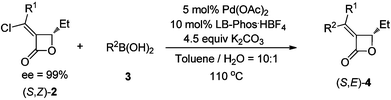
Pd-catalyzed Suzuki coupling reaction of chloroalkylidene-β-lactones with LB-Phos as the ligand†
Pengbin
Li
,
Bo
Lü
,
Chunling
Fu
and
Shengming
Ma
*
Laboratory of Molecular Recognition and synthesis, Department of Chemistry, Zhejiang University, Hangzhou 310027, People's Republic of China. E-mail: masm@sioc.ac.cn; Fax: +86-21-64167510
First published on 14th September 2012
Abstract
In this paper, LB-Phos·HBF4 salt has been applied for the Pd-catalysed Suzuki coupling reactions of optically active (Z)-α-choroalkylidene-β-lactones. Aryl, vinyl, alkyl and heteroaromatic boronic acids may be coupled with optically active β-lactones affording corresponding optically active products highly selectively and efficiently.
Introduction
Much progress has been achieved with C–Cl bond activation reactions in past years thanks to the development of new phosphine ligands.1 Recently, we have developed a new type of monophosphine ligand, which could be used in palladium-catalyzed Suzuki coupling reactions2 and aminations3 of organic chlorides. In the Suzuki coupling of optically active chlorinated 2-(5H)-furanones, LB-Phos showed advantages over some other known ligands by affording the coupling products, i.e. optically active β-substituted butenolides, within 5–10 min without racemization.4 In this paper, we wish to report our recent results on the Suzuki coupling reaction of optically active α-chloroalkylidene-β-lactones derivatives of high ee.Results and discussion
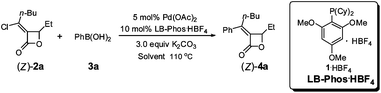
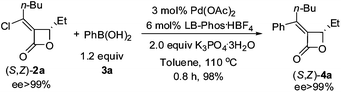
At the beginning, (Z)-α-(1-chloropentylidene)-β-ethyl-β-lactone ((Z)-2a),5 which was prepared by the PdCl2-catalyzed cyclocarbonylation of propargyl alcohols with CuCl2 developed in this group, and phenyl boronic acid (3a) (1.5 equiv) were used to test the catalytic activity of Pd/LB-Phos·HBF4. We found that the Suzuki coupling product (Z)-4a would be formed in 100% yield using 3.0 equiv of K2CO3 at 110 °C in toluene (Table 1, entry 1). Other solvents such as dioxane, DMSO and DMF would lead to lower yields (Table 1, entries 2–4). Reducing the amount of 3a to 1.2 equiv. had a slight negative effect on the yield of (Z)-4a (Table 1, entry 5). However, reducing the loading of Pd(OAc)2, LB-Phos·HBF4, or K2CO3, respectively, would all decrease the yield of (Z)-4a (Table 1, entries 5–8). This problem was circumvented by using 2.0 equiv of K3PO4·3H2O as the base affording (Z)-4a in 95% yield (Table 1, entry 9).|
|
|||||
|---|---|---|---|---|---|
| Entry | 3a (equiv) | Solvent | Time (h) | Yield of (Z)-4aa (%) | Recovery of (Z)-2aa (%) |
| a Determined by 1H NMR analysis using methylene bromide as the internal standard. b K2CO3 (2.0 equiv) was used. c LB-Phos·HBF4 (5 mol%) was used. d Pd(OAc)2 (3 mol%) and LB-Phos·HBF4 (6 mol%) were used. e K3PO4·3H2O (2.0 equiv) was used. | |||||
| 1 | 1.5 | Toluene | 1.0 | 100 | — |
| 2 | 1.5 | Dioxane | 1.0 | 58 | 39 |
| 3 | 1.5 | DMF | 1.5 | — | — |
| 4 | 1.5 | DMSO | 3.2 | — | — |
| 5 | 1.2 | Toluene | 2.6 | 94 | — |
| 6b | 1.2 | Toluene | 1.2 | 77 | 23 |
| 7c | 1.2 | Toluene | 1.0 | 86 | 14 |
| 8d | 1.2 | Toluene | 1.7 | 88 | — |
| 9d,e | 1.2 | Toluene | 0.7 | 95 | — |
Optically active coupling product (S,Z)-4a could also be produced from (S,Z)-2a under our optimized reaction conditions in 98% yield with no racemization (eqn (1)). Thus, it is concluded that even such strained allylic C–O bonds may also be kept untouched.4
 | (1) |
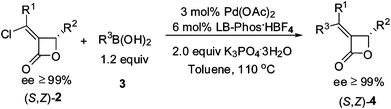
Encouraged by these results, a series of optically active α-chloroalkylidene-β-lactones6 were tested under the optimized conditions (Table 2). Aromatic (Table 2, entries 1–4 and 6–10) and vinylic boronic acids (Table 2, entries 5 and 11) could all be coupled with the starting β-lactones to afford the products within 0.8–3.7 h. Increasing the steric hindrance in the ortho-position of aryl boronic acid led to longer reaction times and lower yields (Table 2, compare entries 1 and 4). 2-Naphthyl boronic acid (3g) was also used to couple with (S,Z)-2a affording (S,Z)-4h in 86% yield (Table 2, entry 8).
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | (S,Z)-2 | 3 | Time (h) | Yieldb (%) | ||
| R1 | R2 | R3 | ||||
| a Standard conditions A: a mixture of 1.0 equiv of (S,Z)-2 (0.2 mmol or 0.4 mmol), 1.2 equiv of 3, 2.0 equiv of K3PO4·3H2O, 0.03 equiv of Pd(OAc)2 and 0.06 equiv of LB-Phos·HBF4 was stirred in toluene (0.1 M) at 110 °C. b Isolated yield. | ||||||
| 1 | (S,Z)-2a | n-Bu | Et | Ph (3a) | 0.8 | 98 ((S,Z)-4a) |
| 2 | (S,Z)-2a | n-Bu | Et | 4-MeOC6H4 (3b) | 2.0 | 84 ((S,Z)-4b) |
| 3 | (S,Z)-2a | n-Bu | Et | 3-MeOC6H4 (3c) | 1.0 | 77 ((S,Z)-4c) |
| 4 | (S,Z)-2a | n-Bu | Et | 2-MeOC6H4 (3d) | 3.7 | 69 ((S,Z)-4d) |
| 5 | (S,Z)-2a | n-Bu | Et | (E)-Styryl (3e) | 2.8 | 91 ((S,Z,E)-4e) |
| 6 | (S,Z)-2a | n-Bu | Et | 4-MeC6H4 (3f) | 1.0 | 93 ((S,Z)-4f) |
| 7 | (S,Z)-2b | n-Bu | Me | 4-MeC6H4 (3f) | 1.0 | 71 ((S,Z)-4g) |
| 8 | (S,Z)-2a | n-Bu | Et | 2-Naphthyl (3g) | 1.0 | 86 ((S,Z)-4h) |
| 9 | (S,Z)-2c | n-Bu | i-Pr | Ph (3a) | 1.5 | 94 ((S,Z)-4i) |
| 10 | (S,Z)-2d | Ph | Et | 4-MeOC6H4 (3b) | 1.5 | 93 ((S,Z)-4j) |
| 11 | (S,Z)-2d | Ph | Et | (E)-1-Pentenyl (3h) | 0.8 | 95 ((S,E,E)-4k) |
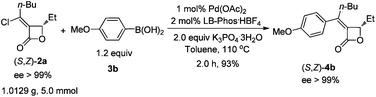
One 5.0 mmol-scale reaction was carried out affording (S,Z)-4b with a relatively higher yield (eqn (2)vs. entry 2, Table 2) within 2.0 h when 1 mol% Pd(OAc)2 together with 2 mol% LB-Phos·HBF4 was used as the catalyst (eqn (2)).
 | (2) |
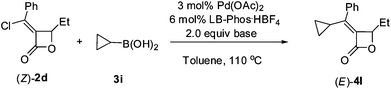
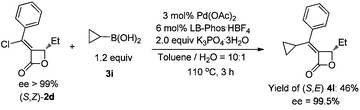
However, when cyclopropylboronic acid 3i and (Z)-2d were coupled under the optimized conditions, the yield of product (E)-4l was only 23% (Table 3, entry 1).6 A higher loading of boronic acid 3i did not help to give a higher yield (Table 3, entries 2–3). The base-sensitive β-lactone (Z)-2d would decompose quickly even in absence of Pd(OAc)2 and LB-Phos·HBF4 (Table 3, entry 5). Using weaker base such as CsF and K2HPO4·3H2O gave no expected product but recovery of (Z)-2d (Table 3, entries 7 and 8).
|
|
|||||
|---|---|---|---|---|---|
| Entry | 3i (equiv) | Base | Time (h) | Yield of (E)-4la (%) | Recovery of (Z)-2da (%) |
| a Determined by 1H NMR analysis. b Neither Pd(OAc)2 nor ligand was added here. c Pd2(dba)3·CHCl3 was used to replace Pd(OAc)2. | |||||
| 1 | 1.2 | K3PO4·3H2O | 1.0 | 23 | 0 |
| 2 | 1.5 | K3PO4·3H2O | 1.8 | 17 | 0 |
| 3 | 3.0 | K3PO4·3H2O | 1.5 | 12 | <4 |
| 4b | 0.0 | K3PO4·3H2O | 1.5 | 0 | 21 |
| 5c | 1.2 | K3PO4·3H2O | 5.0 | 19 | 0 |
| 6 | 1.2 | Cs2CO3 | 1.3 | 12 | 27 |
| 7 | 1.2 | CsF | 1.1 | 0 | 83 |
| 8 | 1.2 | K2HPO4·3H2O | 6.0 | 0 | 97 |
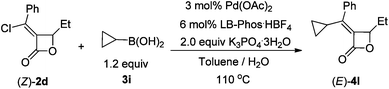
Further screening led to the observation that when toluene–water (v/v = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used as the mixed solvent, (E)-4l was obtained in 29% yield with 50% of (Z)-2d being recovered (Table 4, entry 1). Decreasing the amount of water to 1
1) was used as the mixed solvent, (E)-4l was obtained in 29% yield with 50% of (Z)-2d being recovered (Table 4, entry 1). Decreasing the amount of water to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio (water/toluene (v/v)) would lead to 54% yield (Table 4, entry 2). Further decreasing the amount of water did not improve the yield of (E)-4l (Table 4, entry 3).
10 ratio (water/toluene (v/v)) would lead to 54% yield (Table 4, entry 2). Further decreasing the amount of water did not improve the yield of (E)-4l (Table 4, entry 3).
Under the newly optimized conditions (Table 4, entry 2), cyclopropylboronic acid could couple with optically active (S,Z)-2d affording (S,E)-4l in 46% yield (eqn (3)). However, the coupling of n-butylboronic acid with (S,Z)-2d did not afford the desired coupling product (S,E)-4m. Increasing the loading of K2CO3 to 4.5 equiv solved the problem (Table 5, entry 1). Under the same conditions (standard conditions B), n-hexylboronic acid could easily couple with (S,Z)-2d affording (S,E)-4n in 72% yield (Table 5 entry 2).
 | (3) |
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | (S,Z)-2 | 3 | Time (h) | 4 | ||
| R1 | (equiv) | R2 | Yieldb (%) | ee% | ||
a Standard conditions B: a mixture of 1.0 equiv of (S,Z)-2, 2.0 equiv of 3, 4.5 equiv of K2CO3, 0.05 equiv of Pd(OAc)2 and 0.10 equiv of LB-Phos·HBF4 were stirred in toluene–H2O = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 110 °C.
b Isolated yield.
c PdCl2(LB-Phos)2 (5 mol%) was used as catalyst. 1 at 110 °C.
b Isolated yield.
c PdCl2(LB-Phos)2 (5 mol%) was used as catalyst.
|
||||||
| 1 | Ph (S,Z)-2d | 2.0 | n-Bu (3j) | 3.0 | 73 ((S,E)-4m) | 99.3 |
| 2 | Ph (S,Z)-2d | 2.0 | n-Hex (3k) | 6.0 | 72 ((S,E)-4n) | >99 |
| 3 | n-Bu (Z)-2a | 2.0 | n-Bu (3j) | 8.0 | 23 (4o) | — |
| 4c | n-Bu (S,Z)-2a | 3.0 | n-Bu (3j) | 3.0 | 55 ((S)-4o) | 99.4 |
Furthermore, we found that 2-thienylboronic acid 3l failed to couple with (Z)-2d under the standard conditions A or B (Scheme 1). We reasoned that the formation of Pd–S complex 5 was too stable to prevent the formation of P–Pd species (Scheme 2). Then we synthesized complex PdCl2(LB-Phos)27 by stirring PdCl2(PhCN)2 with LB-Phos in benzene for 3 days at room temperature. Indeed, this problem was also conquered by using PdCl2(LB-Phos)2 (Scheme 3) as the catalyst. (S,Z)-4p8 and (S,Z)-4q could be prepared in 77% and 83% yields, respectively, under these conditions (Scheme 4). The absolute configuration of (S,Z)-4p was further confirmed by X-ray diffraction studies (Fig. 1). It is interesting to note that by using 5 mol% PdCl2(LB-Phos)2 and 3.0 equiv of n-butylboronic acid, the yield of S-4o was also improved to 55% (Table 5, entry 4) as compared with the results presented in entry 3 of Table 5.
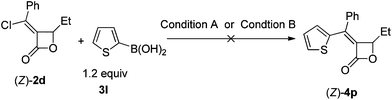 | ||
| Scheme 1 | ||
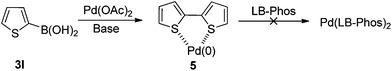 | ||
| Scheme 2 | ||
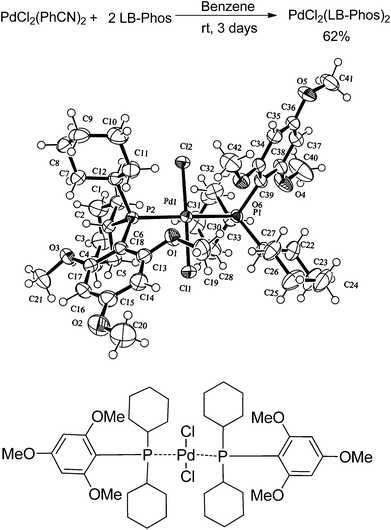 | ||
| Scheme 3 Synthesis and ORTEP representation of trans-PdCl2(LB-Phos)2. | ||
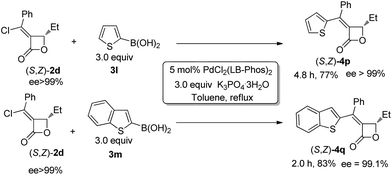 | ||
| Scheme 4 | ||
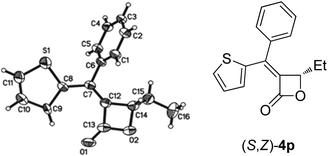 | ||
| Fig. 1 ORTEP representation of (S,Z)-4p.8 | ||
Conclusions
We have prepared a series of optically active α-alkylidene-β-lactones of high ee via the Suzuki coupling reaction of arylic, vinylic boronic acid with optically active α-chloroalkylidene-β-lactones using LB-Phos as ligand.9 When a mixture of toluene and H2O in 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume was used, alkyl boronic acid could also be coupled to afford the products in a moderate yields. 2-Benzothienylboronic acid and 2-thienylboronic acid could also be coupled easily in presence of the pre-formed PdCl2(LB-Phos)2. Further studies in this area are being conducted in our laboratory.
1 in volume was used, alkyl boronic acid could also be coupled to afford the products in a moderate yields. 2-Benzothienylboronic acid and 2-thienylboronic acid could also be coupled easily in presence of the pre-formed PdCl2(LB-Phos)2. Further studies in this area are being conducted in our laboratory.
Experimental section
General information
1H and 13C NMR spectra were recorded on the instruments operated at 300 and 75 MHz, respectively, in CDCl3. Chemical shifts (δ) are given in parts per million (ppm) with the residual peak of CHCl3 at 7.260 ppm or TMS at 0.000 ppm as the internal standard. Infrared spectra were recorded from thin films of pure samples on sodium chloride plates for liquid or in the form of KBr discs for the solid samples. Mass and HRMS spectra were carried out in EI mode. Thin layer chromatography was performed on pre-coated glass-back plates and visualized with UV light at 254 nm. Anhydrous CuCl2 (96%) was purchased from Energy Chemical and heated at 200 °C under vacuum (<1 mmHg) to remove residual water before use. Flash column chromatography was performed on silica gel (10–40 μ). Dioxane and toluene used was refluxed in the presence of sodium wire using diphenyl ketone as indicator and distilled right before use. All the boronic acids were purchased from Alfa Aesar, Aldrich, or J&K chemical LTD.(1) Synthesis of (S,Z)-α-(1-chloropentylidene)-β-isopropyl-β-lactone ((S,Z)-2c). Typical procedure. In a flame-dried flask, a solution of (S)-2-methylnon-4-yn-3-ol (282 mg, 1.8 mmol, ee = 99%) and anhydrous CuCl2 (1.2252 g, 9 mmol) in 20 mL of dry THF was stirred for about 5 min at room temperature followed by the addition of PdCl2 (32.7 mg, 0.18 mmol). Then the flask was transferred to a Parr pressure reactor and charged with 20 atm of CO gas. After the mixture was stirred for 4.3 h at 40 °C, the gas was carefully ventilated, and the residue was diluted with Et2O. Filtration through a short column of silica gel (eluent: Et2O 2 × 70 mL), evaporation, and flash chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 100/1) afforded 237.6 mg (60%) of (S,Z)-2c as a colorless liquid in 99% ee, as determined by HPLC (Chiralpak AS-H, n-hexane–i-PrOH = 98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, λ = 230 nm, tR = 8.4 min (major), 10.2 min (minor)): [α]20D = −45.9 (c = 1.14, CHCl3), 1H NMR (300 MHz, CDCl3) δ 4.89 (d, J = 3.6 Hz, 1H, OCH), 2.34 (t, J = 7.7 Hz, 2H, CH2), 2.24–2.07 (m, 1H, CH), 1.79–1.49 (m, 2H, CH2), 1.44–1.26 (m, 2H, CH2), 1.09 (d, J = 6.9 Hz, 3H, CH3), 0.99 (d, J = 6.9 Hz, 3H, CH3), 0.93 (t, J = 7.2 Hz, 3H. CH3); 13C NMR (75 MHz, CDCl3) δ 161.5, 138.9, 131.9, 83.5, 36.2, 30.9, 28.8, 22.0, 18.4, 15.6, 13.7; IR (neat) ν (cm−1) 2965, 2934, 2876, 1814, 1702, 1467, 1429, 1389, 1370, 1344, 1287, 1216, 1191, 1166, 1109, 1081, 1058; MS (70 ev, EI) m/z (%): 218 (M+(37Cl), 0.33), 216 (M+(35Cl), 0.99), 173 (100); Anal. Calcd For C11H17ClO2: C, 60.97; H, 7.91. Found: C, 61.09; H, 7.97.
2, 0.8 mL min−1, λ = 230 nm, tR = 8.4 min (major), 10.2 min (minor)): [α]20D = −45.9 (c = 1.14, CHCl3), 1H NMR (300 MHz, CDCl3) δ 4.89 (d, J = 3.6 Hz, 1H, OCH), 2.34 (t, J = 7.7 Hz, 2H, CH2), 2.24–2.07 (m, 1H, CH), 1.79–1.49 (m, 2H, CH2), 1.44–1.26 (m, 2H, CH2), 1.09 (d, J = 6.9 Hz, 3H, CH3), 0.99 (d, J = 6.9 Hz, 3H, CH3), 0.93 (t, J = 7.2 Hz, 3H. CH3); 13C NMR (75 MHz, CDCl3) δ 161.5, 138.9, 131.9, 83.5, 36.2, 30.9, 28.8, 22.0, 18.4, 15.6, 13.7; IR (neat) ν (cm−1) 2965, 2934, 2876, 1814, 1702, 1467, 1429, 1389, 1370, 1344, 1287, 1216, 1191, 1166, 1109, 1081, 1058; MS (70 ev, EI) m/z (%): 218 (M+(37Cl), 0.33), 216 (M+(35Cl), 0.99), 173 (100); Anal. Calcd For C11H17ClO2: C, 60.97; H, 7.91. Found: C, 61.09; H, 7.97.
The following compounds were prepared according to this procedure.
(2) (S,Z)-α-(1-Chlorobenzylidene)-β-ethyl-β-lactone ((S,Z)-2d). The mixture of (S)-1-phenylpent-1-yn-3-ol (805.4 mg, 5.0 mmol, ee > 99%) and anhydrous CuCl2 (3.3611 g, 25.0 mmol) were stirred in 50 mL of dry THF at room temperature for about 10 min followed by the addition of PdCl2 (88.3 mg, 0.50 mmol). Then the mixture was stirred for 4.2 h under 20 atm of CO atmosphere at 40 °C to afford (S,Z)-2d (723.3 mg, 65%) (eluent: petroleum ether/ethyl acetate = 8/1, then recrystallization in n-hexane and ethyl acetate): ee > 99% as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, λ = 230 nm, tR = 14.0 min (major)): [α]20D = −96.1 (c = 1.14, CHCl3); white solid, m.p. 83–84 °C (n-hexane/ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.58–7.51 (m, 2H, Ar–H), 7.51–7.42 (m, 3H, Ar–H), 5.35 (dd, J1 = 7.5 Hz, J2 = 3.0 Hz, 1H, OCH) 1.95–1.78 (m, 1H, one proton in CH2), 1.72–1.52 (m, 1H, one proton in CH2), 0.92 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 161.6, 134.2, 133.5, 132.8, 131.4, 128.9, 128.0, 81.2, 24.4, 8.2; IR (KBr) ν (cm−1) 3063, 2976, 2922, 2871, 1806, 1675, 1588, 1579, 1495, 1461, 1445, 1309, 1267, 1236, 1184, 1151, 1129, 1077, 1060, 1024; MS (70 ev, EI), m/z (%): 224 (M+(37Cl), 12.73), 222 (M+(35Cl), 38.89), 221 (100); Anal. Calcd for C12H11ClO2: C, 64.73; H, 4.98. Found: C, 64.75; H, 4.95.
2, 0.8 mL min−1, λ = 230 nm, tR = 14.0 min (major)): [α]20D = −96.1 (c = 1.14, CHCl3); white solid, m.p. 83–84 °C (n-hexane/ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.58–7.51 (m, 2H, Ar–H), 7.51–7.42 (m, 3H, Ar–H), 5.35 (dd, J1 = 7.5 Hz, J2 = 3.0 Hz, 1H, OCH) 1.95–1.78 (m, 1H, one proton in CH2), 1.72–1.52 (m, 1H, one proton in CH2), 0.92 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 161.6, 134.2, 133.5, 132.8, 131.4, 128.9, 128.0, 81.2, 24.4, 8.2; IR (KBr) ν (cm−1) 3063, 2976, 2922, 2871, 1806, 1675, 1588, 1579, 1495, 1461, 1445, 1309, 1267, 1236, 1184, 1151, 1129, 1077, 1060, 1024; MS (70 ev, EI), m/z (%): 224 (M+(37Cl), 12.73), 222 (M+(35Cl), 38.89), 221 (100); Anal. Calcd for C12H11ClO2: C, 64.73; H, 4.98. Found: C, 64.75; H, 4.95.
(3) (Z)-α-(1-Chlorobenzylidene)-β-ethyl-β-lactone ((Z)-2d). The mixture of 1-phenylpent-1-yn-3-ol (799.4 mg, 5.0 mmol) and anhydrous CuCl2 (3.3629 g, 25.0 mmol) were stirred in 50 mL of dry THF at room temperature for about 10 min followed by the addition of PdCl2 (88.5 mg, 0.50 mmol). Then the mixture was stirred for 4.0 h under 20 atm CO atmosphere at 40 °C afforded (Z)-2d (658.5 mg, 59%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 100/1, then recrystallization in n-hexane and ethyl acetate): a white solid: m.p. 54–55 °C (n-hexane/ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.58–7.51 (m, 2H, Ar–H), 7.51–7.42 (m, 3H, Ar–H), 5.35 (dd, J1 = 7.5 Hz, J2 = 3.0 Hz, 1H, OCH), 1.95–1.80 (m, 1H, one proton in CH2), 1.72–1.54 (m, 1H, one proton in CH2), 0.92 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 161.7, 134.2, 133.5, 132.7, 131.4, 128.9, 128.0, 81.2, 24.3, 8.3; IR (KBr) ν (cm−1) 2967, 2936, 2920, 2877, 1797, 1675, 1493, 1447, 1436, 1389, 1311, 1269, 1236, 1161, 1134, 1065; MS (70 ev, EI), m/z (%): 224 (M+(37Cl), 12.73), 222 (M+(35Cl), 39.49), 221 (100); Anal. Calcd for C12H11ClO2: C, 64.73; H, 4.98. Found: C, 64.82; H, 4.97.
petroleum ether/ethyl acetate = 100/1, then recrystallization in n-hexane and ethyl acetate): a white solid: m.p. 54–55 °C (n-hexane/ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.58–7.51 (m, 2H, Ar–H), 7.51–7.42 (m, 3H, Ar–H), 5.35 (dd, J1 = 7.5 Hz, J2 = 3.0 Hz, 1H, OCH), 1.95–1.80 (m, 1H, one proton in CH2), 1.72–1.54 (m, 1H, one proton in CH2), 0.92 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 161.7, 134.2, 133.5, 132.7, 131.4, 128.9, 128.0, 81.2, 24.3, 8.3; IR (KBr) ν (cm−1) 2967, 2936, 2920, 2877, 1797, 1675, 1493, 1447, 1436, 1389, 1311, 1269, 1236, 1161, 1134, 1065; MS (70 ev, EI), m/z (%): 224 (M+(37Cl), 12.73), 222 (M+(35Cl), 39.49), 221 (100); Anal. Calcd for C12H11ClO2: C, 64.73; H, 4.98. Found: C, 64.82; H, 4.97.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 6.12 (s, 4H, Ar–H), 3.89 (s, 12H, 4 × OCH3), 3.83 (s, 6H, 2 × OCH3), 2.96–2.77 (m, 4H, 2 × CH2), 2.24–2.00 (m, 4H, 2 × CH2), 1.90–1.08 (m, 36H, 16 × CH2 and 4 × CH); 13C NMR (75 MHz, CDCl3) δ 164.4, 163.2, 94.5 (t, J = 19.4 Hz), 91.0, 55.8, 55.1, 33.4, 29.7, 28.6, 27.5 (t, J = 5.0 Hz), 27.3 (t, J = 7.1 Hz), 26.5; 31P NMR (121 MHz, CDCl3) δ 17.6; IR (KBr) ν (cm−1) 2925, 2848, 1598, 1580, 1465, 1454, 1405, 1331, 1287, 1266, 1225, 1206, 1180, 1160, 1121, 1089, 1034, 1003. MS (ESI-TOF) 871 (C42H6637ClO6P2Pd+, 100), 869 (C42H6635ClO6P2Pd+, 99.11); Anal. Calcd For C42H66Cl2O6P2Pd: C, 55.66; H, 7.34. Found: C, 55.75; H, 7.44.
ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 6.12 (s, 4H, Ar–H), 3.89 (s, 12H, 4 × OCH3), 3.83 (s, 6H, 2 × OCH3), 2.96–2.77 (m, 4H, 2 × CH2), 2.24–2.00 (m, 4H, 2 × CH2), 1.90–1.08 (m, 36H, 16 × CH2 and 4 × CH); 13C NMR (75 MHz, CDCl3) δ 164.4, 163.2, 94.5 (t, J = 19.4 Hz), 91.0, 55.8, 55.1, 33.4, 29.7, 28.6, 27.5 (t, J = 5.0 Hz), 27.3 (t, J = 7.1 Hz), 26.5; 31P NMR (121 MHz, CDCl3) δ 17.6; IR (KBr) ν (cm−1) 2925, 2848, 1598, 1580, 1465, 1454, 1405, 1331, 1287, 1266, 1225, 1206, 1180, 1160, 1121, 1089, 1034, 1003. MS (ESI-TOF) 871 (C42H6637ClO6P2Pd+, 100), 869 (C42H6635ClO6P2Pd+, 99.11); Anal. Calcd For C42H66Cl2O6P2Pd: C, 55.66; H, 7.34. Found: C, 55.75; H, 7.44.
(1) Synthesis of (S,Z)-α-(1-butylbenzylidene)-β-ethyl-β-lactone ((S,Z)-4a). Typical procedure (condition A). A rubber-capped Schlenk vessel was dried with flame under vacuum and backfilled with nitrogen for three times. Then Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.3 mg, 0.012 mmol), K3PO4·3H2O (107.1 mg, 0.40 mmol), phenylboronic acid (29.4 mg, 0.24 mmol), and 1 mL of toluene were added into the Schlenk vessel sequentially. After being stirred for about 10 min at room temperature, (S,Z)-2a (40.7 mg, 0.20 mmol, ee > 99%) and 1 mL of toluene were added sequentially to the vessel. The resulting mixture was heated at 110 °C with a preheated oil bath. After 45 min, the reaction was complete as monitored by TLC. Then the reaction mixture was cooled to room temperature and diluted by 10 mL of Et2O and filtered through a short column of silica gel (eluent: 2 × 10 mL of Et2O). Evaporation and purification by chromatography (eluent: petroleum ether/ethyl acetate = 60/1) on silica gel afforded (S,Z)-4a (48.4 mg, 98%) as a liquid in ee > 99%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, λ = 230 nm, tR = 9.9 min (major)): [α]20D = −26.1 (c = 1.56, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.57–7.47 (m, 2H, Ar–H), 7.46–7.34 (m, 3H, Ar–H), 5.03 (dd, J1 = 7.5 Hz, J2 = 3.0 Hz, 1H, OCH), 2.60–2.36 (m, 2H, CH2), 2.20–2.04 (m, 1H, one proton in CH2), 1.96–1.78 (m, 1H, one proton in CH2), 1.44–1.24 (m, 4H, 2 × CH2), 1.11 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.3, 148.5, 134.8, 132.1, 129.5, 128.3, 128.0, 79.0, 33.0, 30.0, 26.3, 22.5, 13.7, 8.6; IR (neat) ν (cm−1) 3056, 3028, 2955, 2936, 2873, 1799, 1683, 1577, 1496, 1459, 1446, 1380, 1367, 1312, 1291, 1240, 1159, 1106, 1049, 1014; MS (70 ev, EI) m/z (%): 245 (M+ + 1, 6.32), 244 (M+, 42.33), 145 (100); HRMS Calcd for C16H20O2 (M+): 244.1463. Found 244.1457.
2, 0.8 mL min−1, λ = 230 nm, tR = 9.9 min (major)): [α]20D = −26.1 (c = 1.56, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.57–7.47 (m, 2H, Ar–H), 7.46–7.34 (m, 3H, Ar–H), 5.03 (dd, J1 = 7.5 Hz, J2 = 3.0 Hz, 1H, OCH), 2.60–2.36 (m, 2H, CH2), 2.20–2.04 (m, 1H, one proton in CH2), 1.96–1.78 (m, 1H, one proton in CH2), 1.44–1.24 (m, 4H, 2 × CH2), 1.11 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.3, 148.5, 134.8, 132.1, 129.5, 128.3, 128.0, 79.0, 33.0, 30.0, 26.3, 22.5, 13.7, 8.6; IR (neat) ν (cm−1) 3056, 3028, 2955, 2936, 2873, 1799, 1683, 1577, 1496, 1459, 1446, 1380, 1367, 1312, 1291, 1240, 1159, 1106, 1049, 1014; MS (70 ev, EI) m/z (%): 245 (M+ + 1, 6.32), 244 (M+, 42.33), 145 (100); HRMS Calcd for C16H20O2 (M+): 244.1463. Found 244.1457.
The following compounds were prepared according to these procedures.
(2) (Z)-α-(1-Butylbenzylidene)-β-ethyl-β-lactone ((Z)-4a). The reaction of (Z)-2a (40.5 mg, 0.20 mmol), phenyl boronic acid 3a (29.5 mg, 0.24 mmol), Pd(OAc)2 (1.2 mg, 0.006 mmol), LB-Phos·HBF4 (5.4 mg, 0.012 mmol), and K3PO4·3H2O (106.9 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (Z)-4a (43.6 mg, 89%) (eluent: petroleum ether/ethyl acetate = 60/1) as a liquid. 1H NMR (300 MHz, CDCl3) δ 7.57–7.48 (m, 2H, Ar–H), 7.46–7.36 (m, 3H, Ar–H), 5.03 (dd, J1 = 7.7 Hz, J2 = 3.2 Hz, 1H, OCH), 2.60–2.36 (m, 2H, CH2), 2.20–2.06 (m, 1H, one proton in CH2), 1.96–1.77 (m, 1H, one proton in CH2), 1.44–1.27 (m, 4H, 2 × CH2), 1.11 (t, J = 7.4 Hz, 3H, CH3), 0.86 (t, J = 7.1 Hz, 3H, CH3); 13C NMR δ 163.3, 148.6, 134.9, 132.1, 129.6, 128.3, 128.0, 79.0, 33.0, 30.1, 26.3, 22.6, 13.8, 8.6; IR (neat) ν (cm−1) 3063, 2959, 2931, 2873, 1801, 1683, 1496, 1461, 1446, 1380, 1312, 1240, 1159, 1128, 1104, 1049; MS (70 ev, EI) m/z (%): 245 (M+ + 1, 4.69), 244 (M+, 30.31), 145 (100); HRMS Calcd for C16H20O2 (M+): 244.1463. Found: 244.1460.
(3) (S,Z)-α-(1-Butyl-(4′-methoxyl)benzylidene)-β-ethyl-β-lactone ((S,Z)-4b). The reaction of (S,Z)-2a (39.1 mg, 0.19 mmol, ee > 99%), 4-methoxylphenylboronic acid 3b (37.4 mg, 0.25 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.4 mg, 0.012 mmol), and K3PO4·3H2O (108.6 mg, 0.41 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (S,Z)-4b (44.7 mg, 84%) (eluent: 30–60 °C petroleum ether/ethyl acetate = 20/1) as a liquid in ee = 99.7%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 97
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1.0 mL min−1, 230 nm, tR = 14.9 min (major), 25.8 (minor)); [α]20D = +5.6 (c = 1.01, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.56 (d, J = 9.0 Hz, 2H, Ar–H), 6.93 (d, J = 9.0 Hz, 2H, Ar–H), 5.00 (dd, J1 = 7.7 Hz, J2 = 3.2 Hz, 1H, OCH), 3.83 (s, 3H, OCH3), 2.58–2.29 (m, 2H, CH2), 2.17–2.02 (m, 1H, one proton in CH2), 1.94–1.75 (m, 1H, one proton in CH2), 1.45–1.20 (m, 4H, 2 × CH2), 1.09 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 6.9 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.6, 160.7, 148.1, 130.4, 129.7, 127.0, 113.7, 78.9, 55.2, 32.7, 30.4, 26.4, 22.6, 13.8, 8.6; IR (neat) ν (cm−1) 2958, 2933, 2870, 2838, 1789, 1674, 1605, 1574, 1514, 1463, 1443, 1380, 1301, 1255, 1182, 1157, 1127, 1097, 1040; MS (70 ev, EI) m/z (%): 275 (M+ + 1, 12.52), 274 (M+, 62.30), 175 (100); HRMS Calcd for C17H22O3 (M+): 274.1569. Found 274.1577.
3, 1.0 mL min−1, 230 nm, tR = 14.9 min (major), 25.8 (minor)); [α]20D = +5.6 (c = 1.01, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.56 (d, J = 9.0 Hz, 2H, Ar–H), 6.93 (d, J = 9.0 Hz, 2H, Ar–H), 5.00 (dd, J1 = 7.7 Hz, J2 = 3.2 Hz, 1H, OCH), 3.83 (s, 3H, OCH3), 2.58–2.29 (m, 2H, CH2), 2.17–2.02 (m, 1H, one proton in CH2), 1.94–1.75 (m, 1H, one proton in CH2), 1.45–1.20 (m, 4H, 2 × CH2), 1.09 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 6.9 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.6, 160.7, 148.1, 130.4, 129.7, 127.0, 113.7, 78.9, 55.2, 32.7, 30.4, 26.4, 22.6, 13.8, 8.6; IR (neat) ν (cm−1) 2958, 2933, 2870, 2838, 1789, 1674, 1605, 1574, 1514, 1463, 1443, 1380, 1301, 1255, 1182, 1157, 1127, 1097, 1040; MS (70 ev, EI) m/z (%): 275 (M+ + 1, 12.52), 274 (M+, 62.30), 175 (100); HRMS Calcd for C17H22O3 (M+): 274.1569. Found 274.1577.
(4) (Z)-α-(1-Butyl-(4′-methoxyl)benzylidene)-β-ethyl-β-lactone ((Z)-4b). The reaction of (Z)-2a (40.6 mg, 0.20 mmol), 4-methoxylphenyl boronic acid 3b (36.8 mg, 0.24 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.3 mg, 0.012 mmol), and K3PO4·3H2O (107.1 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (Z)-4b (50.2 mg, 91%) (eluent: petroleum ether/ethyl acetate = 60/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.56 (d, J = 8.7 Hz, 2H, Ar–H), 6.93 (d, J = 8.7 Hz, 2H, Ar–H), 5.00 (dd, J1 = 7.7 Hz, J2 = 3.2 Hz, 1H, OCH), 3.83 (s, 3H, OCH3), 2.57–2.30 (m, 2H, CH2), 2.17–2.03 (m, 1H, one proton in CH2), 1.94–1.76 (m, 1H, one proton in CH2), 1.47–1.23 (m, 4H, 2 × CH2), 1.10 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.7, 160.7, 148.1, 130.4, 129.7, 127.1, 113.7, 78.9, 55.2, 32.7, 30.4, 26.4, 22.6, 13.8, 8.6; IR (neat) ν (cm−1) 2959, 2934, 2873, 2840, 1795, 1676, 1605, 1574, 1514, 1462, 1377, 1301, 1256, 1183, 1157, 1127, 1097, 1040; MS (70 ev, EI) m/z (%): 275 (M+ + 1, 13.42), 274 (M+, 70.85), 175 (100); HRMS Calcd for C17H22O3 (M+): 274.1569. Found: 274.1574.
(5) (S,Z)-α-(1-Butyl-(3′-methoxyl)benzylidene)-β-ethyl-β-lactone ((S,Z)-4c). The reaction of (S,Z)-2a (80.8 mg, 0.40 mmol, ee > 99%), 3-methoxylphenyl boronic acid 3c (73.4 mg, 0.48 mmol), Pd(OAc)2 (2.7 mg, 0.012 mmol), LB-Phos·HBF4 (10.8 mg, 0.024 mmol), and K3PO4·3H2O (212.0 mg, 0.80 mmol) in 4 mL of toluene at 110 °C under nitrogen afforded (S,Z)-4c (83.9 mg, 77%) (eluent: petroleum ether/ethyl acetate = 50/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 97
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 0.8 mL min−1, 230 nm, tR = 10.0 min (major)): [α]20D = −25.8 (c = 0.89, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.31 (t, J = 8.1 Hz, 1H, Ar–H), 7.15 (s, 1H, Ar–H), 7.08 (d, J = 3.9 Hz 1H, Ar–H), 6.97–6.89 (m, 1H, Ar–H), 5.01 (dd, J1 = 7.8 Hz, J2 = 2.7 Hz, 1H, OCH), 3.84 (s, 3H, OCH3), 2.58–2.33 (m, 2H, CH2), 2.21–2.02 (m, 1H, one proton in CH2), 1.97–1.77 (m, 1H, one proton in CH2), 1.50–1.23 (m, 4H, 2 × CH2), 1.10 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 6.6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 159.3, 148.3, 136.1, 132.1, 129.3, 120.1, 115.4, 113.7, 79.0, 55.3, 33.0, 30.1, 26.3, 22.6, 13.8, 8.6; IR (neat) ν (cm−1) 2959, 2930, 2873, 2838, 1801, 1684, 1599, 1579, 1489, 1464, 1430, 1374, 1319, 1290, 1235, 1181, 1153, 1106, 1050; MS (70 ev, EI) m/z (%): 275 (M+ + 1, 18.18), 274 (M+, 100); HRMS Calcd for C17H22O3 (M+): 274.1569. Found 274.1565.
3, 0.8 mL min−1, 230 nm, tR = 10.0 min (major)): [α]20D = −25.8 (c = 0.89, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.31 (t, J = 8.1 Hz, 1H, Ar–H), 7.15 (s, 1H, Ar–H), 7.08 (d, J = 3.9 Hz 1H, Ar–H), 6.97–6.89 (m, 1H, Ar–H), 5.01 (dd, J1 = 7.8 Hz, J2 = 2.7 Hz, 1H, OCH), 3.84 (s, 3H, OCH3), 2.58–2.33 (m, 2H, CH2), 2.21–2.02 (m, 1H, one proton in CH2), 1.97–1.77 (m, 1H, one proton in CH2), 1.50–1.23 (m, 4H, 2 × CH2), 1.10 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 6.6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 159.3, 148.3, 136.1, 132.1, 129.3, 120.1, 115.4, 113.7, 79.0, 55.3, 33.0, 30.1, 26.3, 22.6, 13.8, 8.6; IR (neat) ν (cm−1) 2959, 2930, 2873, 2838, 1801, 1684, 1599, 1579, 1489, 1464, 1430, 1374, 1319, 1290, 1235, 1181, 1153, 1106, 1050; MS (70 ev, EI) m/z (%): 275 (M+ + 1, 18.18), 274 (M+, 100); HRMS Calcd for C17H22O3 (M+): 274.1569. Found 274.1565.
(6) (Z)-α-(1-Butyl-(3′-methoxyl)benzylidene)-β-ethyl-β-lactone ((Z)-4c). The reaction of (Z)-2a (40.4 mg, 0.20 mmol), 3-methoxylphenyl boronic acid 3c (36.5 mg, 0.24 mmol), Pd(OAc)2 (1.2 mg, 0.006 mmol), LB-Phos·HBF4 (5.3 mg, 0.012 mmol), and K3PO4·3H2O (107.2 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (Z)-4c (43.8 mg, 80%) (eluent: petroleum ether/ethyl acetate = 50/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.31 (t, J = 6.0 Hz, 1H, Ar–H), 7.15 (t, J = 2.0 Hz, 1H, Ar–H), 7.08 (d, J = 3.9 Hz, 1H, Ar–H), 6.97–6.87 (m, 1H, Ar–H), 5.02 (dd, J1 = 7.8 Hz, J2 = 3.0 Hz, 1H, OCH), 3.84 (s, 3H, OCH3), 2.57–2.33 (m, 2H, CH2), 2.20–2.03 (m, 1H, one proton in CH2), 1.96–1.77 (m, 1H, one proton in CH2), 1.46–1.23 (m, 4H, 2 × CH2), 1.11 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 159.4, 148.3, 136.1, 132.2, 129.3, 120.1, 115.5, 113.7, 79.0, 55.3, 33.0, 30.2, 26.3, 22.6, 13.8, 8.6; IR (neat) ν (cm−1) 2959, 2935, 2873, 2837, 1801, 1685, 1599, 1579, 1489, 1464, 1430, 1380, 1319, 1289, 1234, 1181, 1153, 1127, 1106, 1049; MS (70 ev, EI) m/z (%): 275 (M+ + 1, 17.84), 274 (M+, 100); HRMS Calcd for C17H22O3 (M+): 274.1569. Found: 274.1569.
(7) (S,Z)-α-(1-Butyl-(2′-methoxyl)benzylidene)-β-ethyl-β-lactone ((S,Z)-4d). The reaction of (S,Z)-2a (81.1 mg, 0.40 mmol, ee > 99%), 2-methoxylphenyl boronic acid 3d (73.3 mg, 0.48 mmol), Pd(OAc)2 (2.7 mg, 0.012 mmol), LB-Phos·HBF4 (10.9 mg, 0.024 mmol), and K3PO4·3H2O (212.9 mg, 0.80 mmol) in 4 mL of toluene at 110 °C under nitrogen afforded (S,Z)-4d (90.5 mg, 82%) (eluent: petroleum ether/ethyl acetate = 40/1) as a liquid in ee = 99.6%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 214 nm, tR = 12.4 min (major), tR = 15.6 min (major)); [α]20D = −16.9 (c = 1.04, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.38–7.27 (m, 1H, Ar–H), 7.16 (dd, J1 = 7.5 Hz, J2 = 1.8 Hz, 1H, Ar–H), 7.02–6.87 (m, 2H, Ar–H), 5.07 (dd, J1 = 7.5 Hz, J2 = 3.0 Hz, 1H, OCH), 3.80 (s, 3H, OCH3), 2.57–2.35 (m, 2H, CH2), 2.20–2.04 (m, 1H, one proton in CH2), 1.97–1.80 (m, 1H, one proton in CH2), 1.36–1.22 (m, 4H, 2 × CH2), 1.11 (t, J = 7.4 Hz, 3H, CH3), 0.84 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 156.3, 147.0, 133.2, 130.1, 130.0, 124.8, 120.3, 110.9, 79.4, 55.4, 33.2, 29.5, 26.2, 22.5, 13.8, 8.4; IR (neat) ν (cm−1) 2955, 2935, 2873, 1806, 1698, 1599, 1580, 1491, 1463, 1435, 1380, 1294, 1243, 1161, 1124, 1093, 1050; MS (70 ev, EI) m/z (%): 275 (M+ + 1, 19.03), 274 (M+, 100); HRMS Calcd for C17H22O3 (M+): 274.1569. Found: 274.1577.
2, 0.8 mL min−1, 214 nm, tR = 12.4 min (major), tR = 15.6 min (major)); [α]20D = −16.9 (c = 1.04, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.38–7.27 (m, 1H, Ar–H), 7.16 (dd, J1 = 7.5 Hz, J2 = 1.8 Hz, 1H, Ar–H), 7.02–6.87 (m, 2H, Ar–H), 5.07 (dd, J1 = 7.5 Hz, J2 = 3.0 Hz, 1H, OCH), 3.80 (s, 3H, OCH3), 2.57–2.35 (m, 2H, CH2), 2.20–2.04 (m, 1H, one proton in CH2), 1.97–1.80 (m, 1H, one proton in CH2), 1.36–1.22 (m, 4H, 2 × CH2), 1.11 (t, J = 7.4 Hz, 3H, CH3), 0.84 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 156.3, 147.0, 133.2, 130.1, 130.0, 124.8, 120.3, 110.9, 79.4, 55.4, 33.2, 29.5, 26.2, 22.5, 13.8, 8.4; IR (neat) ν (cm−1) 2955, 2935, 2873, 1806, 1698, 1599, 1580, 1491, 1463, 1435, 1380, 1294, 1243, 1161, 1124, 1093, 1050; MS (70 ev, EI) m/z (%): 275 (M+ + 1, 19.03), 274 (M+, 100); HRMS Calcd for C17H22O3 (M+): 274.1569. Found: 274.1577.
(8) (Z)-α-(1-Butyl-(2′-methoxyl)benzylidene)-β-ethyl-β-lactone ((Z)-4d). The reaction of (Z)-2a (40.2 mg, 0.20 mmol), 2-methoxylphenyl boronic acid 3d (36.7 mg, 0.48 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.5 mg, 0.012 mmol), and K3PO4·3H2O (107.0 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (Z)-4d (35.7 mg, 65%) (eluent: petroleum ether/ethyl acetate = 40/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.38–7.28 (m, 1H, Ar–H), 7.15 (dd, J1 = 7.5 Hz, J2 = 1.8 Hz, 1H, Ar–H), 7.02–6.86 (m, 2H, Ar–H), 5.07 (dd, J1 = 7.4 Hz, J2 = 3.2 Hz, 1H, OCH), 3.80 (s, 3H, OCH3), 2.58–2.35 (m, 2H, CH2), 2.20–2.04 (m, 1H, one proton in CH2), 1.97–1.80 (m, 1H, one proton in CH2), 1.36–1.20 (m, 4H, 2 × CH2), 1.11 (t, J = 7.5 Hz, 3H, CH3), 0.84 (t, J = 6.9 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 156.3, 147.0, 133.1, 130.1, 130.0, 124.8, 120.3, 110.8, 79.4, 55.4, 33.2, 29.5, 26.2, 22.5, 13.8, 8.4; IR (neat) ν (cm−1) 2955, 2935, 2873, 1806, 1699, 1599, 1580, 1491, 1463, 1435, 1380, 1294, 1267, 1243, 1181, 1161, 1124, 1093, 1050; MS (70 ev, EI) m/z (%): 275 (M+ + 1, 18.92), 274 (M+, 100); HRMS Calcd for C17H22O3 (M+): 274.1569. Found: 274.1569.
(9) (S)-α-(1(Z)-(2′(E)-Phenylethenyl)pentylidene)-β-ethyl-β-lactone ((S,Z,E)-4e). The reaction of (S,Z)-2a (80.4 mg, 0.40 mmol, ee > 99%), E-phenylethenyl boronic acid 3e (71.2 mg, 0.48 mmol), Pd(OAc)2 (2.7 mg, 0.012 mmol), LB-Phos·HBF4 (10.8 mg, 0.024 mmol) and K3PO4·3H2O (212.7 mg, 0.8 mmol) in 4 mL of toluene at 110 °C under nitrogen afforded (S,Z,E)-4e (97.3 mg, 91%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 60/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak AD-H, n-hexane–i-PrOH = 98
petroleum ether/ethyl acetate = 60/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak AD-H, n-hexane–i-PrOH = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 230 nm, tR = 10.4 min (major)); [α]20D = −17.0 (c = 0.88, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.61 (d, J = 16.5 Hz, 1H, C
2, 0.8 mL min−1, 230 nm, tR = 10.4 min (major)); [α]20D = −17.0 (c = 0.88, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.61 (d, J = 16.5 Hz, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.57–7.47 (m, 2H, Ar–H), 7.43–7.27 (m, 3H, Ar–H), 6.90 (d, J = 16.5 Hz, 1H, C
CH), 7.57–7.47 (m, 2H, Ar–H), 7.43–7.27 (m, 3H, Ar–H), 6.90 (d, J = 16.5 Hz, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.01 (dd, J1 = 7.8 Hz, J2 = 3.3 Hz, 1H, OCH), 2.43–2.26 (m, 2H, CH2), 2.15–1.97 (m, 1H, one proton in CH2), 1.92–1.73 (m, 1H, one proton in CH2), 1.69–1.32 (m, 4H, 2 × CH2), 1.08 (t, J = 7.4 Hz, 3H, CH3), 0.96 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 164.1, 144.9, 135.9, 134.5, 133.1, 129.0, 128.7, 127.3, 123.5, 79.6, 31.5, 28.6, 26.3, 23.0, 13.8, 8.7; IR (neat) ν (cm−1) 3079, 3056, 3037, 3018, 2959, 2933, 2873, 1793, 1670, 1627, 1573, 1492, 1464, 1449, 1378, 1366, 1321, 1295, 1236, 1148, 1125, 1054; MS (70 ev, EI) m/z (%): 271 (M+ + 1, 4.69), 270 (M+, 16.98), 213 (100); HRMS Calcd for C18H22O2 (M+): 270.1620. Found 270.1627.
CH), 5.01 (dd, J1 = 7.8 Hz, J2 = 3.3 Hz, 1H, OCH), 2.43–2.26 (m, 2H, CH2), 2.15–1.97 (m, 1H, one proton in CH2), 1.92–1.73 (m, 1H, one proton in CH2), 1.69–1.32 (m, 4H, 2 × CH2), 1.08 (t, J = 7.4 Hz, 3H, CH3), 0.96 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 164.1, 144.9, 135.9, 134.5, 133.1, 129.0, 128.7, 127.3, 123.5, 79.6, 31.5, 28.6, 26.3, 23.0, 13.8, 8.7; IR (neat) ν (cm−1) 3079, 3056, 3037, 3018, 2959, 2933, 2873, 1793, 1670, 1627, 1573, 1492, 1464, 1449, 1378, 1366, 1321, 1295, 1236, 1148, 1125, 1054; MS (70 ev, EI) m/z (%): 271 (M+ + 1, 4.69), 270 (M+, 16.98), 213 (100); HRMS Calcd for C18H22O2 (M+): 270.1620. Found 270.1627.
(10) α-(1(Z)-(2′(E)-Phenylethenyl)pentylidene)-β-ethyl-β-lactone ((Z,E)-4e). The reaction of (Z)-2a (40.6 mg, 0.20 mmol), E-phenylethenyl boronic acid 3e (35.6 mg, 0.24 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), 1·HBF4 (5.4 mg, 0.012 mmol), and K3PO4·3H2O (107.1 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (Z,E)-4e9 (43.7 mg, 81%) (eluent: petroleum ether/ethyl acetate = 60/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.61 (d, J = 16.5 Hz, 1H, C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.57–7.50 (m, 2H, Ar–H), 7.43–7.27 (m, 3H, Ar–H), 6.90 (d, J = 16.5 Hz, 1H, C
CH), 7.57–7.50 (m, 2H, Ar–H), 7.43–7.27 (m, 3H, Ar–H), 6.90 (d, J = 16.5 Hz, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.01 (dd, J1 = 7.8 Hz, J2 = 3.3 Hz, 1H, OCH), 2.44–2.25 (m, 2H, CH2), 2.16–1.97 (m, 1H, one proton in CH2), 1.91–1.73 (m, 1H, one proton in CH2), 1.69–1.34 (m, 4H, 2 × CH2), 1.08 (t, J = 7.4 Hz, 3H, CH3), 0.97 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 164.1, 144.9, 135.9, 134.4, 133.1, 129.0, 128.7, 127.3, 123.4, 79.6, 31.4, 28.6, 26.3, 22.9, 13.8, 8.7; IR (neat) ν (cm−1) 3082, 3060, 3044, 3028, 2959, 2933, 2873, 1791, 1669, 1622, 1576, 1496, 1449, 1374, 1317, 1295, 1236, 1148, 1125, 1094, 1054; MS (70 ev, EI) m/z (%): 271 (M+ + 1, 5.52), 270 (M+, 27.71), 213 (100).
CH), 5.01 (dd, J1 = 7.8 Hz, J2 = 3.3 Hz, 1H, OCH), 2.44–2.25 (m, 2H, CH2), 2.16–1.97 (m, 1H, one proton in CH2), 1.91–1.73 (m, 1H, one proton in CH2), 1.69–1.34 (m, 4H, 2 × CH2), 1.08 (t, J = 7.4 Hz, 3H, CH3), 0.97 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 164.1, 144.9, 135.9, 134.4, 133.1, 129.0, 128.7, 127.3, 123.4, 79.6, 31.4, 28.6, 26.3, 22.9, 13.8, 8.7; IR (neat) ν (cm−1) 3082, 3060, 3044, 3028, 2959, 2933, 2873, 1791, 1669, 1622, 1576, 1496, 1449, 1374, 1317, 1295, 1236, 1148, 1125, 1094, 1054; MS (70 ev, EI) m/z (%): 271 (M+ + 1, 5.52), 270 (M+, 27.71), 213 (100).
(11) (S,Z)-α-(1-Butyl-(4′-methyl)benzylidene)-β-ethyl-β-lactone ((S,Z)-4f). The reaction of (S,Z)-2a (40.4 mg, 0.20 mmol, ee > 99%), 4-methylphenyl boronic acid 3f (32.6 mg, 0.24 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.4 mg, 0.012 mmol), and K3PO4·3H2O (106.7 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (S,Z)-4f9 (48.1 mg, 93%) (eluent: petroleum ether/ethyl acetate = 50/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 230 nm, tR = 9.3 min (major)); [α]20D = −10.8 (c = 0.72, CHCl3, Lit. [α]20D = −10.1 (c = 1.0, CHCl3, ee = 99.9%)9): 1H NMR (300 MHz, CDCl3) δ 7.45 (d, J = 8.1 Hz, 2H, Ar–H), 7.22 (d, J = 8.1 Hz, 2H, Ar–H), 5.01 (dd, J1 = 7.7 Hz, J2 = 2.9 Hz, 1H, OCH), 2.61–2.31 (m, 5H, CH3 and CH2), 2.20–2.04 (m, 1H, one proton in CH2), 1.95–1.77 (m, 1H, one proton in CH2), 1.45–1.24 (m, 4H, 2 × CH2), 1.11 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.4, 148.6, 139.7, 131.9, 131.3, 129.0, 127.9, 79.0, 32.9, 30.2, 26.3, 22.6, 21.3, 13.7, 8.5; IR (neat) ν (cm−1) 3028, 2959, 2926, 2873, 1798, 1681, 1610, 1564, 1513, 1460, 1380, 1312, 1291, 1239, 1158, 1128, 1095, 1048, 1020; MS (70 ev, EI) m/z (%): 259 (M+ + 1, 5.06), 258 (M+, 28.77), 159 (100).
2, 0.8 mL min−1, 230 nm, tR = 9.3 min (major)); [α]20D = −10.8 (c = 0.72, CHCl3, Lit. [α]20D = −10.1 (c = 1.0, CHCl3, ee = 99.9%)9): 1H NMR (300 MHz, CDCl3) δ 7.45 (d, J = 8.1 Hz, 2H, Ar–H), 7.22 (d, J = 8.1 Hz, 2H, Ar–H), 5.01 (dd, J1 = 7.7 Hz, J2 = 2.9 Hz, 1H, OCH), 2.61–2.31 (m, 5H, CH3 and CH2), 2.20–2.04 (m, 1H, one proton in CH2), 1.95–1.77 (m, 1H, one proton in CH2), 1.45–1.24 (m, 4H, 2 × CH2), 1.11 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.4, 148.6, 139.7, 131.9, 131.3, 129.0, 127.9, 79.0, 32.9, 30.2, 26.3, 22.6, 21.3, 13.7, 8.5; IR (neat) ν (cm−1) 3028, 2959, 2926, 2873, 1798, 1681, 1610, 1564, 1513, 1460, 1380, 1312, 1291, 1239, 1158, 1128, 1095, 1048, 1020; MS (70 ev, EI) m/z (%): 259 (M+ + 1, 5.06), 258 (M+, 28.77), 159 (100).
(12) (Z)-α-(1-Butyl-(4′-methyl)benzylidene)-β-ethyl-β-lactone ((Z)-4f). The reaction of (Z)-2a (40.6 mg, 0.20 mmol), 4-methylphenyl boronic acid 3f (33.2 mg, 0.24 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.4 mg, 0.012 mmol), and K3PO4·3H2O (106.4 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (Z)-4f9 (50.1 mg, 97%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 50/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.45 (d, J = 8.4 Hz, 2H, Ar–H), 7.22 (d, J = 7.8 Hz, 2H, Ar–H), 5.01 (dd, J1 = 7.7 Hz, J2 = 3.2 Hz, 1H, OCH), 2.60–2.26 (m, 5H, CH3 and CH2), 2.20–2.03 (m, 1H, one proton in CH2), 1.95–1.77 (m, 1H, one proton in CH2), 1.45–1.24 (m, 4H, 2 × CH2), 1.11 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.4, 148.6, 139.8, 131.9, 131.3, 129.0, 127.9, 79.0, 32.9, 30.2, 26.3, 22.6, 21.3, 13.8, 8.5; IR (neat) ν (cm−1) 3025, 2959, 2930, 2873, 1798, 1681, 1610, 1564, 1513, 1460, 1380, 1312, 1291, 1239, 1158, 1128, 1095, 1048, 1020; MS (70 ev, EI) m/z (%): 259 (M+ + 1, 5.05), 258 (M+, 27.60), 159 (100).
petroleum ether/ethyl acetate = 50/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.45 (d, J = 8.4 Hz, 2H, Ar–H), 7.22 (d, J = 7.8 Hz, 2H, Ar–H), 5.01 (dd, J1 = 7.7 Hz, J2 = 3.2 Hz, 1H, OCH), 2.60–2.26 (m, 5H, CH3 and CH2), 2.20–2.03 (m, 1H, one proton in CH2), 1.95–1.77 (m, 1H, one proton in CH2), 1.45–1.24 (m, 4H, 2 × CH2), 1.11 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.4, 148.6, 139.8, 131.9, 131.3, 129.0, 127.9, 79.0, 32.9, 30.2, 26.3, 22.6, 21.3, 13.8, 8.5; IR (neat) ν (cm−1) 3025, 2959, 2930, 2873, 1798, 1681, 1610, 1564, 1513, 1460, 1380, 1312, 1291, 1239, 1158, 1128, 1095, 1048, 1020; MS (70 ev, EI) m/z (%): 259 (M+ + 1, 5.05), 258 (M+, 27.60), 159 (100).
(13) (S,Z)-α-(1-Butyl-(4′-methyl)benzylidene)-β-methyl-β-lactone ((S,Z)-4g). The reaction of (S,Z)-2b (36.8 mg, 0.20 mmol, ee > 99%), 4-methylphenyl boronic acid 3f (33.0 mg, 0.24 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.4 mg, 0.012 mmol), K3PO4·3H2O (106.6 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (S,Z)-4g (34.1 mg, 71%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 40/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 98
petroleum ether/ethyl acetate = 40/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 230 nm, tR = 9.0 min (major)); [α]20D = +9.5 (c = 0.97, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.46 (d, J = 8.1 Hz, 2H, Ar–H), 7.22 (d, J = 8.1 Hz, 2H, Ar–H), 5.12 (q, J = 6.3 Hz, 1H, OCH), 2.60–2.31 (m, 5H, CH3 and CH2), 1.67 (d, J = 6.0 Hz, 3H, CH3), 1.44–1.24 (m, 4H, 2 × CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.3, 148.4, 139.8, 133.0, 131.8, 129.0, 127.9, 74.2, 32.5, 30.2, 22.5, 21.3, 19.5, 13.7; IR (neat) ν (cm−1) 3021, 2957, 2930, 2869, 1798, 1680, 1610, 1513, 1454, 1378, 1339, 1258, 1157, 1107, 1086, 1061, 1014; MS (70 ev, EI) m/z (%): 245 (M+ + 1, 4.22), 244 (M+, 23.54), 143 (100); HRMS Calcd for C16H20O2 (M+): 244.1463. Found 244.1459.
2, 0.8 mL min−1, 230 nm, tR = 9.0 min (major)); [α]20D = +9.5 (c = 0.97, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.46 (d, J = 8.1 Hz, 2H, Ar–H), 7.22 (d, J = 8.1 Hz, 2H, Ar–H), 5.12 (q, J = 6.3 Hz, 1H, OCH), 2.60–2.31 (m, 5H, CH3 and CH2), 1.67 (d, J = 6.0 Hz, 3H, CH3), 1.44–1.24 (m, 4H, 2 × CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.3, 148.4, 139.8, 133.0, 131.8, 129.0, 127.9, 74.2, 32.5, 30.2, 22.5, 21.3, 19.5, 13.7; IR (neat) ν (cm−1) 3021, 2957, 2930, 2869, 1798, 1680, 1610, 1513, 1454, 1378, 1339, 1258, 1157, 1107, 1086, 1061, 1014; MS (70 ev, EI) m/z (%): 245 (M+ + 1, 4.22), 244 (M+, 23.54), 143 (100); HRMS Calcd for C16H20O2 (M+): 244.1463. Found 244.1459.
(14) (Z)-α-(1-Butyl-(4′-methyl)benzylidene)-β-methyl-β-lactone ((Z)-4g). The reaction of (Z)-2b (38.0 mg, 0.20 mmol), 4-methylphenyl boronic acid 3f (33.2 mg, 0.24 mmol), Pd(OAc)2 (1.4 mg, 0.006 mmol), LB-Phos·HBF4 (5.4 mg, 0.012 mmol), and K3PO4·3H2O (107.2 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (Z)-4g (37.9 mg, 77%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 40/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.51–7.42 (m, 2H, Ar–H), 7.22 (d, J = 7.8 Hz, 2H, Ar–H), 5.12 (q, J = 6.2 Hz, 1H, OCH), 2.60–2.33 (m, 5H, CH3 and CH2), 1.67 (d, J = 6.3 Hz, 3H, CH3), 1.46–1.22 (m, 4H, 2 × CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 148.4, 139.8, 133.0, 131.8, 129.1, 127.9, 74.2, 32.5, 30.2, 22.5, 21.3, 19.5, 13.7; IR (neat) ν (cm−1) 3031, 2957, 2931, 2869, 1798, 1679, 1610, 1513, 1454, 1378, 1339, 1258, 1157, 1107, 1090, 1061, 1014; MS (70 ev, EI) m/z (%): 245 (M+ + 1, 4.54), 244 (M+, 26.94), 143 (100); HRMS Calcd for C16H20O2 (M+): 244.1463. Found: 244.1466.
petroleum ether/ethyl acetate = 40/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.51–7.42 (m, 2H, Ar–H), 7.22 (d, J = 7.8 Hz, 2H, Ar–H), 5.12 (q, J = 6.2 Hz, 1H, OCH), 2.60–2.33 (m, 5H, CH3 and CH2), 1.67 (d, J = 6.3 Hz, 3H, CH3), 1.46–1.22 (m, 4H, 2 × CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 148.4, 139.8, 133.0, 131.8, 129.1, 127.9, 74.2, 32.5, 30.2, 22.5, 21.3, 19.5, 13.7; IR (neat) ν (cm−1) 3031, 2957, 2931, 2869, 1798, 1679, 1610, 1513, 1454, 1378, 1339, 1258, 1157, 1107, 1090, 1061, 1014; MS (70 ev, EI) m/z (%): 245 (M+ + 1, 4.54), 244 (M+, 26.94), 143 (100); HRMS Calcd for C16H20O2 (M+): 244.1463. Found: 244.1466.
(15) (S,Z)-α-(1-(2′-Naphthyl)butylidene)-β-ethyl-β-lactone ((S,Z)-4h). The reaction of (S,Z)-2a (80.6 mg, 0.40 mmol, ee > 99%), 2-naphthyl boronic acid 3g (82.7 mg, 0.48 mmol), Pd(OAc)2 (2.8 mg, 0.012 mmol), LB-Phos·HBF4 (10.9 mg, 0.024 mmol), and K3PO4·3H2O (212.9 mg, 0.80 mmol) in 4 mL of toluene 110 °C under nitrogen afforded (S,Z)-4h (101.0 mg, 86%) (eluent: petroleum ether/methylene dichloride = 2/1) as a solid in ee > 99%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 230 nm, tR = 12.8 min (major)); [α]20D = −5.1 (c = 2.26, CHCl3); m.p. 83–84 °C (n-hexane); 1H NMR (300 MHz, CDCl3) δ 8.03 (s, 1H, Ar–H), 7.97–7.78 (m, 3H, Ar–H), 7.73–7.61 (m, 1H, Ar–H), 7.59–7.43 (m, 2H, Ar–H), 5.08 (dd, J1 = 7.7 Hz, J2 = 3.2 Hz, 1H, OCH), 2.74–2.43 (m, 2H, CH2), 2.27–2.05 (m, 1H, one proton in CH2), 2.04–1.80 (m, 1H, one proton in CH2), 1.53–1.23 (m, 4H, 2 × CH2), 1.16, (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 6.9 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.3, 148.5, 133.7, 132.8, 132.3, 128.5, 128.0, 127.8, 127.6, 126.8, 126.3, 125.4, 79.1, 33.0, 30.1, 26.3, 22.5, 13.7, 8.6; IR (KBr) ν (cm−1) 3057, 2959, 2933, 2872, 1797, 1681, 1597, 1504, 1461, 1380, 1311, 1239, 1155, 1123, 1097, 1048, 1017; MS (70 ev, EI) m/z (%): 295 (M+ + 1, 10.17), 294 (M+, 47.97), 237 (100); Anal. Calcd for C20H22O2: C, 81.60; H, 7.53. Found: C, 81.82; H, 7.58.
2, 0.8 mL min−1, 230 nm, tR = 12.8 min (major)); [α]20D = −5.1 (c = 2.26, CHCl3); m.p. 83–84 °C (n-hexane); 1H NMR (300 MHz, CDCl3) δ 8.03 (s, 1H, Ar–H), 7.97–7.78 (m, 3H, Ar–H), 7.73–7.61 (m, 1H, Ar–H), 7.59–7.43 (m, 2H, Ar–H), 5.08 (dd, J1 = 7.7 Hz, J2 = 3.2 Hz, 1H, OCH), 2.74–2.43 (m, 2H, CH2), 2.27–2.05 (m, 1H, one proton in CH2), 2.04–1.80 (m, 1H, one proton in CH2), 1.53–1.23 (m, 4H, 2 × CH2), 1.16, (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 6.9 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.3, 148.5, 133.7, 132.8, 132.3, 128.5, 128.0, 127.8, 127.6, 126.8, 126.3, 125.4, 79.1, 33.0, 30.1, 26.3, 22.5, 13.7, 8.6; IR (KBr) ν (cm−1) 3057, 2959, 2933, 2872, 1797, 1681, 1597, 1504, 1461, 1380, 1311, 1239, 1155, 1123, 1097, 1048, 1017; MS (70 ev, EI) m/z (%): 295 (M+ + 1, 10.17), 294 (M+, 47.97), 237 (100); Anal. Calcd for C20H22O2: C, 81.60; H, 7.53. Found: C, 81.82; H, 7.58.
(16) (Z)-α-(1-(2′-Naphthyl)butylidene)-β-ethyl-β-lactone ((Z)-4h). The reaction of (Z)-2a (40.7 mg, 0.20 mmol), 2-naphthylboronic acid 3g (41.6 mg, 0.24 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.4 mg, 0.012 mmol), K3PO4·3H2O (106.4 mg, 0.4 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (Z)-4h (52.7 mg, 89%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 40/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 8.03 (d, J = 1.2 Hz, 1H, Ar–H), 7.94–7.79 (m, 3H, Ar–H), 7.71–7.61 (m, 1H, Ar–H), 7.57–7.46 (m, 2H, Ar–H), 5.08 (dd, J1 = 7.8 Hz, J2 = 3.0 Hz, 1H, OCH), 2.72–2.45 (m, 2H, CH2), 2.25–2.06 (m, 1H, one proton in CH2), 2.01–1.82 (m, 1H, one proton in CH2), 1.50–1.24 (m, 4H, 2 × CH2), 1.15 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.3, 148.5, 133.7, 132.9, 132.4, 132.3, 128.6, 128.0, 127.8, 127.6, 126.9, 126.3, 125.4, 79.1, 33.0, 30.2, 26.4, 22.6, 13.8, 8.6; IR (neat) ν (cm−1) 3057, 2959, 2933, 2873, 1798, 1681, 1600, 1501, 1461, 1374, 1308, 1239, 1155, 1123, 1097, 1048, 1014; MS (70 ev, EI) m/z (%): 295 (M+ + 1, 10.23), 294 (M+, 47.88), 237 (100); HRMS Calcd for C20H22O2 (M+): 294.1620. Found: 294.1615.
petroleum ether/ethyl acetate = 40/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 8.03 (d, J = 1.2 Hz, 1H, Ar–H), 7.94–7.79 (m, 3H, Ar–H), 7.71–7.61 (m, 1H, Ar–H), 7.57–7.46 (m, 2H, Ar–H), 5.08 (dd, J1 = 7.8 Hz, J2 = 3.0 Hz, 1H, OCH), 2.72–2.45 (m, 2H, CH2), 2.25–2.06 (m, 1H, one proton in CH2), 2.01–1.82 (m, 1H, one proton in CH2), 1.50–1.24 (m, 4H, 2 × CH2), 1.15 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.3, 148.5, 133.7, 132.9, 132.4, 132.3, 128.6, 128.0, 127.8, 127.6, 126.9, 126.3, 125.4, 79.1, 33.0, 30.2, 26.4, 22.6, 13.8, 8.6; IR (neat) ν (cm−1) 3057, 2959, 2933, 2873, 1798, 1681, 1600, 1501, 1461, 1374, 1308, 1239, 1155, 1123, 1097, 1048, 1014; MS (70 ev, EI) m/z (%): 295 (M+ + 1, 10.23), 294 (M+, 47.88), 237 (100); HRMS Calcd for C20H22O2 (M+): 294.1620. Found: 294.1615.
(17) (S,Z)-α-(1-Butylbenzylidene)-β-isopropyl-β-lactone ((S,Z)-4i). The reaction of (S,Z)-2c (86.6 mg, 0.40 mmol, ee = 99%), phenyl boronic acid 3a (59.1 mg, 0.48 mmol), Pd(OAc)2 (2.8 mg, 0.012 mmol), LB-Phos·HBF4 (10.9 mg, 0.024 mmol), K3PO4·3H2O (213.6 mg, 0.80 mmol) in 4 mL of toluene at 110 °C under nitrogen afforded (S,Z)-4i (97.1 mg, 94%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 40/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 98
petroleum ether/ethyl acetate = 40/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 230 nm, tR = 8.1 min (major)): [α]20D = −6.8 (c = 1.02, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.58–7.47 (m, 2H, Ar–H), 7.46–7.32 (m, 3H, Ar–H), 4.98 (d, J = 2.7 Hz, 1H, OCH), 2.63–2.49 (m, 1H, one proton in CH2), 2.49–2.36 (m, 1H, one proton in CH2), 2.36–2.18 (m, 1H, CH), 1.47–1.23 (m, 4H, 2 × CH2), 1.17 (d, J = 7.2 Hz, 3H, CH3), 1.05 (d, J = 6.9 Hz, 3H, CH3), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.3, 148.6, 134.9, 131.3, 129.4, 128.2, 127.9, 82.3, 33.2, 30.6, 29.8, 22.5, 18.7, 14.9, 13.6; IR (neat) ν (cm−1) 3059, 3022, 2963, 2932, 2873, 1801, 1682, 1573, 1494, 1466, 1445, 1387, 1375, 1341, 1296, 1244, 1187, 1148, 1105, 1072, 1038: MS (70 ev, EI) m/z (%): 259 (M+ + 1, 3.06), 258 (M+, 21.03), 145 (100); HRMS Calcd for C17H22O2 (M+): 258.1620. Found: 258.1609.
2, 0.8 mL min−1, 230 nm, tR = 8.1 min (major)): [α]20D = −6.8 (c = 1.02, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.58–7.47 (m, 2H, Ar–H), 7.46–7.32 (m, 3H, Ar–H), 4.98 (d, J = 2.7 Hz, 1H, OCH), 2.63–2.49 (m, 1H, one proton in CH2), 2.49–2.36 (m, 1H, one proton in CH2), 2.36–2.18 (m, 1H, CH), 1.47–1.23 (m, 4H, 2 × CH2), 1.17 (d, J = 7.2 Hz, 3H, CH3), 1.05 (d, J = 6.9 Hz, 3H, CH3), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.3, 148.6, 134.9, 131.3, 129.4, 128.2, 127.9, 82.3, 33.2, 30.6, 29.8, 22.5, 18.7, 14.9, 13.6; IR (neat) ν (cm−1) 3059, 3022, 2963, 2932, 2873, 1801, 1682, 1573, 1494, 1466, 1445, 1387, 1375, 1341, 1296, 1244, 1187, 1148, 1105, 1072, 1038: MS (70 ev, EI) m/z (%): 259 (M+ + 1, 3.06), 258 (M+, 21.03), 145 (100); HRMS Calcd for C17H22O2 (M+): 258.1620. Found: 258.1609.
(18) (Z)-α-(1-Butylbenzylidene)-β-isopropyl-β-lactone ((Z)-4i). The reaction of (Z)-2c (43.5 mg, 0.20 mmol), phenylboronic acid 3a (30.1 mg, 0.25 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.4 mg, 0.012 mmol), K3PO4·3H2O (107.1 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (Z)-4i (45.0 mg, 87%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 50/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.55–7.46 (m, 2H, Ar–H), 7.46–7.35 (m, 3H, Ar–H), 4.97 (d, J = 3.0 Hz, 1H, OCH), 2.63–2.34 (m, 2H, CH2), 2.34–2.16 (m, 1H, CH), 1.44–1.23 (m, 4H, 2 × CH2), 1.16 (d, J = 6.9 Hz, 3H, CH3), 1.04 (d, J = 6.9 Hz, 3H, CH3), 0.86 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.4, 148.7, 135.0, 131.4, 129.5, 128.3, 128.0, 82.4, 33.4, 30.7, 29.9, 22.6, 18.8, 15.0, 13.7; IR (neat) ν (cm−1) 2963, 2932, 2873, 1801, 1682, 1489, 1465, 1445, 1383, 1338, 1244, 1185, 1147, 1105, 1068, 1038: MS (70 ev, EI) m/z (%): 259 (M+ + 1, 3.42), 258 (M+, 21.00), 145 (100); HRMS Calcd for C17H22O2 (M+): 258.1620. Found: 258.1612.
petroleum ether/ethyl acetate = 50/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.55–7.46 (m, 2H, Ar–H), 7.46–7.35 (m, 3H, Ar–H), 4.97 (d, J = 3.0 Hz, 1H, OCH), 2.63–2.34 (m, 2H, CH2), 2.34–2.16 (m, 1H, CH), 1.44–1.23 (m, 4H, 2 × CH2), 1.16 (d, J = 6.9 Hz, 3H, CH3), 1.04 (d, J = 6.9 Hz, 3H, CH3), 0.86 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.4, 148.7, 135.0, 131.4, 129.5, 128.3, 128.0, 82.4, 33.4, 30.7, 29.9, 22.6, 18.8, 15.0, 13.7; IR (neat) ν (cm−1) 2963, 2932, 2873, 1801, 1682, 1489, 1465, 1445, 1383, 1338, 1244, 1185, 1147, 1105, 1068, 1038: MS (70 ev, EI) m/z (%): 259 (M+ + 1, 3.42), 258 (M+, 21.00), 145 (100); HRMS Calcd for C17H22O2 (M+): 258.1620. Found: 258.1612.
(19) (S,Z)-α-(1-(4′-Methoxylphenyl)benzylidene)-β-ethyl-β-lactone ((S,Z)-4j). The reaction of (S,Z)-2d (88.9 mg, 0.40 mmol, ee > 99%), 4-methoxylphenyl boronic acid 3a (73.4 mg, 0.48 mmol), Pd(OAc)2 (2.7 mg, 0.012 mmol), LB-Phos·HBF4 (10.8 mg, 0.024 mmol), K3PO4·3H2O (212.8 mg, 0.80 mmol) in 4 mL of toluene at 110 °C under nitrogen afforded (S,Z)-4j (109.2 mg, 93%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 20/1) as a solid in ee > 99%, as determined by HPLC (Chiralpak OJ-H, n-hexane–i-PrOH = 98
petroleum ether/ethyl acetate = 20/1) as a solid in ee > 99%, as determined by HPLC (Chiralpak OJ-H, n-hexane–i-PrOH = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 230 nm, tR = 35.8 min (major)): [α]20D = +65.1 (c = 1.08, CHCl3); m.p. 68–69 °C (n-hexane/ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.55–7.47 (m, 2H, Ar–H), 7.45–7.35 (m, 3H, Ar–H), 7.29–7.18 (m, 2H, Ar–H), 6.94–6.85 (m, 2H, Ar–H), 5.17 (dd, J1 = 7.2 Hz, J2 = 3.3 Hz, 1H, OCH), 3.84 (s, 3H, OCH3), 1.64–1.47 (m, 1H, one proton in CH2), 1.46–1.30 (m, 1H, one proton in CH2), 0.85 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.8, 161.1, 146.7, 137.7, 131.8, 130.8, 129.3, 129.2, 128.6, 128.2, 113.6, 79.6, 55.3, 24.7, 8.1; IR (KBr) ν (cm−1) 3053, 2971, 2931, 2908, 2876, 2841, 1783, 1650, 1604, 1513, 1493, 1459, 1445, 1297, 1257, 1174, 1161, 1128, 1077, 1064, 1023; MS (70 ev, EI) m/z (%): 295 (M+ + 1, 18.33), 294 (M+, 90.71), 265 (100); HRMS Calcd for C19H18O3 (M+): 294.1256. Found: 294.1255.
2, 0.8 mL min−1, 230 nm, tR = 35.8 min (major)): [α]20D = +65.1 (c = 1.08, CHCl3); m.p. 68–69 °C (n-hexane/ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.55–7.47 (m, 2H, Ar–H), 7.45–7.35 (m, 3H, Ar–H), 7.29–7.18 (m, 2H, Ar–H), 6.94–6.85 (m, 2H, Ar–H), 5.17 (dd, J1 = 7.2 Hz, J2 = 3.3 Hz, 1H, OCH), 3.84 (s, 3H, OCH3), 1.64–1.47 (m, 1H, one proton in CH2), 1.46–1.30 (m, 1H, one proton in CH2), 0.85 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.8, 161.1, 146.7, 137.7, 131.8, 130.8, 129.3, 129.2, 128.6, 128.2, 113.6, 79.6, 55.3, 24.7, 8.1; IR (KBr) ν (cm−1) 3053, 2971, 2931, 2908, 2876, 2841, 1783, 1650, 1604, 1513, 1493, 1459, 1445, 1297, 1257, 1174, 1161, 1128, 1077, 1064, 1023; MS (70 ev, EI) m/z (%): 295 (M+ + 1, 18.33), 294 (M+, 90.71), 265 (100); HRMS Calcd for C19H18O3 (M+): 294.1256. Found: 294.1255.
(20) (Z)-α-(1-(4′-Methoxylphenyl)benzylidene)-β-ethyl-β-lactone ((Z)-4j). The reaction of (Z)-2d (44.7 mg, 0.20 mmol), 4-methoxylphenyl boronic acid 3a (36.9 mg, 0.24 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.4 mg, 0.012 mmol), K3PO4·3H2O (106.8 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (Z)-4j (57.7 mg, 97%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 40/1) as a solid: m.p. 97–98 °C (n-hexane/ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.56–7.47 (m, 2H, Ar–H), 7.45–7.35 (m, 3H, Ar–H), 7.29–7.19 (m, 2H, Ar–H), 6.94–6.85 (m, 2H, Ar–H), 5.17 (dd, J1 = 7.2 Hz, J2 = 3.6 Hz, 1H, OCH), 3.84 (s, 3H, OCH3), 1.64–1.48 (m, 1H, one proton in CH2) 1.48–1.30 (m, 1H, one proton in CH2), 0.85 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.8, 161.1, 146.7, 137.7, 131.8, 130.8, 129.3, 129.2, 128.6, 128.2, 113.6, 79.5, 55.3, 24.7, 8.1; IR (KBr) ν (cm−1) 2996, 2968, 2962, 2932, 2873, 2834, 1802, 1670, 1606, 1514, 1456, 1441, 1389, 1320, 1272, 1250, 1182, 1161, 1125, 1077, 1053, 1029; MS (70 ev, EI) m/z (%): 295 (M+ + 1, 19.56), 294 (M+, 92.91), 265 (100); Anal. Calcd for C19H18O3 (M+): C: 77.53, H: 6.16. Found: C: 77.44, H, 5.99.
petroleum ether/ethyl acetate = 40/1) as a solid: m.p. 97–98 °C (n-hexane/ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.56–7.47 (m, 2H, Ar–H), 7.45–7.35 (m, 3H, Ar–H), 7.29–7.19 (m, 2H, Ar–H), 6.94–6.85 (m, 2H, Ar–H), 5.17 (dd, J1 = 7.2 Hz, J2 = 3.6 Hz, 1H, OCH), 3.84 (s, 3H, OCH3), 1.64–1.48 (m, 1H, one proton in CH2) 1.48–1.30 (m, 1H, one proton in CH2), 0.85 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.8, 161.1, 146.7, 137.7, 131.8, 130.8, 129.3, 129.2, 128.6, 128.2, 113.6, 79.5, 55.3, 24.7, 8.1; IR (KBr) ν (cm−1) 2996, 2968, 2962, 2932, 2873, 2834, 1802, 1670, 1606, 1514, 1456, 1441, 1389, 1320, 1272, 1250, 1182, 1161, 1125, 1077, 1053, 1029; MS (70 ev, EI) m/z (%): 295 (M+ + 1, 19.56), 294 (M+, 92.91), 265 (100); Anal. Calcd for C19H18O3 (M+): C: 77.53, H: 6.16. Found: C: 77.44, H, 5.99.
(21) (S)-α-(1(E)-(Pent-1′(E)-enyl)benzylidene)-β-ethyl-β-lactone ((S,E,E)-4k). The reaction of (S,Z)-2d (88.5 mg, 0.40 mmol, ee > 99%), (1E)-pentenyl boronic acid 3h (55. 3 mg, 0.48 mmol), Pd(OAc)2 (2.7 mg, 0.012 mmol), LB-Phos·HBF4 (10.7 mg, 0.024 mmol), K3PO4·3H2O (213.5 mg, 0.80 mmol) in 4 mL of toluene at 110 °C under nitrogen afforded (S,E,E)-4k (96.6 mg, 95%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 40/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak AD-H, n-hexane–i-PrOH = 98
petroleum ether/ethyl acetate = 40/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak AD-H, n-hexane–i-PrOH = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 230 nm, tR = 8.0 min (major), 8.5 min (minor)): [α]20D = −120.7 (c = 1.04, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.45–7.32 (m, 3H, Ar–H), 7.25–7.15 (m, 2H, Ar–H), 7.10 (d, J = 15.9 Hz, 1H, C
2, 0.8 mL min−1, 230 nm, tR = 8.0 min (major), 8.5 min (minor)): [α]20D = −120.7 (c = 1.04, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.45–7.32 (m, 3H, Ar–H), 7.25–7.15 (m, 2H, Ar–H), 7.10 (d, J = 15.9 Hz, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.84 (dt, J1 = 15.9 Hz, J2 = 7.4 Hz, 1H, C
CH), 5.84 (dt, J1 = 15.9 Hz, J2 = 7.4 Hz, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.97 (dd, J1 = 7.1 Hz, J2 = 3.5 Hz, 1H, OCH), 2.18 (q, J = 7.3 Hz, 2H, CH2), 1.59–1.21 (m, 4H, 2 × CH2), 0.89 (t, J = 7.4 Hz, 3H, CH3), 0.78 (t, J = 7.5 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 164.1, 145.0, 143.2, 134.6, 131.3, 128.8, 128.5, 126.7, 79.9, 35.1, 25.3, 22.0, 13.7, 8.2; IR (neat) ν (cm−1), 2964, 2932, 2874, 1795, 1664, 1462, 1444, 1322, 1310, 1301, 1236, 1163, 1106, 1054; MS (70 ev, EI) m/z (%): 257 (M+ + 1, 2.24), 256 (M+, 11.22), 185 (100); HRMS Calcd for C17H20O2 (M+): 256.1463. Found: 256.1458.
CH), 4.97 (dd, J1 = 7.1 Hz, J2 = 3.5 Hz, 1H, OCH), 2.18 (q, J = 7.3 Hz, 2H, CH2), 1.59–1.21 (m, 4H, 2 × CH2), 0.89 (t, J = 7.4 Hz, 3H, CH3), 0.78 (t, J = 7.5 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 164.1, 145.0, 143.2, 134.6, 131.3, 128.8, 128.5, 126.7, 79.9, 35.1, 25.3, 22.0, 13.7, 8.2; IR (neat) ν (cm−1), 2964, 2932, 2874, 1795, 1664, 1462, 1444, 1322, 1310, 1301, 1236, 1163, 1106, 1054; MS (70 ev, EI) m/z (%): 257 (M+ + 1, 2.24), 256 (M+, 11.22), 185 (100); HRMS Calcd for C17H20O2 (M+): 256.1463. Found: 256.1458.
(22) α-(1(E)-(Pent-1′(E)-enyl)benzylidene)-β-ethyl-β-lactone ((E,E)-4k). The reaction of (Z)-2d (45.1 mg, 0.40 mmol), (1E)-pentenylboronic acid 3h (27.9 mg, 0.24 mmol), Pd(OAc)2 (1.3 mg, 0.006 mmol), LB-Phos·HBF4 (5.4 mg, 0.012 mmol), K3PO4·3H2O (107.6 mg, 0.40 mmol) in 2 mL of toluene at 110 °C under nitrogen afforded (E,E)-4k (48.1 mg, 92%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 40/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.52–7.32 (m, 3H, Ar–H), 7.25–7.15 (m, 2H, Ar–H), 7.10 (d, J = 15.6 Hz, 1H, C
petroleum ether/ethyl acetate = 40/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.52–7.32 (m, 3H, Ar–H), 7.25–7.15 (m, 2H, Ar–H), 7.10 (d, J = 15.6 Hz, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.94–5.74 (m, 1H, C
CH), 5.94–5.74 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.97 (dd, J1 = 7.1 Hz, J2 = 3.8 Hz, 1H, OCH), 2.18 (q, J = 7.1 Hz, 2H, CH2), 1.57–1.19 (m, 4H, 2 × CH2), 0.89 (t, J = 7.5 Hz, 3H, CH3), 0.78 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 164.1, 145.0, 143.2, 134.6, 131.3, 128.8, 128.5, 126.7, 79.9, 35.1, 25.3, 22.0, 13.7, 8.3; IR (neat) ν (cm−1) 2964, 2931, 2869, 1794, 1665, 1629, 1462, 1444, 1382, 1322, 1304, 1293, 1236, 1163, 1106, 1073, 1054; MS (70 ev, EI) m/z (%): 257 (M+ + 1, 1.52), 256 (M+, 8.33), 185 (100); HRMS Calcd for C17H20O2 (M+): 256.1463. Found: 256.1469.
CH), 4.97 (dd, J1 = 7.1 Hz, J2 = 3.8 Hz, 1H, OCH), 2.18 (q, J = 7.1 Hz, 2H, CH2), 1.57–1.19 (m, 4H, 2 × CH2), 0.89 (t, J = 7.5 Hz, 3H, CH3), 0.78 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 164.1, 145.0, 143.2, 134.6, 131.3, 128.8, 128.5, 126.7, 79.9, 35.1, 25.3, 22.0, 13.7, 8.3; IR (neat) ν (cm−1) 2964, 2931, 2869, 1794, 1665, 1629, 1462, 1444, 1382, 1322, 1304, 1293, 1236, 1163, 1106, 1073, 1054; MS (70 ev, EI) m/z (%): 257 (M+ + 1, 1.52), 256 (M+, 8.33), 185 (100); HRMS Calcd for C17H20O2 (M+): 256.1463. Found: 256.1469.
(1) Synthesis of (S,E)-α-(1-cyclopropylbenzylidene)-β-ethyl-β-lactone ((S,E)-4l). Typical procedure A rubber-capped Schlenk vessel was dried with flame under vacuum and backfilled with nitrogen for three times. Then Pd(OAc)2 (2.6 mg, 0.012 mmol), LB-Phos·HBF4 (10.9 mg, 0.024 mmol), K3PO4·3H2O (212.8 mg, 0.80 mmol), cyclopropylboronic acid 3i (41.4 mg, 0.48 mmol), 2 mL of toluene and 0.4 mL of H2O were added into the Schlenk vessel sequentially. Then (S,Z)-2d (89.1 mg, 0.40 mmol, ee > 99%) and 2 mL of toluene were added sequentially to the vessel. The resulting mixture was heated at 110 °C with a preheated oil bath. After 3.0 h, the reaction was complete as monitored by TLC. Then the reaction mixture was diluted by 10 mL of Et2O and filtered through a short column of silica gel (eluent: 2 × 10 mL of Et2O). Evaporation and purification by chromatography (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 30/1) on silica gel and recrystallization (8 mL of n-hexane) afforded (S,E)-4l (42.4 mg, 46%) as a solid in 99.5% ee, as determined by HPLC (Chiralpak OJ-H, n-hexane–i-PrOH = 98
petroleum ether/ethyl acetate = 30/1) on silica gel and recrystallization (8 mL of n-hexane) afforded (S,E)-4l (42.4 mg, 46%) as a solid in 99.5% ee, as determined by HPLC (Chiralpak OJ-H, n-hexane–i-PrOH = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, λ = 230 nm, tR = 16.6 min (major), 15.6 min (minor)); [α]20D = −29.0 (c = 1.02, CHCl3): m.p. 87–88 °C (n-hexane); 1H NMR (300 MHz, CDCl3) δ 7.42–7.29 (m, 3H, Ar–H), 7.14–7.00 (m, 2H, Ar–H), 4.78 (dd, J1 = 7.2 Hz, J2 = 3.6 Hz, 1H, OCH), 2.66–2.48 (m, 1H, CH), 1.53–1.21 (m, 2H, CH2), 0.99–0.84 (m, 2H, CH2), 0.84–0.63 (m, 4H, CH3 and one proton in CH2), 0.53–0.37 (m, 1H, one proton in CH2); 13C NMR (75 MHz, CDCl3) δ 164.8, 151.7, 133.5, 131.9, 128.5, 128.3, 127.8, 79.9, 25.5, 14.9, 8.3, 6.9, 6.2; IR (KBr) ν (cm−1) 3087, 3012, 2987, 2963, 2940, 2922, 2878, 1792, 1697, 1489, 1463, 1441, 1420, 1306, 1235, 1194, 1168, 1119, 1055, 1011; MS (70 ev, EI) m/z (%): 229 (M+ + 1, 2.36), 228 (M+, 14.38), 153 (100); Anal. Calcd for C15H16O2: C, 78.92; H, 7.06. Found: C, 79.08; H, 7.36.
2, 0.8 mL min−1, λ = 230 nm, tR = 16.6 min (major), 15.6 min (minor)); [α]20D = −29.0 (c = 1.02, CHCl3): m.p. 87–88 °C (n-hexane); 1H NMR (300 MHz, CDCl3) δ 7.42–7.29 (m, 3H, Ar–H), 7.14–7.00 (m, 2H, Ar–H), 4.78 (dd, J1 = 7.2 Hz, J2 = 3.6 Hz, 1H, OCH), 2.66–2.48 (m, 1H, CH), 1.53–1.21 (m, 2H, CH2), 0.99–0.84 (m, 2H, CH2), 0.84–0.63 (m, 4H, CH3 and one proton in CH2), 0.53–0.37 (m, 1H, one proton in CH2); 13C NMR (75 MHz, CDCl3) δ 164.8, 151.7, 133.5, 131.9, 128.5, 128.3, 127.8, 79.9, 25.5, 14.9, 8.3, 6.9, 6.2; IR (KBr) ν (cm−1) 3087, 3012, 2987, 2963, 2940, 2922, 2878, 1792, 1697, 1489, 1463, 1441, 1420, 1306, 1235, 1194, 1168, 1119, 1055, 1011; MS (70 ev, EI) m/z (%): 229 (M+ + 1, 2.36), 228 (M+, 14.38), 153 (100); Anal. Calcd for C15H16O2: C, 78.92; H, 7.06. Found: C, 79.08; H, 7.36.
The following compounds were prepared according to this procedure.
(2) (E)-α-(1-Cyclopropylbenzylidene)-β-ethyl-β-lactone ((E)-4l). The reaction of (Z)-2d (90.1 mg, 0.41 mmol), n-butyl boronic acid 3i (41.6 mg, 0.48 mmol), Pd(OAc)2 (2.6 mg, 0.012 mmol), LB-Phos·HBF4 (10.8 mg, 0.024 mmol), K3PO4·3H2O (213.2 mg, 0.80 mmol) in the mixture solvent of 4 mL of toluene and 0.4 mL of H2O at 110 °C under nitrogen afforded (E)-4l (22.6 mg, 24%) (eluent:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 30/1, then recrystallization in 8 mL n-hexane) as a solid: m.p. 68–69 °C (n-hexane); 1H NMR (300 MHz, CDCl3) δ 7.42–7.28 (m, 3H, Ar–H), 7.14–6.98 (m, 2H, Ar–H), 4.78 (dd, J1 = 7.2 Hz, J2 = 3.9 Hz, 1H, OCH), 2.66–2.49 (m, 1H, CH), 1.53–1.18 (m, 2H, CH2), 0.99–0.83 (m, 2H, CH2), 0.83–0.63 (m, 4H, CH3 and one proton in CH2), 0.53–0.35 (m, 1H, one proton in CH2); 13C NMR (75 MHz, CDCl3) δ 164.8, 151.7, 133.5, 131.9, 128.5, 128.3, 127.8, 79.9, 25.5, 14.9, 8.3, 6.9, 6.2; IR (KBr) ν (cm−1) 3009, 2965, 2940, 2917, 2873, 1790, 1695, 1603, 1496, 1461, 1435, 1312, 1261, 1235, 1194, 1169, 1119, 1094, 1056, 1042, 1012; MS (70 ev, EI) m/z (%): 229 (M+ + 1, 2.15), 228 (M+, 13.49), 153 (100); Anal. Calcd for C15H16O2: C, 78.92. H, 7.06. Found: C, 78.94; H, 7.14.
petroleum ether/ethyl acetate = 30/1, then recrystallization in 8 mL n-hexane) as a solid: m.p. 68–69 °C (n-hexane); 1H NMR (300 MHz, CDCl3) δ 7.42–7.28 (m, 3H, Ar–H), 7.14–6.98 (m, 2H, Ar–H), 4.78 (dd, J1 = 7.2 Hz, J2 = 3.9 Hz, 1H, OCH), 2.66–2.49 (m, 1H, CH), 1.53–1.18 (m, 2H, CH2), 0.99–0.83 (m, 2H, CH2), 0.83–0.63 (m, 4H, CH3 and one proton in CH2), 0.53–0.35 (m, 1H, one proton in CH2); 13C NMR (75 MHz, CDCl3) δ 164.8, 151.7, 133.5, 131.9, 128.5, 128.3, 127.8, 79.9, 25.5, 14.9, 8.3, 6.9, 6.2; IR (KBr) ν (cm−1) 3009, 2965, 2940, 2917, 2873, 1790, 1695, 1603, 1496, 1461, 1435, 1312, 1261, 1235, 1194, 1169, 1119, 1094, 1056, 1042, 1012; MS (70 ev, EI) m/z (%): 229 (M+ + 1, 2.15), 228 (M+, 13.49), 153 (100); Anal. Calcd for C15H16O2: C, 78.92. H, 7.06. Found: C, 78.94; H, 7.14.
(1) Synthesis of (S,E)-α-(1-butylbenzylidene)-β-ethyl-β-lactone ((S,E)-4m). Typical procedure (condition B). A rubber-capped Schlenk vessel was dried with flame under vacuum and backfilled with nitrogen for three times. Then Pd(OAc)2 (2.2 mg, 0.01 mmol), LB-Phos·HBF4 (9.1 mg, 0.02 mmol), K2CO3 (124.1 mg, 0.90 mmol), n-butylboronic acid 3j (40.8 mg, 0.40 mmol), 1 mL of toluene and 0.2 mL of H2O were added into the Schlenk vessel sequentially and stirred. Then (S,Z)-2d (44.3 mg, 0.20 mmol, ee > 99%) and 1 mL of toluene were added sequentially to the vessel. The resulting mixture was heated at 110 °C with a preheated oil bath. After 3.0 h, the reaction was complete as monitored by TLC. Then the reaction mixture was diluted by 10 mL of Et2O and filtered through a short column of silica gel (eluent: 2 × 10 mL of Et2O). Evaporation and purification by chromatography ((eluent: n-hexane–CH2Cl2 = 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (300 mL), then n-hexane/ethyl acetate = 100/1)) afforded (S,E)-4m (35.5 mg, 73%) as a liquid in ee 99.3%, as determined by HPLC (Chiralpak AD-H, n-hexane–i-PrOH = 98
1 (300 mL), then n-hexane/ethyl acetate = 100/1)) afforded (S,E)-4m (35.5 mg, 73%) as a liquid in ee 99.3%, as determined by HPLC (Chiralpak AD-H, n-hexane–i-PrOH = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 230 nm, tR = 10.7 min (major)): [α]20D = +23.4 (c = 0.94, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.47–7.35 (m, 3H, Ar–H), 7.34–7.24 (m, 2H, Ar–H), 5.20 (dd, J1 = 7.2 Hz, J2 = 3.3 Hz, 1H, OCH), 3.28–3.07 (m, 1H, one proton in CH2), 2.82–2.65 (m, 1H, one proton in CH2), 1.78–1.57 (m, 1H, one proton in CH2), 1.56–1.24 (m, 5H, 2 × CH2 and one proton in CH2), 0.94–0.71 (m, 6H, 2 × CH3); 13C NMR (75 MHz, CDCl3) δ 164.7, 149.1, 136.3, 132.4, 129.3, 128.8, 127.2, 80.5, 31.5, 30.5, 24.7, 22.2, 13.7, 8.3; IR (neat) ν (cm−1) 2960, 2930, 2870, 2860, 1797, 1687, 1459, 1442, 1378, 1309, 1239, 1161, 1131, 1088, 1054; MS (70 ev, EI) m/z (%): 245 (M+ + 1, 2.76), 244 (M+, 20.81), 129 (100); HRMS Calcd for C16H20O2 (M+): 244.1463. Found: 244.1458.
2, 0.8 mL min−1, 230 nm, tR = 10.7 min (major)): [α]20D = +23.4 (c = 0.94, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.47–7.35 (m, 3H, Ar–H), 7.34–7.24 (m, 2H, Ar–H), 5.20 (dd, J1 = 7.2 Hz, J2 = 3.3 Hz, 1H, OCH), 3.28–3.07 (m, 1H, one proton in CH2), 2.82–2.65 (m, 1H, one proton in CH2), 1.78–1.57 (m, 1H, one proton in CH2), 1.56–1.24 (m, 5H, 2 × CH2 and one proton in CH2), 0.94–0.71 (m, 6H, 2 × CH3); 13C NMR (75 MHz, CDCl3) δ 164.7, 149.1, 136.3, 132.4, 129.3, 128.8, 127.2, 80.5, 31.5, 30.5, 24.7, 22.2, 13.7, 8.3; IR (neat) ν (cm−1) 2960, 2930, 2870, 2860, 1797, 1687, 1459, 1442, 1378, 1309, 1239, 1161, 1131, 1088, 1054; MS (70 ev, EI) m/z (%): 245 (M+ + 1, 2.76), 244 (M+, 20.81), 129 (100); HRMS Calcd for C16H20O2 (M+): 244.1463. Found: 244.1458.
The following compounds were prepared according to these procedure.
(2) (E)-α-(1-Butylbenzylidene)-β-ethyl-β-lactone ((E)-4m). The reaction of (Z)-2d (89.6 mg, 0.40 mmol), n-butylboronic acid 3j (81.7 mg, 0.80 mmol), Pd(OAc)2 (4.6 mg, 0.02 mmol), LB-Phos·HBF4 (18.0 mg, 0.04 mmol), K2CO3 (248.4 mg, 1.80 mmol) in the mixture solvent of 4 mL of toluene and 0.4 mL of H2O at 110 °C under nitrogen afforded (E)-4m (73.9 mg, containing 0.5% 1,3,5-trimethoxylbenzene as determined by 1H NMR analysis, yield 75%) (eluent: 30–60 °C petroleum ether/CH2Cl2 = 2/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.47–7.35 (m, 3H, Ar–H), 7.34–7.22 (m, 2H, Ar–H), 5.20 (dd, J1 = 7.1 Hz, J2 = 3.2 Hz, 1H, OCH), 3.26–3.09 (m, 1H, one proton in CH2), 2.81–2.65 (m, 1H, one proton in CH2), 1.78–1.58 (m, 1H, one proton in CH2), 1.56–1.24 (m, 5H, 2 × CH2 and one proton in CH2), 0.93–0.75 (m, 6H, 2 × CH3); 13C NMR (75 MHz, CDCl3) δ 164.7, 149.1, 136.3, 132.4, 129.4, 128.8, 127.2, 80.5, 31.6, 30.5, 24.7, 22.2, 13.7, 8.3; IR (neat) ν (cm−1) 2960, 2930, 2870, 2860, 1797, 1688, 1460, 1442, 1309, 1239, 1161, 1131, 1088, 1054; MS (70 ev, EI) m/z (%): 245 (M+ + 1, 2.59), 244 (M+, 19.19), 129 (100); HRMS Calcd for C16H20O2 (M+): 244.1463. Found 244.1464.
(3) (S,E)-α-(1-Hexylbenzylidene)-β-ethyl-β-lactone ((S,E)-4n). The reaction of (S,Z)-2d (44.6 mg, 0.20 mmol, ee > 99%), n-hexylboronic acid 3k (51.4 mg, 0.40 mmol), Pd(OAc)2 (2.3 mg, 0.01 mmol), LB-Phos·HBF4 (9.0 mg, 0.02 mmol), and K2CO3 (125.0 mg, 0.90 mmol) in the mixture solvent of 2 mL of toluene and 0.2 mL of H2O at 110 °C under nitrogen afforded (S,E)-4n (39.9 mg, containing 0.8% 1,3,5-trimethoxylbenzene as determined by 1H NMR analysis, yield 72%) (eluent: petroleum ether/ethyl acetate = 100/1) as a liquid in ee > 99%, as determined by HPLC (Chiralpak AS-H, n-hexane–i-PrOH = 98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 230 nm, tR = 7.2 min (major)): [α]20D = +20.6 (c = 1.18, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.48–7.36 (m, 3H, Ar–H), 7.35–7.24 (m, 2H, Ar–H), 5.20 (dd, J1 = 7.1 Hz, J2 = 3.5 Hz, 1H, OCH), 3.25–3.09 (m, 1H, one proton in CH2), 2.83–2.66 (m, 1H, one proton in CH2), 1.77–1.58 (m, 1H, one proton in CH2), 1.53–1.14 (m, 9H, 4 × CH2 and one proton in CH2), 0.93–0.77 (m, 6H, 2 × CH3); 13C NMR (75 MHz, CDCl3) δ 164.7, 149.1, 136.3, 132.4, 129.3, 128.8, 127.2, 80.5, 31.8, 31.4, 28.7, 28.4, 24.7, 22.4, 14.0, 8.3; IR (neat) ν (cm−1) 3059, 2957, 2929, 2858, 1798, 1688, 1603, 1578, 1495, 1461, 1445, 1379, 1309, 1239, 1160, 1130, 1090, 1055; MS (70 ev, EI) m/z (%): 273 (M+ + 1, 1.33), 272 (M+, 8.97), 143 (100); HRMS Calcd for C18H24O2 (M+): 272.1776. Found: 272.1777.
2, 0.8 mL min−1, 230 nm, tR = 7.2 min (major)): [α]20D = +20.6 (c = 1.18, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.48–7.36 (m, 3H, Ar–H), 7.35–7.24 (m, 2H, Ar–H), 5.20 (dd, J1 = 7.1 Hz, J2 = 3.5 Hz, 1H, OCH), 3.25–3.09 (m, 1H, one proton in CH2), 2.83–2.66 (m, 1H, one proton in CH2), 1.77–1.58 (m, 1H, one proton in CH2), 1.53–1.14 (m, 9H, 4 × CH2 and one proton in CH2), 0.93–0.77 (m, 6H, 2 × CH3); 13C NMR (75 MHz, CDCl3) δ 164.7, 149.1, 136.3, 132.4, 129.3, 128.8, 127.2, 80.5, 31.8, 31.4, 28.7, 28.4, 24.7, 22.4, 14.0, 8.3; IR (neat) ν (cm−1) 3059, 2957, 2929, 2858, 1798, 1688, 1603, 1578, 1495, 1461, 1445, 1379, 1309, 1239, 1160, 1130, 1090, 1055; MS (70 ev, EI) m/z (%): 273 (M+ + 1, 1.33), 272 (M+, 8.97), 143 (100); HRMS Calcd for C18H24O2 (M+): 272.1776. Found: 272.1777.
(4) (E)-α-(1-Hexylbenzylidene)-β-ethyl-β-lactone ((E)-4n). The reaction of (Z)-2d (88.3 mg, 0.40 mmol), n-hexylboronic acid 3k (104.3 mg, 0.80 mmol), Pd(OAc)2 (2.7 mg, 0.012 mmol), LB-Phos·HBF4 (10.8 mg, 0.024 mmol), and K2CO3 (244.4 mg, 1.77 mmol) in the mixture solvent of 4 mL of toluene and 0.4 mL of H2O at 110 °C under nitrogen afforded (E)-4n (87.0 mg, containing 1.0% 1,3,5-trimethoxylbenzene as determined by 1H NMR analysis, yield 80%) (eluent: petroleum ether/ethyl acetate = 80/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 7.48–7.35 (m, 3H, Ar–H), 7.34–7.24 (m, 2H, Ar–H), 5.20 (dd, J1 = 7.4 Hz, J2 = 3.5 Hz, 1H, OCH), 3.25–3.08 (m, 1H, one proton in CH2), 2.82–2.65 (m, 1H, one proton in CH2), 1.78–1.58 (m, 1H, one proton in CH2), 1.54–1.12 (m, 9H, 4 × CH2 and one proton in CH2), 0.96–0.72 (m, 6H, 2 × CH3); 13C NMR (75 MHz, CDCl3), δ 164.7, 149.1, 136.3, 132.4, 129.3, 128.8, 127.2, 80.5, 31.8, 31.4, 28.7, 28.4, 24.7, 22.4, 14.0, 8.3; IR (neat) ν (cm−1) 3059, 2957, 2929, 2858, 1798, 1688, 1492, 1460, 1445, 1382, 1309, 1239, 1160, 1131, 1090, 1055; MS (70 ev, EI) m/z (%): 273 (M+ + 1, 3.93), 272 (M+, 21.87), 143 (100); HRMS Calcd for C18H24O2 (M+): 272.1776. Found: 272.1776.
(5) (S)-α-(5-Nonylidene)-β-ethyl-β-lactone ((S)-4o). The reaction of (S,Z)-2a (80.1 mg, 0.40 mmol, ee > 99%), n-butylboronic acid 3j (123.4 mg, 1.21 mmol), PdCl2(LB-Phos)2 (18.3 mg, 0.02 mmol), and K2CO3 (248.4 mg, 1.80 mmol) in the mixture solvent of 4 mL of toluene and 0.4 mL of H2O at 110 °C under nitrogen afforded (S)-4o (48.7 mg, 55%) (eluent: 30–60 °C petroleum ether/CH2Cl2 = 2/1) as a liquid in ee = 99.4%, as determined by HPLC (Chiralpak AS-H, n-hexane–i-PrOH = 98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.8 mL min−1, 230 nm, tR = 6.3 min (major), 8.4 min (minor)): [α]20D = −64.5 (c = 1.21, CHCl3); 1H NMR (300 MHz, CDCl3) δ 4.89 (dd, J1 = 7.5 Hz, J2 = 3.3 Hz, 1H, OCH), 2.60–2.39 (m, 2H, CH2), 2.14–1.90 (m, 3H, CH2 and one proton in CH2), 1.84–1.64 (m, 1H, one proton in CH2), 1.56–1.18 (m, 8H, 4 × CH2), 1.03 (t, J = 7.4 Hz, 3H, CH3), 0.97–0.79 (m, 6H, 2 × CH3); 13C NMR (75 MHz, CDCl3) δ 164.6, 152.0, 131.4, 79.5, 31.7, 30.7, 30.1, 29.5, 26.3, 22.7, 22.4, 13.8, 8.6; IR (neat) ν (cm−1) 2959, 2933, 2874, 2861, 1800, 1709, 1462, 1380, 1310, 1243, 1147, 1123, 1095, 1053, 1013; MS (70 ev, EI) m/z (%): 224 (M+, 2.75), 195 (100); HRMS Calcd for C14H24O2 (M+): 224.1776. Found: 224.1780.
2, 0.8 mL min−1, 230 nm, tR = 6.3 min (major), 8.4 min (minor)): [α]20D = −64.5 (c = 1.21, CHCl3); 1H NMR (300 MHz, CDCl3) δ 4.89 (dd, J1 = 7.5 Hz, J2 = 3.3 Hz, 1H, OCH), 2.60–2.39 (m, 2H, CH2), 2.14–1.90 (m, 3H, CH2 and one proton in CH2), 1.84–1.64 (m, 1H, one proton in CH2), 1.56–1.18 (m, 8H, 4 × CH2), 1.03 (t, J = 7.4 Hz, 3H, CH3), 0.97–0.79 (m, 6H, 2 × CH3); 13C NMR (75 MHz, CDCl3) δ 164.6, 152.0, 131.4, 79.5, 31.7, 30.7, 30.1, 29.5, 26.3, 22.7, 22.4, 13.8, 8.6; IR (neat) ν (cm−1) 2959, 2933, 2874, 2861, 1800, 1709, 1462, 1380, 1310, 1243, 1147, 1123, 1095, 1053, 1013; MS (70 ev, EI) m/z (%): 224 (M+, 2.75), 195 (100); HRMS Calcd for C14H24O2 (M+): 224.1776. Found: 224.1780.
(6) α-(5-Nonylidene)-β-ethyl-β-lactone (4o). The reaction of (Z)-2a (40.4 mg, 0.20 mmol), n-butylboronic acid 3j (61.0 mg, 0.60 mmol), PdCl2(LB-Phos)2 (9.2 mg, 0.01 mmol), and K2CO3 (125.5 mg, 0.91 mmol) in the mixture solvent of 2 mL of toluene and 0.2 mL of H2O at 110 °C under nitrogen afforded 4o (25.3 mg, 56%) (eluent: 30–60 °C petroleum ether/CH2Cl2 = 2/1) as a liquid: 1H NMR (300 MHz, CDCl3) δ 4.89 (dd, J1 = 7.5 Hz, J2 = 3.0 Hz, 1H, OCH), 2.60–2.39 (m, 2H, CH2), 2.14–1.90 (m, 3H, CH2 and one proton in CH2), 1.84–1.64 (m, 1H, one proton in CH2), 1.56–1.17 (m, 8H, 4 × CH2), 1.02 (t, J = 7.5 Hz, 3H, CH3), 0.97–0.79 (m, 6H, 2 × CH3); 13C NMR (75 MHz, CDCl3) δ 164.6, 152.1, 131.4, 79.5, 31.7, 30.7, 30.1, 29.4, 26.3, 22.7, 22.4, 13.8, 8.6; IR (neat) ν (cm−1) 2959, 2932, 2874, 2860, 1799, 1709, 1463, 1380, 1310, 1257, 1243, 1147, 1123, 1095, 1053, 1013; MS (70 ev, EI) m/z (%): 224 (M+, 2.58), 195 (100); HRMS Calcd for C14H24O2 (M+) : 224.1776. Found: 224.1777.
(1) Synthesis of (S,Z)-α-(1-(2′-thienyl)benzylidene)-β-ethyl-β-lactone ((S,Z)-4p). Typical procedure. A rubber-capped Schlenk vessel was dried with flame under vacuum and backfilled with nitrogen for three times. Then PdCl2(LB-Phos)2 (18.2 mg, 0.02 mmol), K3PO4·3H2O (319.1 mg, 1.20 mmol), 2-thienylboronic acid 3l (153.6 mg, 1.20 mmol), and 2 mL of toluene were added into the Schlenk vessel sequentially and stirred. Then (S,Z)-2d (88.7 mg, 0.40 mmol, ee > 99%) and 2 mL of toluene were added sequentially to the vessel. The resulting mixture was heated to reflux with a preheated oil bath. After 4.8 h, the reaction was complete as monitored by TLC. Then the reaction mixture was cooled and diluted by 10 mL of Et2O and filtered through a short column of silica gel (eluent: 2 × 10 mL of Et2O). Evaporation and purification by chromatography (petroleum ether/ethyl acetate = 30/1) afforded (S,Z)-4p (83.3 mg, 77%) as a yellow solid in ee > 99%, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1.0 mL min−1, 230 nm, tR = 10.0 min (major), 14.7 min (minor)); [α]20D = −104.6 (c = 1.02, CHCl3); m.p. 72–73 °C (n-hexane and ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.89 (dd, J1 = 3.8 Hz, J2 = 0.8 Hz, 1H, Ar–H), 7.53–7.39 (m, 4H, Ar–H), 7.39–7.30 (m, 2H, Ar–H), 7.16–7.08 (m, 1H, Ar–H), 5.00 (dd, J1 = 7.2 Hz, J2 = 3.6 Hz, 1H, OCH), 1.54–1.20 (m, 2H, CH2), 0.83 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 139.5, 139.1, 136.9, 132.2, 130.4, 129.8, 129.4, 128.65, 128.62, 128.1, 79.2, 25.0, 8.1; IR (KBr) ν (cm−1) 2969, 2936, 2871, 1787, 1650, 1462, 1441, 1415, 1374, 1293, 1226, 1154, 1125, 1101, 1052; MS (70 ev, EI) m/z (%): 271 (M+ + 1, 12.60), 270 (M+, 65.41), 240 (100); Anal. Calcd for C16H14O2S: C, 71.08; H, 5.22. Found: C, 70.62; H, 5.16.
2, 1.0 mL min−1, 230 nm, tR = 10.0 min (major), 14.7 min (minor)); [α]20D = −104.6 (c = 1.02, CHCl3); m.p. 72–73 °C (n-hexane and ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.89 (dd, J1 = 3.8 Hz, J2 = 0.8 Hz, 1H, Ar–H), 7.53–7.39 (m, 4H, Ar–H), 7.39–7.30 (m, 2H, Ar–H), 7.16–7.08 (m, 1H, Ar–H), 5.00 (dd, J1 = 7.2 Hz, J2 = 3.6 Hz, 1H, OCH), 1.54–1.20 (m, 2H, CH2), 0.83 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 139.5, 139.1, 136.9, 132.2, 130.4, 129.8, 129.4, 128.65, 128.62, 128.1, 79.2, 25.0, 8.1; IR (KBr) ν (cm−1) 2969, 2936, 2871, 1787, 1650, 1462, 1441, 1415, 1374, 1293, 1226, 1154, 1125, 1101, 1052; MS (70 ev, EI) m/z (%): 271 (M+ + 1, 12.60), 270 (M+, 65.41), 240 (100); Anal. Calcd for C16H14O2S: C, 71.08; H, 5.22. Found: C, 70.62; H, 5.16.
The following compounds were prepared according to this procedure.
(2) (Z)-α-(1-(2′-Thienyl)benzylidene)-β-ethyl-β-lactone ((Z)-4p). The reaction of (Z)-2d (44.4 mg, 0.20 mmol), 2-thienylboronic acid 3k (76.7 mg, 0.60 mmol), PdCl2(LB-Phos)2 (9.1 mg, 0.01 mmol), K3PO4·3H2O (159.2 mg, 0.60 mmol) in 2 mL of toluene afford (Z)-4p (41.1 mg, 76%) (eluent: petroleum ether/ethyl acetate = 30/1) as a yellow solid: m.p. 75–76 °C (n-hexane and ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.90 (dd, J1 = 3.8 Hz, J2 = 1.1 Hz, 1H, Ar–H), 7.52–7.39 (m, 4H, Ar–H), 7.39–7.31 (m, 2H, Ar–H), 7.14–7.07 (m, 1H, Ar–H), 4.99 (dd, J1 = 7.1 Hz, J2 = 3.5 Hz, 1H, OCH), 1.53–1.18 (m, 2H, CH2), 0.82 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.2, 139.4, 139.1, 136.9, 132.2, 130.4, 129.7, 129.4, 128.61, 128.59, 128.1, 79.2, 24.9, 8.1; IR (KBr) ν (cm−1) 3097, 2969, 2936, 2878, 1789, 1650, 1489, 1460, 1444, 1415, 1368, 1299, 1244, 1227, 1154, 1125, 1100, 1052; MS (70 ev, EI) m/z (%): 271 (M+ + 1, 13.24), 270 (M+, 13.24), 241 (100). Anal. Calcd for C16H14O2S: C, 71.08; H, 5.22. Found: C, 71.03; H, 5.19.
(3) (S,Z)-α-(1-(2′-Benzothienyl)benzylidene)-β-ethyl-β-lactone ((S,Z)-4q). The reaction of (S,Z)-2d (89.2 mg, 0.40 mmol, ee > 99%), 2-benzothienylboronic acid 3m (214.4 mg, 1.20 mmol), PdCl2(LB-Phos)2 (18.2 mg, 0.02 mmol), and K3PO4·3H2O (319.6 mg, 1.20 mmol) in 4 mL of toluene under nitrogen afforded (S,Z)-4q (106.3 mg, 83%) (eluent: petroleum ether/ethyl acetate = 50/1) as a yellow solid in 99.1% ee, as determined by HPLC (Chiralpak OD-H, n-hexane–i-PrOH = 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1.0 mL min−1, 230 nm, tR = 9.16 min (major), 17.9 min (minor)): [α]20D = −13.7 (c = 1.12, CHCl3); m.p. 102–103 °C (n-hexane and ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.94 (s, 1H, Ar–H), 7.84–7.74 (m, 2H, Ar–H), 7.54–7.43 (m, 3H, Ar–H), 7.43–7.33 (m, 4H, Ar–H), 5.05 (dd, J1 = 7.2 Hz, J2 = 3.3 Hz, 1H, OCH), 1.58–1.18 (m, 2H, CH2), 0.86 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 162.8, 141.7, 139.5, 139.2, 138.8, 136.5, 132.3, 129.6, 129.3, 128.7, 126.0, 124.8, 124.7, 122.1, 79.4, 24.9, 8.2; IR (KBr) ν (cm−1) 3058, 3027, 2970, 2937, 2877, 1790, 1651, 1594, 1504, 1457, 1443, 1431, 1343, 1299, 1264, 1239, 1192, 1145, 1120, 1099, 1057, 1030; MS (70 ev, EI) m/z (%): 321 (M+ + 1, 20.28), 320 (M+, 100); Anal. Calcd for C20H16O2S: C, 74.97; H, 5.03. Found: C, 74.77; H, 5.01.
10, 1.0 mL min−1, 230 nm, tR = 9.16 min (major), 17.9 min (minor)): [α]20D = −13.7 (c = 1.12, CHCl3); m.p. 102–103 °C (n-hexane and ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.94 (s, 1H, Ar–H), 7.84–7.74 (m, 2H, Ar–H), 7.54–7.43 (m, 3H, Ar–H), 7.43–7.33 (m, 4H, Ar–H), 5.05 (dd, J1 = 7.2 Hz, J2 = 3.3 Hz, 1H, OCH), 1.58–1.18 (m, 2H, CH2), 0.86 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 162.8, 141.7, 139.5, 139.2, 138.8, 136.5, 132.3, 129.6, 129.3, 128.7, 126.0, 124.8, 124.7, 122.1, 79.4, 24.9, 8.2; IR (KBr) ν (cm−1) 3058, 3027, 2970, 2937, 2877, 1790, 1651, 1594, 1504, 1457, 1443, 1431, 1343, 1299, 1264, 1239, 1192, 1145, 1120, 1099, 1057, 1030; MS (70 ev, EI) m/z (%): 321 (M+ + 1, 20.28), 320 (M+, 100); Anal. Calcd for C20H16O2S: C, 74.97; H, 5.03. Found: C, 74.77; H, 5.01.
(4) (Z)-α-(1-(2′-Benzothienyl)benzylidene)-β-ethyl-β-lactone ((Z)-4q). The reaction of (Z)-2d (44.8 mg, 0.20 mmol), 2-benzothienylboronic acid 3m (106.8 mg, 0.60 mmol), PdCl2(LB-Phos)2 (9.2 mg, 0.01 mmol), and K3PO4·3H2O (159.5 mg, 0.60 mmol) in 2 mL of toluene under nitrogen afforded (Z)-4q (59.3 mg, 92%) (eluent: petroleum ether/ethyl acetate = 30/1) as a yellow solid: m.p. 145–146 °C (n-hexane and ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.94 (s, 1H, Ar–H), 7.85–7.71 (m, 2H, Ar–H), 7.55–7.42 (m, 3H, Ar–H), 7.42–7.33 (m, 4H, Ar–H), 5.04 (dd, J1 = 7.1 Hz, J2 = 3.5 Hz, 1H, OCH), 1.60–1.20 (m, 2H, CH2), 0.86 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 162.8, 141.7, 139.5, 139.2, 138.8, 136.6, 132.3, 129.6, 129.3, 128.7, 126.0, 124.8, 124.7, 122.2, 79.4, 24.9, 8.1; IR (KBr) ν (cm−1) 2964, 2928, 1793, 1651, 1590, 1501, 1455, 1443, 1378, 1365, 1300, 1253, 1237, 1190, 1160, 1146, 1118, 1075, 1058, 1029; MS (70 ev, EI) m/z (%): 321 (M+ + 1, 31.86), 320 (M+, 100). Anal. Calcd for C20H16O2S: C, 74.97; H, 5.03. Found: C, 74.73; H, 5.04.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1.0 mL min−1, λ = 230 nm, tR = 9.7 min (major)): [α]20D = +6.5 (c = 0.99, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.62–7.46 (m, 2H, Ar–H), 6.97–6.83 (m, 2H, Ar–H), 5.00 (dd, J1 = 7.7 Hz, J2 = 2.9 Hz, 1H, OCH), 3.83 (s, 3H, OCH3), 2.58–2.29 (m, 2H, CH2), 2.17–2.02 (m, 1H, one proton in CH2), 1.94–1.75 (m, 1H, one proton in CH2), 1.45–1.20 (m, 4H, 2 × CH2), 1.09 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 6.8 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.7, 160.7, 148.1, 130.4, 129.7, 127.0, 113.7, 78.9, 55.2, 32.7, 30.4, 26.4, 22.6, 13.8, 8.6.
5, 1.0 mL min−1, λ = 230 nm, tR = 9.7 min (major)): [α]20D = +6.5 (c = 0.99, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.62–7.46 (m, 2H, Ar–H), 6.97–6.83 (m, 2H, Ar–H), 5.00 (dd, J1 = 7.7 Hz, J2 = 2.9 Hz, 1H, OCH), 3.83 (s, 3H, OCH3), 2.58–2.29 (m, 2H, CH2), 2.17–2.02 (m, 1H, one proton in CH2), 1.94–1.75 (m, 1H, one proton in CH2), 1.45–1.20 (m, 4H, 2 × CH2), 1.09 (t, J = 7.4 Hz, 3H, CH3), 0.87 (t, J = 6.8 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 163.7, 160.7, 148.1, 130.4, 129.7, 127.0, 113.7, 78.9, 55.2, 32.7, 30.4, 26.4, 22.6, 13.8, 8.6.
Acknowledgements
Financial support from the National Basic Research Program of China (2009CB825300) and National Natural Science Foundation of China (21172192) are greatly appreciated. Shengming Ma is a Qiu Shi Adjunct Professor at Zhejiang University. We thank Jingqiang Kuang in our group for reproducing the results presented in Table 2, entries 4, 11 and Table 5, entry 2.References
- (a) W. Shen, Tetrahedron Lett., 1997, 38, 5575 CrossRef CAS; (b) A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 1998, 37, 3387 CrossRef CAS; (c) J. Lemo, K. Heuzé and D. Astruc, Chem. Commun., 2007, 4351 RSC; (d) T. Fujihara, S. Yoshida, J. Terao and Y. Tsuji, Org. Lett., 2009, 11, 2121 CrossRef CAS; (e) D. W. Old, J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1998, 120, 9722 CrossRef CAS; (f) J. P. Wolfe and S. L. Buchwald, Angew. Chem., Int. Ed., 1999, 38, 2413 CrossRef CAS; (g) X. Huang, K. W. Anderson, D. Zim, L. Jiang, A. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 6653 CrossRef CAS; (h) J. E. Milne and S. L. Buchwald, J. Am. Chem. Soc., 2004, 126, 13028 CrossRef CAS; (i) S. D. Walker, T. E. Barder, J. R. Martinelli and S. L. Buchwald, Angew. Chem., Int. Ed., 2004, 43, 1871 CrossRef CAS; (j) F. Y. Kwong, W. H. Lam, C. H. Yeung, K. S. Chan and A. S. C. Chan, Chem. Commun., 2004, 1922 RSC; (k) C. M. So, C. C. Yeung, C. P. Lau and F. Y. Kwong, J. Org. Chem., 2008, 73, 7803 CrossRef CAS; (l) P. Y. Wong, W. K. Chow, K. H. Chung, C. M. So, C. P. Lau and F. Y. Kwong, Chem. Commun., 2011, 47, 8328 RSC; (m) N. Kataoka, Q. Shelby, J. P. Stambuli and J. F. Hartwig, J. Org. Chem., 2002, 67, 5553 CrossRef CAS; (n) C. Baillie, L. Zhang and J. Xiao, J. Org. Chem., 2004, 69, 7779 CrossRef CAS; (o) X. Bei, H. W. Turner, W. H. Weinberg and A. S. Guram, J. Org. Chem., 1999, 64, 6797 CrossRef CAS; (p) F. Rataboul, A. Zapf, R. Jackstell, S. Harkal, T. Riermeier, A. Monsees, U. Dingerdissen and M. Beller, Chem.–Eur. J., 2004, 10, 2983 CrossRef CAS; (q) F. Y. Kwong and A. S. C. Chan, Synlett, 2008, 1440 CrossRef CAS.
- B. Lü, C. Fu and S. Ma, Tetrahedron Lett., 2010, 51, 1284 Search PubMed.
- B. Lü, P. Li, C. Fu, L. Xue, Z. Lin and S. Ma, Adv. Synth. Catal., 2011, 353, 100 Search PubMed.
- B. Lü, C. Fu and S. Ma, Chem.–Eur. J., 2010, 16, 6434 Search PubMed.
- (a) S. Ma, B. Wu and S. Zhao, Org. Lett., 2003, 5, 4429 CrossRef CAS; (b) S. Ma, B. Wu, X. Jiang and S. Zhao, J. Org. Chem., 2005, 70, 2568 CrossRef CAS.
- (a) For review of Suzuki coupling within alkyl boronic acid see: H. Douncet, Eur. J. Org. Chem., 2008, 2013 Search PubMed; (b) K. Matos and J. A. Soderquist, J. Org. Chem., 1998, 63, 461 CrossRef CAS.
- Crystal data for compound trans-PdCl2(LB-Phos)2: C46H76Cl2O7P2Pd, MW = 980.31, triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , final R indices [I > 2σ(I)], R1 = 0.0290, wR2 = 0.0839; R indices (all data), R1 = 0.0323, wR2 = 0.0881; a = 12.8490(5) Å, b = 13.1603(5) Å, c = 17.5483(6) Å, α = 73.847(1)°, β = 68.570(1)°, γ = 65.921(1)°, V = 2492.57(16) Å3. T = 296 K, wavelength: 0.71073 Å, Z = 2, reflections collected/unique: 29003/8738. (Rint = 0.0178); number of observations [>2σ(I)] 8018, parameters: 526. Supplementary crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, CCDC: 888344.
, final R indices [I > 2σ(I)], R1 = 0.0290, wR2 = 0.0839; R indices (all data), R1 = 0.0323, wR2 = 0.0881; a = 12.8490(5) Å, b = 13.1603(5) Å, c = 17.5483(6) Å, α = 73.847(1)°, β = 68.570(1)°, γ = 65.921(1)°, V = 2492.57(16) Å3. T = 296 K, wavelength: 0.71073 Å, Z = 2, reflections collected/unique: 29003/8738. (Rint = 0.0178); number of observations [>2σ(I)] 8018, parameters: 526. Supplementary crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, CCDC: 888344. - Crystal data for compound (S,Z)-4p: C16H14O2S, MW = 270.33, monoclinic, space group P 21, final R indices [I > 2σ(I)], R1 = 0.0685, wR2 = 0.1807; R indices (all data), R1 = 0.0880, wR2 = 0.2043; a = 9.3629(6) Å, b = 16.6080(8) Å, c = 9.8929(5) Å, α = 90°, β = 115.250 (7)°, γ = 90°, V = 1391.37(17) Å3. T = 293 K, wavelength: 1.54184 Å, Z = 4, reflections collected/unique: 13412/4948 (Rint = 0.0516); number of observations [>2σ(I)] 3803, parameters: 346. Supplementary crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, CCDC: 888345.
- S. Ma, X. Jiang, X. Cheng and H. Hou, Adv. Synth. Catal., 2006, 348, 2114 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 888344, 888345. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ob26365c |
| This journal is © The Royal Society of Chemistry 2013 |