMR signals of the non-protonated aromatic carbons of PBxIsyT (a), PBxGalxyT (b), and PBxManxyT (c) copolyesters. The assignations correspond to terephthalic units attached to: B, butanediol; I, Isosorbide; G, Galx; M, Manx. | ||
3.2. Thermal properties
The thermal behavior of PBxIsyT, PBxGalxyT and PBxManxyT copolyesters was systematically studied by TGA and DSC under an inert atmosphere. The thermal parameters resulting from these analyses are given in Table 2, where the corresponding data for the parent homopolyesters PBT, PIsT, PGalxT and PManxT are also included for comparison purposes. The thermal decomposition of all these polyesters occurs in a single step leaving less than 10% of the residue upon heating at 600 °C. PBT starts to decompose well above 300 °C with 5% of the initial weight being lost at 371 °C (°T5%) and with a maximum decomposition rate at 408 °C (maxTd). The onset decomposition temperatures of PBxIsyT, PBxGalxyT and PBxManxyT copolyesters and their corresponding homopolyesters indicative of the thermal stability are comparatively depicted in Fig. 4. In the PBxIsyT series, °T5% and maxTd increase with the content in Is to reach values of 374 and 424 °C respectively in the PIsT homopolyester. Strangely copolyesters containing minor amounts of Is show values slightly below those of PBT; the low molecular weight of these copolyesters may be the reason for such behavior. Regarding PBxGalxyT copolyesters, both °T5% and maxTd steadily increase with the content in sugar units with values between those displayed by the two parent homopolyesters PBT and PGalxT. The same trend is observed for the PBxManxyT. These results are in full accordance with the high thermal stability observed for PGalxT and PManxT homopolyesters compared to PBT. The valuable conclusion drawn from this comparative thermogravimetric study is that the insertion of bicyclic sugar units into aromatic polyesters instead of reducing their decomposition temperatures contributes significantly to increasing their thermal stability. Since the melting temperature in these copolyesters decreases with composition, the gap between melting and decomposition temperatures becomes wider allowing a more comfortable melt processing. This is an issue of prime importance for the appraisal of these bio-based polymers as potential thermoplastic materials.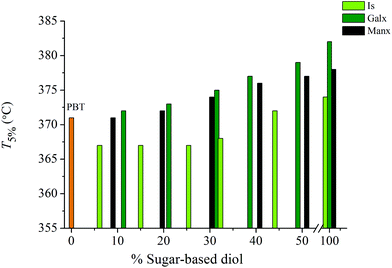 | ||
| Fig. 4 Temperature for 5% weight loss versus composition plot for PBT copolyesters containing Is, Galx and Manx. | ||
The glass transition temperatures (Tg) of PBxIsyT, PBxGalxyT and PBxManxyT copolyesters were measured as the inflection point of the heating DSC traces of samples quenched from the melt, and values are compared in Table 2 and depicted in Fig. 5 together with those of their corresponding homopolyesters. The Tg of PBxIsyT copolyesters increased as butanediol was replaced by Is units, even at low Is contents despite the lower molecular weights of PBxIsyT copolyesters compared to that of PBT. Such behavior evidences the great positive effect that the fused bicyclic tetrahydrofuran structure exerts on restricting the mobility of the polyester chain. In the PBxGalxyT series the Tg also increased with the amount of replaced 1,4-butanediol with minimum and maximum values corresponding to their respective PBT and PGalxT homopolyesters. However the effect of Galx was less pronounced than that of Is for any composition with the difference becoming larger for higher degrees of replacement. Regarding PBxManxyT copolyesters, the Tg again increased as 1,4-butanediol was replaced by Manx units going from 31 °C for PBT up to 137 °C for PManxT. The effect of Manx on Tg is also slightly weaker than that of Is but in this case differences almost fade away for low contents in bicyclic units; in fact, a similar increase in Tg was attained by incorporating either Is or Manx units as far as the content of 1,4-butanediol remained above 60%. The conclusion drawn from this comparative study is that although the all three bicyclic carbohydrate-based diols, Is, Galx and Manx, are capable of raising the Tg of PBT, the effect depends on the stiffness of the bicyclic structure inserted into the copolyester chain. As expected, the fused rings of both Is and Manx exert an effect on the Tg more pronounced than the non-fused two 1,3-dioxolane rings present in Galx. Is appears to be the most effective Tg increasing comonomer because its bicyclic structure made of almost rigid tetrahydrofuran rings is more conformationally impeded than that of Manx made of 1,3-dioxanes. Nevertheless, an almost lineal increasing variation of Tg with the content in bicyclic units takes place in the three cases allowing a fine tuning of the property by proper adjusting of the copolyester composition.
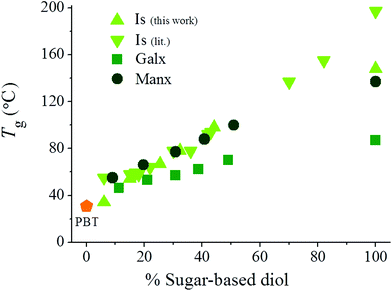 | ||
| Fig. 5 Glass-transition temperature versus composition plot for PBT copolyesters containing Is (data from this work and from the literature29,32), Galx and Manx. | ||
With regard to the melting and crystallization behavior, the DSC analysis of the polyesters showed noteworthy qualitative and quantitative differences among the three series (Table 2). PBT is a semicrystalline polyester able to crystallize very fast and to a large degree under a wide variety of conditions, and it may serve as a reference. PBxIsyT and PBxManxyT copolyesters were found to be semicrystalline for low to medium contents in the bicyclic diol whereas PBxGalxyT were able to crystallize for the whole range of compositions. In fact, PBxIsyT copolyesters were semicrystalline up to 32% of Is, whereas PBxManxyT showed endothermic peaks characteristic of melting for Manx contents below or equal to 41%. Nevertheless, the insertion of Is, Galx and Manx units invariably gave place to a noticeable decrease in both Tm and ΔHm (Fig. 6), bringing into evidence the repressing effect of the bicyclic diols on PBT crystallinity. The three homopolyesters PIsT, PGalxT and PManxT were amorphous, at least under the conditions here studied. The depressing effect of copolymerization on crystallinity appears to be stronger when bicyclic fused rings are incorporated into the polymer chain. The conclusion is that although the incorporation of the three bicyclic diols induced changes in the melting-crystallization behavior of PBT, the symmetric fused Manx unit seems to be capable of preserving the crystallinity more than the asymmetric fused Is, but less than the symmetric non-fused Galx.
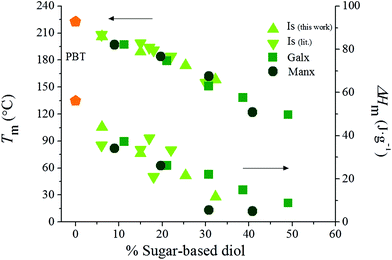 | ||
| Fig. 6 Melting temperature and enthalpy versus composition plot for PBT copolyesters containing Is (data from this work and from the literature29,32), Galx and Manx. | ||
4. Conclusions
Three different carbohydrate-based bicyclic diols, Is, which is a dianhydride derived from D-glucose, and Galx and Manx, which are internal diacetals derived from D-galactose and D-fructose, respectively, have been comparatively studied as comonomers of 1,4-butanediol in the preparation of PBT copolyesters by polycondensation in the melt. Exactly the same reaction conditions were used to prepare the three series of copolyesters and they were selected to afford high yields and molecular weights with a minimum degradation of the carbohydrate-based compounds. Copolyesters with pretty high molecular weights and compositions very close to their corresponding feeds have been obtained in the copolymerization with Galx and Manx whereas copolyesters made from Is displayed much lower molecular weights and losses in the sugar-derived comonomer of up to 40%. The three bicyclic monomers were able to enhance the thermal stability of PBT to a similar degree. Isosorbide is the bicyclic monomer that increases the glass-transition temperature of PBT most efficiently, although Manx is capable of affording almost the same effect as far as sugar-based contents are less than 40%. The three bicyclic sugar derived monomers decrease the crystallinity of PBT to a degree that depends on both symmetry and stiffness of the bicyclic structure; copolyesters of Is and Manx are semicrystalline for contents of up to 32% and 41% in these units, respectively, whereas those containing Galx preserve the crystallinity for all the studied compositions. The three homopolyesters entirely made of sugar-derived diols are amorphous.Acknowledgements
Financial support for this work was provided by MICINN (Spain) with Grant MAT2009-14053-CO2-01 and by AGAUR (Catalonia) with grant 2009SGR1469. The authors are also indebted to MEC (Spain) for the FPU grant awarded to Cristina Lavilla.References
- F. Welle, Resour. Conserv. Recycl., 2011, 55, 865–875 CrossRef.
- G. Grause, A. Buekens, Y. Sakata, A. Okuwaki and T. Yoshioka, J. Mater. Cycles Waste, 2011, 13, 265–282 CrossRef.
- R. Wool and S. Sun, Biobased Polymers and Composites, Academic Press, New York, 2005 Search PubMed.
- M. N. Belgacem and A. Gandini, Monomers, Polymers and Composites from Renewable Resources, Elselvier, Oxford, 2008 Search PubMed.
- C. K. Williams and M. A. Hillmyer, Polym. Rev., 2008, 48, 1–10 CrossRef CAS.
- R. Narain, Engineered Carbohydrate-Based Materials for Biomedical Applications: Polymers, Surfaces, Dendrimers, Nanoparticles and Hydrogels, Wiley, Hoboken, 2011 Search PubMed.
- A. Gandini, Green Chem., 2011, 13, 1061–1083 RSC.
- J. A. Galbis and M. G. García-Martín, Sugars as Monomers, Monomers, Polymers and Composites from Renewable Resources, ed. M. N. Belgacem and A. Gandini, Elselvier, Oxford, 2008, ch. 5, pp. 89–114 Search PubMed.
- P. Rychter, M. Kawalec, M. Sobota, P. Kurcok and M. Kowalczuk, Biomacromolecules, 2010, 11, 839–847 CrossRef CAS.
- R. J. Müller, I. Kleeberg and W. D. Deckwer, J. Biotechnol., 2001, 86, 87–95 CrossRef.
- G. Q. Chen and M. K. Patel, Chem. Rev., 2012, 112, 2082–2099 CrossRef CAS.
- C. Delhomme, D. Weuster-Botz and F. E. Kuhn, Green Chem., 2009, 11, 13–26 RSC.
- C. Berti, E. Binassi, M. Colonna, M. Fiorini, G. Kannan, S. Karanam, M. Mazzacurati, I. Odeh and M. Vannini (SABIC-IP), U.S. Pat. Appl. Publ.2010168371, 2010 Search PubMed.
- N. González-Vidal, A. Martínez de Ilarduya and S. Muñoz-Guerra, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5954–5966 CrossRef.
- S. R. Turner, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5847–5852 CrossRef CAS.
- C. J. Booth, M. Kindinger, H. R. McKenzie, J. Handcock, A. V. Bray and G. W. Beall, Polymer, 2006, 47, 6398–6405 CrossRef CAS.
- A. Gandini, D. Coelho, M. Gomes, B. Reis and A. J. D. Silvestre, J. Mater. Chem., 2009, 19, 8656–8664 RSC.
- M. Gomes, A. Gandini, A. J. D. Silvestre and B. Reis, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3759–3768 CrossRef CAS.
- R. Storbeck and M. Ballauff, J. Appl. Polym. Sci., 1996, 59, 1199–1202 CrossRef CAS.
- H. R. Kricheldorf, J. Macromol. Sci. Rev. Macromol. Chem. Phys., 1997, C37, 599–631 CrossRef CAS.
- C. Lavilla, A. Alla, A. Martínez de Ilarduya, E. Benito, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Polymer, 2012, 53, 3432–3445 CrossRef CAS.
- C. Lavilla, A. Martínez de Ilarduya, A. Alla, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Macromolecules, 2012, 45, 8257–8266 CrossRef CAS.
- C. Japu, A. Alla, A. Martínez de Ilarduya, M. G. García-Martín, E. Benito, J. A. Galbis and S. Muñoz-Guerra, Polym. Chem., 2012, 3, 2092–2101 RSC.
- C. Lavilla, A. Martínez de Ilarduya, A. Alla and S. Muñoz-Guerra, Polym. Chem., 2012 10.1039/c2py20531a.
- F. Fenouillot, A. Rousseau, G. Colomines, R. Saint-Loup and J. P. Pascault, Prog. Polym. Sci., 2010, 35, 578–622 CrossRef CAS.
- B. A. J. Noordover, R. Duchateau, R. A. T. M. van Benthem, W. Ming and C. E. Koning, Biomacromolecules, 2007, 8, 3860–3870 CrossRef CAS.
- B. A. J. Noordover, V. G. van Staalduinen, R. Duchateau, C. E. Koning, R. A. T. M. van Benthem, M. Mak, A. Heise, A. E. Frissen and J. van Haveren, Biomacromolecules, 2006, 7, 3406–3416 CrossRef CAS.
- C. Lavilla, A. Alla, A. Martínez de Ilarduya, E. Benito, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Biomacromolecules, 2011, 12, 2642–2652 CrossRef CAS.
- H. R. Kricheldorf, G. Behnken and M. Sell, J. Macromol. Sci., Part A: Pure Appl. Chem., 2007, 44, 679–684 CrossRef CAS.
- S. Inkinen, M. Stolt and A. Södergard, Biomacromolecules, 2010, 11, 1196–1201 CrossRef CAS.
- A. Fradet and M. Tessier, Polyesters, in Synthetic Methods in Step-Growth Polymers, ed. M. E. Rogers and T. E. Long, Wiley, New Jersey, 2003, pp. 17–134 Search PubMed.
- R. Sablong, R. Duchateau, C. E. Koning, G. de Wit, D. van Es, R. Koelewijn and J. van Haveren, Biomacromolecules, 2008, 9, 3090–3097 CrossRef CAS.
- J. C. Randal, Polymer Sequence Determination, Academic Press, New York, 1977, pp. 41–69 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2gc36480h |
| This journal is © The Royal Society of Chemistry 2013 |
