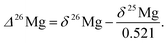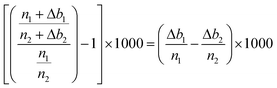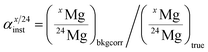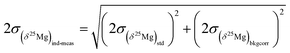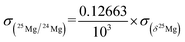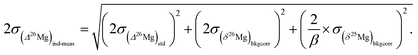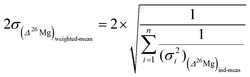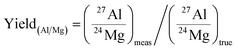High precision Mg isotope measurements of meteoritic samples by secondary ion mass spectrometry
Tu-Han
Luu
*ab,
Marc
Chaussidon
a,
Ritesh Kumar
Mishra
a,
Claire
Rollion-Bard
a,
Johan
Villeneuve
c,
Gopalan
Srinivasan
d and
Jean-Louis
Birck
b
aCentre de Recherches Pétrographiques et Géochimiques (CRPG) – INSU CNRS – Université de Lorraine – UPR 2300, 15 Rue Notre-Dame des Pauvres, BP20, 54501 Vandoeuvre-lès-Nancy Cedex, France. E-mail: luu@crpg.cnrs-nancy.fr
bLaboratoire de Géochimie et Cosmochimie, Institut de Physique du Globe de Paris (IPGP), Sorbonne Paris Cité, 1 rue Jussieu, 75238 Paris Cedex 05, France
cInstitut des Sciences de la Terre d'Orléans – UMR 6113 – CNRS/Université d'Orléans, 1A rue de la Férollerie, 45071 Orléans Cedex 2, France
dCenter for Earth Sciences, Indian Institute of Science, Bangalore 560012, India
First published on 19th October 2012
Abstract
The possibility of establishing an accurate relative chronology of the early solar system events based on the decay of short-lived 26Al to 26Mg (half-life of 0.72 Myr) depends on the level of homogeneity (or heterogeneity) of 26Al and Mg isotopes. However, this level is difficult to constrain precisely because of the very high precision needed for the determination of isotopic ratios, typically of ±5 ppm. In this study, we report for the first time a detailed analytical protocol developed for high precision in situ Mg isotopic measurements (25Mg/24Mg and 26Mg/24Mg ratios, as well as 26Mg excess) by MC-SIMS. As the data reduction process is critical for both accuracy and precision of the final isotopic results, factors such as the Faraday cup (FC) background drift and matrix effects on instrumental fractionation have been investigated. Indeed these instrumental effects impacting the measured Mg-isotope ratios can be as large or larger than the variations we are looking for to constrain the initial distribution of 26Al and Mg isotopes in the early solar system. Our results show that they definitely are limiting factors regarding the precision of Mg isotopic compositions, and that an under- or over-correction of both FC background instabilities and instrumental isotopic fractionation leads to important bias on δ25Mg, δ26Mg and Δ26Mg values (for example, olivines not corrected for FC background drifts display Δ26Mg values that can differ by as much as 10 ppm from the truly corrected value). The new data reduction process described here can then be applied to meteoritic samples (components of chondritic meteorites for instance) to accurately establish their relative chronology of formation.
1 Introduction
Variations of the Mg isotopic composition of meteoritic materials can be understood at first order to be the sum of (i) mass-dependent isotopic fractionations due to processes such as evaporation or condensation and (ii) the decay of short-lived 26Al to 26Mg (half-life of 0.72 Myr). Calcium-, aluminum-rich inclusions (CAIs), that are the oldest dated solids formed in the accretion disk around the early sun,1–3 display large 26Mg excesses.4 They can be used to define the initial 26Al/27Al ratio (5.23 (±0.13) × 10−5 (ref. 5 and 6)) that anchors the 26Al-based chronology. However, the 26Al–26Mg system can be used as a chronometer only under the assumption that 26Al and Mg isotopes were homogenized early in the accretion disk.The level of homogeneity (or heterogeneity) is difficult to constrain precisely. One way is to be able to compare 26Mg excesses measured with high precision in samples formed at various ages in the accretion disk with 26Mg excesses predicted assuming homogeneity. For a solar 27Al/24Mg ratio of 0.101 (ref. 7) (this ratio being estimated from CI chondrites, and not the solar photosphere), 26Mg produced from the total decay of an initial 26Al/27Al ratio of 5.23 × 10−5 increases the 26Mg/24Mg ratio by ∼38 ppm. The magnitude of 26Mg excesses measured in situ by MC-SIMS (multi-collection secondary ion mass spectrometry) in ferromagnesian chondrules (chondrules are mm sized objects which were melted and quenched in the accretion disk and they constitute the major high-temperature component of primitive meteorites) from ordinary chondrites does support a ±10% homogeneous distribution of 26Al and Mg isotopes at the time of CAI formation in the disk.8 This view has been challenged9 from very high precision bulk analyses (±2.5 ppm for 26Mg excesses10) of refractory components of carbonaceous chondrites (CAIs and amoeboid olivine aggregates) by HR-MC-ICPMS (high-resolution multi-collector inductively coupled plasma source mass spectrometry).
One key to the debate is the development of high precision for Mg isotopic measurements, both bulk and in situ. In fact, the studied objects (i.e. CAIs) underwent, after their formation from precursors condensed from the gas, several high temperature events including melting and re-crystallization. If melting/crystallization occurred in a closed system, it did not modify the bulk compositions (Mg and Al isotopes and Al/Mg ratio) so that a bulk 26Al isochron gives theoretically access to the Al and Mg isotopic compositions of the precursors and thus dates condensation. At variance in situ analysis by MC-SIMS allows us to look for the existence of a 26Al mineral isochron within one object, which would date the partitioning of Al and Mg between the different constituent minerals during the last melting/crystallization event. The combination of bulk and in situ data should allow us to reconstruct the history of the high temperature components of meteorites, from early condensation events to late melting and re-melting processes.
A high precision Mg isotopic measurement method has already been developed for HR-MC-ICPMS.10 However, bulk analyses by HR-MC-ICPMS require a large sample size and do not allow us to determine mineral isochrons because of the high spatial resolution required in the case of the early solar system objects.
Here we describe the analytical protocol developed for high precision Mg isotopic measurements (25Mg/24Mg and 26Mg/24Mg ratios, as well as 26Mg excess) of meteoritic samples by MC-SIMS on ∼30–40 μm analytical spots. This protocol is a further refinement of that developed by Villeneuve et al.8,11 Other groups are developing these measurements12–15 but their procedure is not yet described in full detail. The factors limiting the precision are assessed. An example of application to the study of several components of chondritic meteorites is given.
2 Data acquisition
Mg-isotope ratios and Al/Mg ratios are measured using the CRPG-CNRS (Nancy) CAMECA large radius ims 1270 and ims 1280HR2 ion microprobes (some instrument configuration and capabilities of MC-SIMS can be found in Benninghoven et al.16 and de Chambost17). Gold coated or carbon coated polished thick sections of samples are sputtered by a 13 kV O− static primary beam and positive secondary ions of Al and Mg isotopes are extracted and accelerated at 10 kV. The intensity of the primary beam is set to produce the highest possible count rate for secondary ions (i.e. >1 × 109 counts per second (cps) on 24Mg+, in olivines), while keeping the beam diameter small enough to allow the analysis of individual mineral phases in chondrules or CAIs: for instance a ∼30 nA primary beam intensity corresponds to a ∼30–40 μm spot size. The secondary ions are analyzed at a mass resolution M/ΔM = 2500 (using exit slit #1 of the multicollector) in multicollection mode using four Faraday cups (FCs): L′2, C, H1 and H′2, for 24Mg, 25Mg, 26Mg and 27Al, respectively. Such a low mass resolution is chosen to maximize the flatness of the three Mg peaks though the interference of the hydride 24MgH+ on 25Mg (with vacuum in the sample chamber below 3 × 10−9 Torr, the contribution of 24MgH+ to 25Mg is less than 10−6 relative) is not totally resolved (a M/ΔM of 3559 would be required). However measurements made at higher mass resolution (M/ΔM = 6000 using exit slit #2) have shown that the contribution of the hydride to 25Mg remains less than a few cps, i.e. <∼10−8 relative for 25Mg (whose intensity is greater than a few 108 cps in olivines) if the vacuum in the chamber is <3 × 10−9 Torr.Each analysis of a new sample mount starts by a manual setting of the Z position of the mount to keep constant the distance between the sample surface and the front plate of the immersion lens. When possible, different grains of different international and in-house standards are included with the sample(s) to analyze in the same mount. In addition, different mounts containing standards are also analyzed in between mounts containing samples and standards. Generally, analyses are automatically chained. A chain of analyses can include both depth profiles (in that case no more than 7 measurements are done at the same spot) and analyses at different spots on a same grain or on different grains (standard or sample) close to each other in the mount (in that case the sample stage is moved, only over a short distance, but allowing much more analyses to be done, the number depending on the spot size). Whatever the case the primary beam never moves. One typical analysis lasts for 425 s, including a total of 150 s presputtering and 275 s simultaneous counting of the intensities of 24Mg+, 25Mg+, 26Mg+ and 27Al+ (25 cycles of 10 s counting time separated by 1 s waiting time). During presputtering, first the background of each FC is measured (the secondary beam is deflected from the entrance of the magnet by the deflector Y of the coupling lens LC1C) and, second, an automatic centering of the secondary beam is performed (using secondary intensity measured for 24Mg) either in the field aperture (using transfer lenses deflectors LTdefxy) with the ims 1270 or in both the field aperture and the contrast aperture (using transfer lenses deflectors DTFAxy and DTCAxy) with the ims 1280HR2. This automatic centering allows the correction of the secondary beam trajectory for possible small deviations due to imperfect alignment or flatness of the sample. In addition to these centerings, the charging of the sample is automatically monitored (and the secondary high voltage readjusted of a few volts if necessary) by scanning the energy distribution of secondary ions. The nuclear magnetic resonance (NMR) field sensor is used on the ims 1280HR2 to control and stabilize the magnetic field since no peak jumping is required because of the use of multi-collection.
3 Data reduction
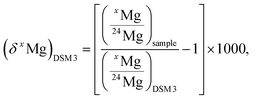
At the level of precision required for Mg isotope analysis of extraterrestrial materials (e.g. 10 ppm or better on 26Mg excesses noted Δ26Mg, see Section 3.5 for definition) the procedure of data reduction is critical to avoid introduction of any analytical bias in the final isotopic results. This is particularly critical for MC-SIMS analysis for two major reasons. First, in MC-SIMS all measurements are direct measurements of isotopic compositions while in HR-MC-ICPMS, thanks to the standard–sample bracketing technique which cannot be used for SIMS, only differences of isotopic compositions are measured, thus eliminating most of the instrumental isotopic fractionations. Second, significant matrix effects are present and have a major influence on instrumental fractionation. However, MC-SIMS has the advantage of a much lower instrumental fractionation, one order of magnitude less, than HR-MC-ICPMS. The approach developed to calibrate precisely and to correct for instrumental fractionation is described in the following, as well as the propagation of errors due to these different corrections.In the following, Mg isotopic compositions will be expressed either as isotopic ratios or as delta values. The δ25,26Mg notation is the relative deviation, in per mil (‰), of the 25,26Mg/24Mg ratio from a reference isotopic composition (noted (δxMg)DSM 3 when the DSM 3 international standard is used).
The reference isotopic composition used to calculate the raw Mg-isotope ratios is that of SRM 980, with 25Mg/24Mg = 0.12663 and 26Mg/24Mg = 0.13932,18 because the DSM 3 Mg-isotope ratios are determined relative to the SRM 980 international standard. A re-evaluation of these ratios has recently been published (25Mg/24Mg = 0.126896 and 26Mg/24Mg = 0.139652 (ref. 10)). However, as explained in the following, because most of the data reduction is made using isotopic ratios and not delta values, and because instrumental fractionation is calibrated from the analyses of different standards, the final corrected delta values are independent of the values taken as the reference isotopic ratios.
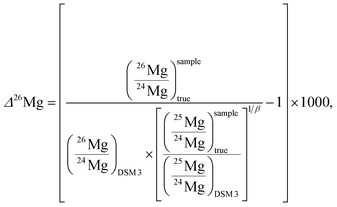
The capital delta notation (Δ26Mg, in ‰) will also be used hereafter to express 26Mg excesses or deficits relative to a given mass fractionation law. In the case of a mass fractionation law for Mg isotopes characterized by a coefficient of 0.521 (see Section 3.5 for more details), the Δ26Mg value is calculated according to:
Note that Ogliore et al.19 have recently shown that in SIMS the mean of isotopic ratios determined from individual measurement cycles at low count rates is biased, yielding a long-run averaged ratio that is systematically higher than the true ratio. However, this effect is completely negligible at the high count rates used to measure the Mg-isotope ratios discussed in this paper.
3.1 Raw data and outlier rejection
The measured isotopic ratios averaged over 25 cycles and corrected only for the yields (determined from the Cameca calibration routine20 at the beginning of each analytical session) and the backgrounds (determined during pre-sputtering) of the four Faraday cups are named raw data (e.g. (25Mg/24Mg)raw). Several instrumental parameters are automatically registered with the raw data. Thus for each measurement raw data are accompanied by (i) the x and y sample positions (in μm) and a picture of the sample in reflected light through the ion probe microscope at the beginning of sputtering, (ii) the sample chamber pressure (in Torr), (iii) the primary beam intensity (in A), (iv) the transfer deflectors values (LTdefxy for the ims 1270; DTFAxy and DTCAxy for the ims 1280HR2) which are automatically centered, (v) the drift of the secondary high voltage (in V) which is determined automatically, (vi) the background of the four FCs (in cps), (vii) the secondary intensities of 24Mg+, 25Mg+, 26Mg+ and 27Al+ (in cps), (viii) the 25Mg/24Mg, 26Mg/24Mg and 26Mg/25Mg isotopic ratios, expressed in the δxMg notation (with x = 25 or 26 for ratios to 24Mg) with their associated 1σ error (1 s.e., n = 25), a 2 standard deviation (2 s.d.) threshold being used to reject outliers within the 25 cycles (rejections of δ25Mg, δ26Mg and 27Al/24Mg are independent so that rejected δ25Mg and δ26Mg, if any, could correspond to different cycles), and (ix) the 27Al/24Mg ratio and its associated 1σ error.Results for which anomalies were observed during the analytical procedure were systematically discarded. They are identified from any of the following criteria: (i) secondary intensity normalized to the primary beam intensity lower (by 20% or more) than the typical value observed on standards of similar matrix, (ii) spikes in the background measured for the FCs during pre-sputtering, (iii) anomalous charge (more than 15 V) of the sample (if any, it likely results from an incomplete charge compensation due to ageing of the mount metallization or its local removal when spots are close to each other), (iii) anomalously large re-centering of the transfer deflectors (in excess of ±7 V), (iv) low statistic on either the δ25Mg or δ26Mg values (worse than 0.05‰, 1 s.e.). In addition, the samples are systematically observed after analyses with optical microscopy (or secondary electron microscopy) to discard analyses which would correspond to spots not entirely within a grain or spots touching a crack or an inclusion.
3.2 FC background drift
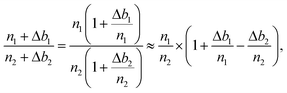
Significant drifts of FC backgrounds take place, primarily due to cyclic temperature variations (worst conditions for the air conditioning system result in an amplitude lower than ±0.4 °C over one day) in the ion probe room. As FC backgrounds are measured during the pre-sputtering at the beginning of each measurement, the raw 25Mg/24Mg and 26Mg/24Mg ratios are systematically corrected for the background drift using a linear interpolation between two successive analyses. Note that the background variations of L′2, H1 and H′2 are correlated within each other, whereas they can be anti-correlated with the background variations of C.If n1 and n2 are two count rates (in cps) for two Mg isotopes and Δb1 and Δb2 the drifts (in cps) estimated for their background variations from linear interpolation (Δ = measured background − extrapolated background), then the corrected n1/n2 isotope ratio writes:
It then comes that the per mil variations of the n1/n2 ratio can be expressed as:
So that finally, the delta values can be corrected for the background instabilities according to: 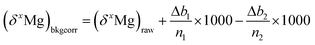 , with x = 25 or 26.
, with x = 25 or 26.
Two effects of this correction for drifts of background are significant (Fig. 1). Firstly, because the magnitude of the correction increases when count rates on the different Mg isotopes decrease, this correction will have a larger impact on Mg-poor minerals or glasses or in the case of lower Mg secondary yield. This is the case for pyroxene and spinel relative to olivine. Secondly, because the count rates are about eight times lower on 25Mg and 26Mg relative to 24Mg, the effect of the correction is non-mass dependent and impacts upon the magnitude of the 26Mg excesses that can be calculated from the 25Mg/24Mg and 26Mg/24Mg ratios (with a maximum of 10 ppm change for olivines, Fig. 1).
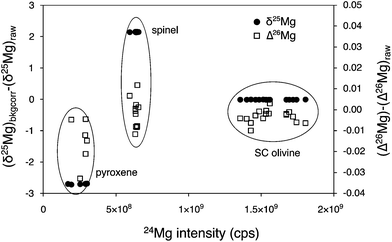 | ||
| Fig. 1 The correction for drifts of Faraday cup backgrounds using a linear interpolation between two successive analyses impacts upon both δ25Mg (black dots) and δ26Mg values (not shown), with a more important effect on Mg-poor minerals (spinel, pyroxene) compared to Mg-rich minerals (olivines for instance). The background correction also affects Δ26Mg values (open squares): this non-mass dependent correction is due to lower count rates on 25Mg and 26Mg compared to 24Mg, with a maximum of 10 ppm change for olivines for instance. | ||
3.3 Instrumental fractionation
Instrumental isotopic fractionation is produced in SIMS analysis during the extraction and acceleration of secondary ions from the sample, their analysis (transfer optic, electrostatic and magnetic sectors) in the mass spectrometer and their counting in the collectors of the multicollector. Instrumental fractionation resulting from differential breaking of chemical bonds in the sample depending on their vibration energies is a mass-dependent fractionation. Some phenomena which are minor contributors to the isotopic fractionation taking place in the spectrometer can be mass-independent, for example when improper tuning of the transfer optic results in the 24Mg+, 25Mg+ and 26Mg+ ion beams not to be perfectly centered in the cross-over plane where they can be cut differently by the entrance slit of the spectrometer. In addition, improper tuning of the coupling optic (which refocuses the secondary beam between the electrostatic and magnetic sectors) may result in different off-axis aberrations of the secondary beam in the focal plane of the magnet (where the collectors are) and thus in slight differences of peak shapes for the three Mg isotopes and consequently some mass-independent fractionation. Because instrumental isotopic fractionation is primarily mass-dependent, it is generally named instrumental mass fractionation, but one important criterion of proper tuning of the spectrometer is to minimize the mass-independent component of instrumental fractionation, which is indicated by the intercept in its calibration (see below).Instrumental fractionation (αinst) is defined for Mg isotopes as:
| Standards | SiO2e (wt%) | Al2O3e (wt%) | MgOe (wt%) | 27Al/24Mge (atomic) | 27Al/24Mgf (ion microprobe) | Al/Mg yield |
|---|---|---|---|---|---|---|
| a Chemical composition data coming from this study. b Chemical composition data coming from Decitre (2000). c Chemical composition data coming from Ionov et al. (1993). d Chemical composition data coming from Gao et al. (2002). e Indicates that the 1σ error is typically ±2% for SiO2, Al2O3 and MgO analyses and for the resulting (27Al/24Mg)atomic ratio. f Indicates that the 1σ error is typically better than ±1.5% for (27Al/24Mg)ion microprobe measurements. g The 1σ error on the Al/Mg yield of Eagle Station olivine is ±0.30. | ||||||
| San Carlos olivinea | 40.33 | 0.03 | 48.87 | 6.14 × 10−4 | 4.67 × 10−4 | 0.76 |
| Eagle Station (MNHN) olivinea | 39.29 | 2.72 × 10−3 | 42.69 | 6.37 × 10−5 | 6.36 × 10−5 | 1.00g |
| Clinopyroxene BZCG (Zabargad)b | 50.35 | 7.05 | 14.11 | 0.50 | 0.38 | 0.76 |
| Clinopyroxene BZ 226 (Zabargad)b | 51.36 | 4.49 | 15.35 | 0.29 | 0.23 | 0.80 |
| Clinopyroxene 313-3 (Vitim)c | 52.84 | 5.81 | 15.52 | 0.37 | 0.28 | 0.75 |
| Orthopyroxene BZ 226 (Zabargad)b | 54.14 | 3.85 | 31.20 | 0.12 | 0.10 | 0.78 |
| Orthopyroxene 313-3 (Vitim)c | 55.16 | 3.73 | 32.94 | 0.11 | 0.09 | 0.77 |
| Spinel (Burma)a | 0.02 | 71.66 | 27.88 | 2.57 | 1.86 | 0.72 |
| Spinel 86-1 (Vitim)c | 0.06 | 57.33 | 20.9 | 2.75 | 2.07 | 0.75 |
| Basaltic glass MORB CLDR015Va | 50.43 | 15.83 | 8.43 | 1.88 | 1.41 | 0.75 |
| Basaltic glass BHVO (Hawaii)a | 49.90 | 13.50 | 7.23 | 1.87 | 1.41 | 0.75 |
| Basaltic glass BCR2 (Columbia river)a | 54.10 | 13.50 | 3.59 | 3.76 | 2.97 | 0.79 |
| Synthetic anorthitic glassa | 44.05 | 34.66 | 1.79 | 19.41 | 14.83 | 0.76 |
| Synthetic melilitic glassa | 41.00 | 11.00 | 7.00 | 1.57 | 1.10 | 0.70 |
| Synthetic pyroxenic glassa | 44.38 | 14.78 | 11.21 | 1.32 | 1.04 | 0.79 |
| Synthetic glass Bacatia | 31.01 | 30.74 | 10.29 | 2.99 | 2.41 | 0.81 |
| Synthetic glass Al20a | 48.68 | 20.09 | 9.52 | 2.11 | 1.66 | 0.79 |
| Synthetic glass Al10a | 55.05 | 10.41 | 11.37 | 0.92 | 0.74 | 0.81 |
| Synthetic glass Al5a | 58.31 | 5.11 | 11.17 | 0.46 | 0.38 | 0.83 |
| Synthetic glass NIST SRM 614d | 71.83 | 2.29 | 0.01 | 435.85 | 336.90 | 0.77 |
| Synthetic glass NIST SRM 610d | 69.06 | 2.20 | 0.08 | 28.84 | 21.82 | 0.76 |
| Hibonite (Madagascar)a | 0.88 | 73.70 | 2.91 | 25.35 | 15.95 | 0.63 |
Matrix effects on instrumental fractionation have been shown to follow a systematic often similar to that observed for natural isotopic fractionations between mineral or between minerals and fluids, as shown for example for D/H in amphiboles and micas.26 By analogy with the exponential law (α25/24inst = (α26/24inst)β) generally used27 to express Mg isotopic fractionations for terrestrial or meteoritic samples, the instrumental fractionation law is determined from a linear regression (with the Isoplot 3.00 software28) between ln(α25/24inst) and ln(α26/24inst) measured for the different international and in-house standards. The instrumental law is always of the following form:29
| ln(α26/24inst) = [ln(α25/24inst) − b]/βinst |
| α25/24inst = exp(b) × (α26/24inst)βinst |
For b very close to 0, one can approximate the above equation by:
| α25/24inst ≈ (α26/24inst)βinst. |
Large variations of α25/24inst due to matrix effects, i.e. caused by variations of vibrational energies of the bonds involving Mg isotopes in minerals or glasses having different chemical compositions, are present among silicates and oxides (Fig. 2). In the case of olivines for instance, a similar effect as previously reported for O isotopes30 exists for Mg isotopes. Values of α25/24inst increase by ∼1‰/amu from Fo#79 (olivine from the Eagle Station pallasite) to Fo#88 (San Carlos olivine), and this trend can be linearly extrapolated to determine α25/24inst for olivines with Fo# > 88. Similarly for melilite, a change of 1.9‰/amu is observed between two melilite glasses having different Al/Mg ratios. Matrix effects between silicates and oxides are of similar magnitude, e.g. ∼2‰/amu between pyroxene and spinel.
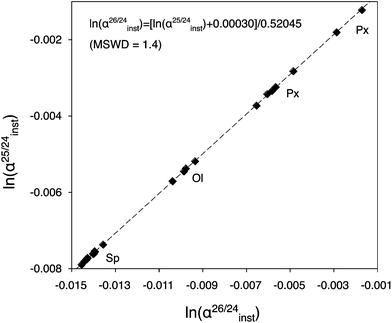 | ||
| Fig. 2 Example of an Mg isotopic instrumental fractionation law, calibrated using reference materials with different compositions (San Carlos olivine, Burma spinel and synthetic pyroxene). Slope and intercept are calculated using the Isoplot 3.00 software.28 Large variations of ln(α25/24inst) values are present among silicates and oxides, and are linked to matrix effects which result from variations of vibrational energies of the bonds involving Mg isotopes in minerals or glasses having different chemical compositions. | ||
When the instrumental fractionation law has been properly determined, the (25Mg/24Mg)bkgcorr ratio of a given sample is corrected for the appropriate value of α25/24inst determined from the calibration based on standards with different compositions. Then, the corresponding value of α26/24inst is calculated from the instrumental fractionation law (using the values determined for βinst and b) and is used to correct the (26Mg/24Mg)bkgcorr ratio. The two Mg-isotope ratios obtained are considered as the “true isotopic ratios” of the sample, in the sense that they are corrected for all ion probe instrumental effects and seem the closest possible to the true values.
3.4 Determination of (δ25Mg)DSM 3 and (δ26Mg)DSM 3
The (δ25Mg)DSM 3 and (δ26Mg)DSM 3 values are calculated from the (25Mg/24Mg)true and (26Mg/24Mg)true ratios, respectively (see above), and are expressed with respect to the DSM 3 standard.The 2σ error on the δ25Mg value of an individual measurement 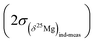 is calculated as the quadratic sum of (i) the external reproducibility determined from repetitive analyses of standards of same matrix as the sample
is calculated as the quadratic sum of (i) the external reproducibility determined from repetitive analyses of standards of same matrix as the sample 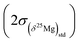 and (ii) the internal error due to the counting statistic
and (ii) the internal error due to the counting statistic 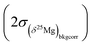 according to:
according to:
The component which dominates by far in the error is the external reproducibility (typically not better than ±0.150‰ for olivine for instance) which is one order of magnitude higher than the counting statistic error (typically better than ±0.021‰ for olivine for instance). The 2σ error on the δ26Mg value of an individual measurement 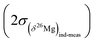 is calculated similarly.
is calculated similarly.
3.5 Calculation of 26Mg excess or deficit and error propagation
The 26Mg excesses or deficits, written in capital delta notation Δ26Mg (in ‰), are calculated directly from the true isotopic ratios using the following relationship:with β = βEarth or βmet (see below), (25Mg/24Mg)DSM 3 = 0.126887, (26Mg/24Mg)DSM 3 = 0.139863. These ratios were calculated from (δ26Mg)DSM 3SRM 980 = 3.90 (±0.03)‰ (2 s.e.), (δ26Mg)DSM 3SRM 980 standing for the δ26Mg of DSM 3, expressed with respect to the SRM 980 international standard.31,32 However, the isotopic homogeneity of the SRM 980 standard has been challenged.33 Calculation with (δ26Mg)DSM 3SRM 980 = 3.40 (±0.13)‰ (ref. 33) results in a 0.007‰ decrease in Δ26Mg values for extraterrestrial materials (that remains within the error bar of the Δ26Mg value calculated with (δ26Mg)DSM 3SRM 980 = 3.9‰), whereas no change is seen for terrestrial materials. This is because the β value used to calculate Δ26Mg values for terrestrial samples is 0.521 (βEarth, corresponding to equilibrium Mg isotopic fractionations34), and 0.514 for meteoritic olivines (βmet).35 This value of 0.514 for βmet has been shown to describe at best most cosmochemical Mg isotope fractionations since they are kinetic, occurring mostly through evaporation and/or condensation processes.36,37
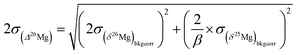
The 2σ internal error (2 s.e. or 2σ) on Δ26Mg due to counting statistic errors on the 26Mg/24Mg and 25Mg/24Mg ratios is given by:
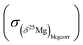 and
and 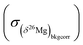 the errors on the isotope ratios due to counting statistic, typically for an olivine ±0.014‰ and ±0.021‰, respectively. To be conservative, the correlation of errors between the 26Mg/24Mg and 25Mg/24Mg ratios are not taken into account since it could tend to artificially decrease the errors. Note that the relationship between the errors on the isotope ratios and the errors on the delta values is given, for instance for δ25Mg, by:
the errors on the isotope ratios due to counting statistic, typically for an olivine ±0.014‰ and ±0.021‰, respectively. To be conservative, the correlation of errors between the 26Mg/24Mg and 25Mg/24Mg ratios are not taken into account since it could tend to artificially decrease the errors. Note that the relationship between the errors on the isotope ratios and the errors on the delta values is given, for instance for δ25Mg, by:The 2σ error on the Δ26Mg value of an individual measurement 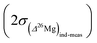 is then calculated as the quadratic sum of (i) the external reproducibility determined from repetitive analyses of standards
is then calculated as the quadratic sum of (i) the external reproducibility determined from repetitive analyses of standards 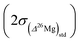 which is of ±0.010‰ for the session shown in Fig. 2 for example, and (ii) the internal error due to the counting statistic according to:
which is of ±0.010‰ for the session shown in Fig. 2 for example, and (ii) the internal error due to the counting statistic according to:
When several measurements (n), e.g. different spots in the same object (such as an isolated olivine or a chondrule), give Δ26Mg values which are identical within ±2σ then a mean Δ26Mg value is calculated for this sample as the weighted mean of the n measurements. The 2σ error associated with this weighted mean is given by:
Finally an important comment must be made concerning the differences between the errors on the δ25Mg and δ26Mg values and the error on the Δ26Mg value. Because variations of mass fractionation follow the instrumental fractionation law (in the three Mg isotopes diagram) they do not introduce errors on Δ26Mg values. Thus for olivines values of 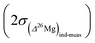 are typically of 0.06‰ while values of
are typically of 0.06‰ while values of 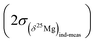 or
or 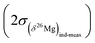 are of 0.20‰ and 0.39‰, respectively, for the session shown in Fig. 2. An interesting application of this observation is that several Δ26Mg measurements can be made on a small grain (e.g. <150 μm) successively at the same spot (i.e. by depth profiling). This is a way to improve the precision on the Δ26Mg value when the grain is too small to make several analyses at different locations. Fig. 3b shows the results of seven such depth profiles made on San Carlos olivines (each depth profile corresponds to five to seven successive analyses). Each depth profile gives Δ26Mg values of 0‰ within their 2 s.e. of typically ±0.015‰ (the 2 s.d. for each spot varying from 0.028‰ to 0.034‰) despite a significant change of instrumental fractionation with depth in the sample, which results in a range of variation for δ25Mg and δ26Mg values of 0.4‰ and 0.8‰, respectively (Fig. 3a and Table 2).
are of 0.20‰ and 0.39‰, respectively, for the session shown in Fig. 2. An interesting application of this observation is that several Δ26Mg measurements can be made on a small grain (e.g. <150 μm) successively at the same spot (i.e. by depth profiling). This is a way to improve the precision on the Δ26Mg value when the grain is too small to make several analyses at different locations. Fig. 3b shows the results of seven such depth profiles made on San Carlos olivines (each depth profile corresponds to five to seven successive analyses). Each depth profile gives Δ26Mg values of 0‰ within their 2 s.e. of typically ±0.015‰ (the 2 s.d. for each spot varying from 0.028‰ to 0.034‰) despite a significant change of instrumental fractionation with depth in the sample, which results in a range of variation for δ25Mg and δ26Mg values of 0.4‰ and 0.8‰, respectively (Fig. 3a and Table 2).
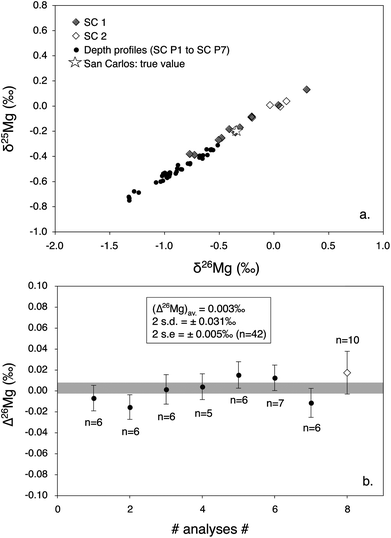 | ||
| Fig. 3 Mg isotopic compositions of San Carlos olivines (Fo#88) measured using two different protocols: either single measurements at different spots (black (SC1) and open (SC2) diamonds, corresponding to two different separated San Carlos olivine grains) or depth profiles (black dots). All data are corrected for matrix effects. The true Mg-isotope composition of San Carlos olivines is also plotted (open star). (a) Three Mg-isotope diagram showing that the two types of data follow the same fractionation law (even if the fractionation is in average stronger for depth profiles). Error bars, typically better than ±0.11‰ on δ25Mg values and ±0.22‰ on δ26Mg values for this analytical session, are not shown for simplicity. (b) Averages of analyses made by depth profiles at different spots (n is the number of analyses in a given depth profile, black dots) compared to the average of the single analyses (n = 10) made at different spots (open diamond). Both types of measurements show Δ26Mg values correctly determined at 0‰ within 2 s.e. Thus, depth profiles can be used in small samples to obtain a precision on Δ26Mg values similar to that obtained from averaging several analyses made at different locations. | ||
| Name | Description | δ 25Mg (‰) | 2 s.e | δ 26Mg (‰) | 2 s.e | Δ 26Mg (‰) | 2 s.e | n |
|---|---|---|---|---|---|---|---|---|
| SC 1 | Separated grain | −0.180 | 0.103 | −0.343 | 0.206 | 0.017 | 0.020 | 10 |
| SC 2 | Separated grain | −0.058 | 0.059 | −0.118 | 0.126 | −0.006 | 0.023 | 7 |
| Average | 0.007 | 0.015 | 17 | |||||
| SC P1 | Separated grain – profile no. 1 | −0.514 | 0.029 | −0.912 | 0.067 | −0.007 | 0.012 | 6 |
| SC P2 | Separated grain – profile no. 2 | −0.522 | 0.032 | −0.939 | 0.078 | −0.015 | 0.012 | 6 |
| SC P3 | Separated grain – profile no. 3 | −0.493 | 0.049 | −0.862 | 0.092 | 0.002 | 0.014 | 6 |
| SC P4 | Separated grain – profile no. 4 | −0.346 | 0.025 | −0.582 | 0.043 | 0.004 | 0.012 | 5 |
| SC P5 | Separated grain – profile no. 5 | −0.379 | 0.024 | −0.631 | 0.035 | 0.015 | 0.013 | 6 |
| SC P6 | Separated grain – profile no. 6 | −0.552 | 0.026 | −0.969 | 0.048 | 0.013 | 0.012 | 7 |
| SC P7 | Separated grain – profile no. 7 | −0.694 | 0.042 | −1.263 | 0.079 | −0.011 | 0.014 | 6 |
| Average | 0.003 | 0.005 | 42 | |||||
| Average | 0.002 | 0.009 |
3.6 Precision reached for the determination of 26Mg excess or deficit
Fig. 4 shows typical results of Δ26Mg measurements for two international and one in-house standards run during one analytical session. The standards show no significant excess or deficit in 26Mg, consistent with their terrestrial origin. The external reproducibility is better than ±0.04‰ (2 s.d., n = 23) and the 2σ error on the mean of all analyses of standards is ±0.018‰ (2 s.e., n = 23).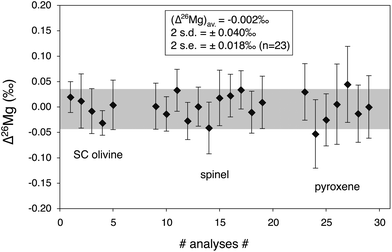 | ||
| Fig. 4 26Mg excess or deficit (expressed with the Δ26Mg notation, see text) obtained for three reference materials with different chemical compositions (the same as in Fig. 2) measured within one analytical session (n = 23). They show no significant excess or deficit in 26Mg, consistent with their terrestrial origin. The typical external reproducibility (2 s.d.) is better than ±0.04‰ while the 2σ error of each individual measurement is typically better than ±0.06‰. The 2σ error on the mean of all analyses of reference materials (2 s.e.) is better than ±0.02‰. | ||
The major source of errors on Δ26Mg values that can be identified in the procedure described here, if an improper treatment of the data is performed, is the correction for instrumental fractionation. Because the instrumental fractionation law is always slightly different from the cosmochemical mass fractionation law and from the terrestrial mass fractionation law, an over-correction or an under-correction of instrumental fractionation (due to a poor calibration of matrix effects) will result in an error on Δ26Mg value (Fig. 5). For instance, correcting for an improper Fo content (e.g. Fo#100 instead of Fo#88 for San Carlos olivines) leads to an absolute error on Δ26Mg value of ∼±5 ppm. Similarly, using an improper β value (e.g. βmet instead of βEarth for San Carlos olivines) can lead to a maximum absolute error of ∼±30 ppm on Δ26Mg (Fig. 5 and Table 3).
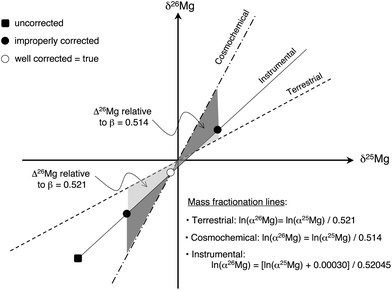 | ||
| Fig. 5 Schematic effect in a three Mg isotope diagram of an improper correction for instrumental isotopic fractionation on the determination of the Δ26Mg value of meteoritic samples. The open dot stands for the isotopic composition after appropriate corrections (see text). In this example no 26Mg excess is obtained since the open dot is sitting on the cosmochemical fractionation line (see text). The two black dots represent wrong corrections of instrumental fractionation in which the matrix effect was under- or over-estimated: this results in “wrong” apparent 26Mg excess or deficit relative to the cosmochemical line (dark grey field). Using erroneously the terrestrial line instead of the cosmochemical line also results in “wrong” 26Mg excess or deficit (light grey field). | ||
| δ 25Mg (‰) | Δ 26Mg (‰) | ||
|---|---|---|---|
| β Earth = 0.521 | β met = 0.514 | ||
| Fo#79 | −0.997 | −0.010 | 0.016 |
| Fo#88 | −0.058 | −0.006 | −0.004 |
| Fo#100 | 1.199 | −0.001 | −0.031 |
3.7 Al and Mg relative ion yields
When the 26Mg excesses in meteoritic samples are presumed to be due to the in situ decay of short-lived 26Al, isochron diagrams are built to determine the 26Al/27Al ratio at the time of isotopic closure. For that, the 27Al/24Mg ratios must be determined very precisely in order to minimize the error on the 26Al/27Al (e.g. an error of ±1.3% (see below) in a CAI with 26Al/27Al = 5 × 10−5 introduces an error of ∼±1.3% on the 26Al/27Al ratio). Because elemental secondary ion yields show strong differences between different elements (and different matrices) that cannot be predicted precisely enough from theoretical grounds,38 they must be calibrated precisely using a set of standards that covers the chemical variability of the samples to analyze.The Al/Mg relative yield is defined by the ratio between the measured and the true Al/Mg (or 27Al/24Mg) ratios:
Thus the Al/Mg yield is determined from analyses of international and in-house standards and can then be used to correct measurements of samples. Results of the calibration of the Al/Mg yield for various silicates and oxides are then shown in Table 1. Note that all silicates show (for this analytical session) an averaged Al/Mg yield of 0.77 (±0.05, 2 s.d.) with an associated 2 s.e. of 1.3% (n = 28), while oxides such as hibonites show significantly different yields (0.629 (±0.001, 1 s.e.) in this session). Minerals that contain trace amounts of Al, such as olivine, show the same Al/Mg yield as Al-rich silicates within the error (1.00 (±0.30, 1 s.e.) for olivine from the Eagle Station pallasite, having a Al2O3 content of ∼0.0027 wt%).
The 2σ error on the 27Al/24Mg ratio is calculated for an individual measurement by summing in a quadratic way the counting error (typically ±2% (2 s.e.) relative in an olivine and ±0.2% (2 s.e.) in a mineral like spinel where Al and Mg are major elements) and the two sigma external reproducibility on the standards (typically ±8% (2 s.e.) in an olivine and ± 1.2% (2 s.e.) in an Al-rich mineral).
4 Examples of the implications of high precision Mg isotopic analyses of components of chondritic meteorites
Using this method, the construction of high precision 26Al isochrons for chondrules and CAIs is within the reach of in situ analysis using ion microprobes. Both the slope (from which the initial (26Al/27Al)0 ratio is deduced) and the (δ26Mg*)0 intercept (this notation standing for 26Mg excesses or deficits linked to 26Al in situ decay) can be precisely determined. This gives theoretically access to the crystallization age (calculated from the (26Al/27Al)0 ratio), and to the 26Al model age of the precursors (calculated from the (δ26Mg*)0 value with an appropriate evolution model).Fig. 6 shows as an example of two 26Al isochrons measured for one CAI from the Efremovka CV3 carbonaceous chondrite (data from Mishra and Chaussidon39) and for one chondrule from the Semarkona LL3 ordinary chondrite.8 The CAI isochron has a (26Al/27Al)0 of 4.76 (±0.15) × 10−5 and a (δ26Mg*)0 of 0.146 (±0.041)‰, while the chondrule isochron has a (26Al/27Al)0 of 8.92 (±0.91) × 10−6 and a (δ26Mg*)0 of −0.0024 (±0.0075)‰. If interpreted in a simple model considering that there was a time zero when the inner accretion disk was homogenized to (26Al/27Al)i = 5.23 × 10−5 and (δ26Mg*)i = −0.038‰,5,6,8 the two isochrons imply that the last melting/crystallization event for the CAI and the chondrule took place 0.10+0.03−0.03 Myr and 1.86+0.11−0.10 Myr, respectively, after the time zero. A 1.2 to 4 Myr age difference between CAIs and chondrules is a general conclusion of 26Al studies interpreted under the assumption of an homogeneous distribution of 26Al in the inner solar system,8 which would be consistent with the latest accretion models considering progressive gravitational collapse of mm sized particles concentrated by turbulence in the nebular gas (see review by Dauphas and Chaussidon40).
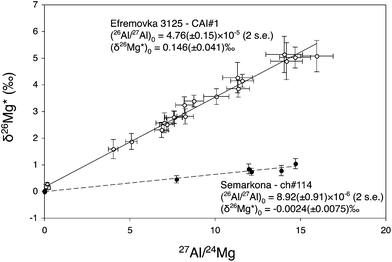 | ||
| Fig. 6 Two 26Al isochrons measured for one CAI from the Efremovka CV3 carbonaceous chondrite (MSWD = 1.15, data from Mishra and Chaussidon, 2012) and for one chondrule from the Semarkona LL3 ordinary chondrite (MSWD = 0.71, data from Villeneuve et al., 2009). A gap ranging from 1.2 to 4 Myr between the 26Al ages of CAIs and chondrules is generally deduced from 26Al studies assuming a homogeneous distribution of 26Al and Mg isotopes in the accretion disk. | ||
The different (δ26Mg*)0 observed for the CAI and the chondrule can be understood as reflecting different origins and histories for their precursors. The simplest model would be for the chondrule that the precursors were condensed from the nebular gas at 1.86 Myr (age given by the (26Al/27Al)0 of the chondrule isochron): 26Al decay in the nebular gas with a 27Al/24Mg of 0.101 for 1.86 Myr would result in a δ26Mg* of −0.007‰. For the CAI, the simplest model is that its precursors were condensed at time zero and then evolved in a closed system with the bulk 27Al/24Mg ratio of the CAI of 6.3, leading to the build up of a δ26Mg* of 0.146‰ in 0.10 Myr. However more complicated scenarios are possible13,35 depending on the model considered.
High precision Mg-isotope measurements are also possible for Mg-rich and Al-poor phases (i.e. phases with a low Al/Mg ratio). This is the case for Mg-rich refractory olivines (either isolated olivines or olivines in porphyritic type I chondrules), whose 26Al model ages could be constrained. These Mg-rich olivines may have various origins. Because they are virtually devoid of Al no radiogenic in-growth of 26Mg takes place, so that their Mg isotopic composition will reflect that of their source, i.e. the nebular gas from which they condensed, the chondrule melt from which they crystallized,41–43 or the planetesimal mantle from which they crystallized.44–48 For a given model, the precision on the 26Al model age calculations of these Mg-rich refractory olivines is highly dependent on the precision on both the Δ26Mg value and the 27Al/24Mg ratio. For instance for a parent melt with a 27Al/24Mg ratio of 2.5, a precision of ±0.016‰ on Δ26Mg leads to a precision on the age of ±0.02 Ma (data from Luu et al.49).
5 Conclusion
The analytical protocol and data reduction process described in this study allow high precision to be reached for Mg isotopic measurements (25Mg/24Mg and 26Mg/24Mg ratios, as well as 26Mg excess) by MC-SIMS. This method minimizes analytical bias on the final Mg-isotope results, which is very important at the level of precision targeted in cosmochemistry (better than 10 ppm absolute error on the calculation of the final 26Mg excess or deficit).This new possibility of reaching very high precision for Mg-isotope analyses opens new perspectives in geo- and cosmochemistry fields. For instance natural processes such as biomineralization could be better understood by more accurately constraining the induced fractionation.
Acknowledgements
The authors want to thank Laurent Tissandier for providing the Bacati glass. This work was supported by grant from European Research Council (ERC grant FP7/2007–2013 Grant Agreement no. [226846] Cosmochemical Exploration of the first two Million Years of the Solar System—CEMYSS). This is CRPG-CNRS contribution n°2206.References
- A. Bouvier and M. Wadhwa, Nat. Geosci., 2010, 3, 637–641 CrossRef CAS.
- Y. Amelin, A. N. Krot, I. D. Hutcheon and A. A. Ulyano, Science, 2002, 297, 1678–1683 CrossRef CAS.
- J. Connelly, Y. Amelin, A. N. Krot and M. Bizzarro, Astrophys. J., 2008, 675, L121–L124 CrossRef CAS.
- T. Lee, D. A. Papanastassiou and G. J. Wasserburg, Geophys. Res. Lett., 1976, 3, 109–112 CrossRef CAS.
- K. Thrane, M. Bizzarro and J. A. Baker, Astrophys. J., 2006, 646, L159–L162 CrossRef CAS.
- B. Jacobsen, Q. Z. Yin, F. Moynier, Y. Amelin, A. N. Krot, K. Nagashima, I. D. Hutcheon and H. Palme, Earth Planet. Sci. Lett., 2008, 272, 353–364 CrossRef CAS.
- K. Lodders, Astrophys. J., 2003, 591, 1220–1247 CrossRef CAS.
- J. Villeneuve, M. Chaussidon and G. Libourel, Science, 2009, 325, 985–988 CrossRef CAS.
- K. K. Larsen, A. Trinquier, C. Paton, M. Schiller, D. Wielandt, M. A. Ivanova, J. N. Connelly, A. Nordlund, A. N. Krot and M. Bizzarro, Astrophys. J., 2011, 735, L37 CrossRef.
- M. Bizzarro, C. Paton, K. Larsen, M. Schiller, A. Trinquier and D. Ulfbeck, J. Anal. At. Spectrom., 2011, 26, 565–577 RSC.
- J. Villeneuve, M. Chaussidon and G. Libourel, Earth Planet. Sci. Lett., 2011, 301, 107–116 CrossRef CAS.
- G. J. MacPherson, E. S. Bullock, P. E. Janney, N. T. Kita, T. Ushikubo, A. M. Davis, M. Wadhwa and A. N. Krot, Astrophys. J., 2010, 711, L117–L121 CrossRef CAS.
- G. J. MacPherson, N. T. Kita, T. Ushikubo, E. S. Bullock and A. M. Davis, Earth Planet. Sci. Lett., 2012, 331–332, 43–54 CrossRef CAS.
- N. T. Kita and T. Ushikubo, Meteorit. Planet. Sci., 2012, 47, 1108–1119 CrossRef CAS.
- N. T. Kita, T. Ushikubo, K. B. Knight, R. A. Mendybaev, A. M. Davis, F. M. Richter and J. H. Fournelle, Geochim. Cosmochim. Acta, 2012, 86, 37–51 CrossRef CAS.
- Secondary Ion Mass Spectrometry: Basic Concepts, Instrumental Aspects, Applications, and Trends, ed. A. Benninghoven, F. G. Rüdenauer and H. W. Werner, Wiley, New York, 1987, p. 1227 Search PubMed.
- E. de Chambost, F. Hillion, B. Rasser and H. N. Migeon, in SIMS VIII Proceedings, ed. A. Benninghoven, K. T. F. Janssen, J. Tumpner and H. W. Werner, New York, John Wiley, 1991, pp. 207–210 Search PubMed.
- E. J. Catanzaro, T. J. Murphy, E. L. Garner and W. R. Shields, J. Res. Natl. Bur. Stand., Sect. A, 1966, 70, 453–458 CAS.
- R. C. Ogliore, G. R. Huss and K. Nagashima, Nucl. Instrum. Methods Phys. Res., Sect. B, 2011, 269, 1910–1918 CrossRef.
- E. de Chambost, User's Guide for Multicollector Cameca IMS 1270, Cameca, Courbevoie, France, 1997 Search PubMed.
- D. A. Ionov, I. V. Ashchepkov, H.-G. Stosch, G. Witt-Eickschen and H. A. Seck, J. Petrol., 1993, 34, 1141–1175 CAS.
- S. Decitre, PhD thesis, Centre de Recherches Pétrographiques et Géochimiques (CRPG-CNRS), Nancy, 2000.
- S. Gao, X. Liu, H. Yuan, B. Hattendorf, D. Günther, L. Chen and S. Hu, Geostand. Newsl., 2002, 26, 181–196 CrossRef CAS.
- W. Yang, F.-Z. Teng and H.-F. Zhang, Earth Planet. Sci. Lett., 2009, 288, 475–482 CrossRef CAS.
- F.-Z. Teng, W.-Y. Li, S. Ke, B. Marty, N. Dauphas, S. Huang, F.-Y. Wu and A. Pourmand, Geochim. Cosmochim. Acta, 2010, 74, 4150–4166 CrossRef CAS.
- E. Deloule, C. France-Lanord and F. Albarède, in Stable Isotope Geochemistry: A Tribute to Samuel Epstein, ed. H. P. Taylor, Jr, J. R. O'Neil and I. R. Kaplan, 1991, pp. 53–62 Search PubMed.
- E. D. Young and A. Galy, Rev. Min. Geochem., 2004, 55, 197–230 CrossRef CAS.
- K. R. Ludwig, ISOPLOT 3: a Geochronological Toolkit for Microsoft Excel, Berkeley Geochronology Centre Special Publication, vol. 4, 2003, p. 74 Search PubMed.
- F. Albarède and B. Beard, Rev. Mineral. Geochem., 2004, 55, 113–152 CrossRef.
- L. A. Leshin, A. E. Rubin and K. D. McKeegan, Geochim. Cosmochim. Acta, 1997, 61, 835–845 CrossRef CAS.
- A. Galy, N. S. Belshaw, L. Halicz and R. K. O'Nions, Int. J. Mass Spectrom., 2001, 208, 89–98 CrossRef CAS.
- E. B. Boulou-Bi, N. Vigier, A. Brenot and A. Poszwa, Geostand. Geoanal. Res., 2009, 33, 95–109 CrossRef.
- A. Galy, O. Yoffe, P. E. Janney, R. W. Williams, C. Cloquet, O. Alard, L. Halicz, M. Wadhwa, I. D. Hutcheon, E. Ramon and J. Carignan, J. Anal. At. Spectrom., 2003, 18, 1352–1356 RSC.
- E. D. Young, A. Galy and H. Nagahara, Geochim. Cosmochim. Acta, 2002, 66, 1095–1104 CrossRef CAS.
- A. M. Davis, F. M. Richter, R. A. Mendybaev, P. E. Janney, M. Wadhwa and K. D. McKeegan, 36th Lunar Planet. Inst. Conf., League City, Texas, 2005 Search PubMed.
- A. M. Davis, A. Hashimoto, R. N. Clayton and T. K. Mayeda, Nature, 1990, 347, 655–658 CrossRef CAS.
- A. M. Davis and F. M. Richter, in Treatise on Geochemistry, Vol. 1: Meteorites, Planets, and Comets, ed. A. M. Davis, H. D. Holland and K. K. Turekian, Oxford, Elsevier-Pergamon, 2003, pp. 407–430 Search PubMed.
- R. W. Hinton, Chem. Geol., 1990, 83, 11–25 CrossRef CAS.
- R. K. Mishra and M. Chaussidon, 43rd Lunar Planet. Sc. Conf., Texas, 2012 Search PubMed.
- N. Dauphas and M. Chaussidon, Annu. Rev. Earth Planet. Sci., 2011, 39, 351–386 CrossRef CAS.
- N. G. Rudraswami, T. Ushikubo, D. Nakashima and N. T. Kita, Geochim. Cosmochim. Acta, 2011, 75, 7596–7611 CrossRef CAS.
- H. Nagahara, Nature, 1981, 292, 135–136 CrossRef CAS.
- R. H. Jones, in International Conference: Chondrules and the Protoplanetary Disk, ed. R. H. Hewins, R. H. Jones and E. R. D. Scott, Cambridge University Press, 1996, pp. 163–172 Search PubMed.
- L. Tissandier, G. Libourel and F. Robert, Meteorit. Planet. Sci., 2002, 37, 1377–1389 CrossRef CAS.
- G. Libourel, A. N. Krot and L. Tissandier, Earth Planet. Sci. Lett., 2006, 251, 232–240 CrossRef CAS.
- G. Libourel and A. N. Krot, Earth Planet. Sci. Lett., 2007, 254, 1–8 CrossRef CAS.
- M. Chaussidon, G. Libourel and A. N. Krot, Geochim. Cosmochim. Acta, 2008, 72, 1924–1938 CrossRef CAS.
- G. Libourel and M. Chaussidon, Earth Planet. Sci. Lett., 2011, 301, 9–21 CrossRef CAS.
- T.-H. Luu, M. Chaussidon and J.-L. Birck, 43rd Lunar Planet. Sc. Conf., Texas, 2012 Search PubMed.
| This journal is © The Royal Society of Chemistry 2013 |