Organocatalytic conversion of cellulose into a platform chemical†
Benjamin R.
Caes‡
a,
Michael J.
Palte
b and
Ronald T.
Raines
*ac
aDepartment of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706, USA. E-mail: rtraines@wisc.edu
bMedical Scientist Training Program and Molecular & Cellular Pharmacology Graduate Training Program, University of Wisconsin-Madison, 1300 University Avenue, Madison, WI 53706, USA
cDepartment of Biochemistry, University of Wisconsin-Madison, 433 Babcock Drive, Madison, WI 53706, USA
First published on 3rd October 2012
Abstract
The search for a source of fuels and chemicals that is both abundant and renewable has become of paramount importance. The polysaccharide cellulose meets both criteria, and methods have been developed for its transformation into the platform chemical 5-(hydroxymethyl)furfural (HMF). These methods typically employ harsh reaction conditions or toxic heavy metal catalysts, deterring large-scale implementation. Here, we describe a low-temperature, one-pot route that uses ortho-carboxyl-substituted phenylboronic acids as organocatalysts in conjunction with hydrated magnesium chloride and mineral acids to convert cellulose and cellulose-rich municipal waste to HMF in yields comparable to processes that use toxic heavy metal catalysts. Isotopic labeling studies indicate that the key aldose-to-ketose transformation occurs via an enediol intermediate. The route, which also allows for facile catalyst recovery and recycling, provides a green prototype for cellulose conversion.
Introduction
The dependence on fossil fuels for energy and chemicals has become unsustainable.1 Abundant, renewable biomass resources could meet the fuel and chemical demands of the future,2 but must recapitulate the wide array of products now derived from fossil fuels. The six-carbon furanic 5-(hydroxymethyl)furfural (HMF) has the potential to meet this challenge.3 HMF can be transformed into a variety of useful products, such as common polyester building blocks4 and the promising liquid fuel 2,5-dimethylfuran.5 The carbon skeleton of HMF is identical to those of the hexose sugars that are the primary components of cellulose and hemicelluloses in biomass. Still, accessing this resource requires a process that efficiently transforms these carbohydrates into HMF.The conversion of cellulose to HMF can proceed via three steps: hydrolysis of cellulose to glucose, isomerization of glucose to fructose, and dehydration of fructose to HMF (Fig. 1). Although several methods exist for transforming glucose and fructose into HMF,6 few are capable of producing HMF in high yields directly from cellulose.3 Solid acids7 and heavy metals6b,8 are the best extant catalysts for this conversion. The inefficiency of solid acid catalysts and the toxicity of heavy metals could diminish their impact.9 Considering the ever-growing need for green chemistry, we sought a conversion process that uses benign and recyclable reagents, catalysts, and solvents, as well as mild reaction conditions. Here, we report on our discovery of such a process.
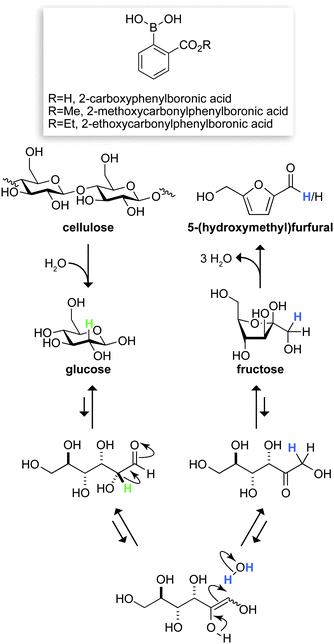 | ||
| Fig. 1 Putative route for the conversion of cellulose to 5-(hydroxymethyl)furfural, mediated by the depicted boronic acids. Labeled hydrogens in glucose (green) and water (blue) have the indicated fates. | ||
Results and discussion
Our initial experiments focused on discovering an alternative to heavy metals for accessing HMF from glucose. As a solvent, we choose N,N-dimethylacetamide (DMA), a polar aprotic solvent that has served as the medium for other biomass conversions.8b,10 We screened a variety of metal chlorides (Table S1†) and found that magnesium, nickel, zinc, cerium, or lanthanum, like chromium, provides appreciable yields of HMF (Table 1). We chose to employ magnesium due to its being one of the most abundant elements in Earth's crust11 and the human body.12|
|
||||
|---|---|---|---|---|
| Metal chloride, mol% | Boronic acid, wt% | T (°C) | Time (h) | HMF yield (%) |
| a Glucose was at 10 wt% relative to DMA. Mol% and HMF yield (HPLC) are relative to glucose. | ||||
| — | — | 100 | 6 | 0 |
| CrCl2, 25 | — | 100 | 6 | 58 |
| MgCl2·6H2O, 200 | — | 120 | 6 | 29 |
| NiCl2·6H2O, 300 | — | 120 | 3 | 32 |
| ZnCl2, 300 | — | 120 | 6 | 21 |
| CeCl3·7H2O, 300 | — | 120 | 3 | 22 |
| LaCl3·7H2O, 300 | — | 120 | 6 | 22 |
| — | 2-Carboxyphenyl, 100 | 120 | 6 | 2 |
| MgCl2·6H2O, 200 | 2-Carboxyphenyl, 100 | 120 | 4 | 54 |
| MgCl2·6H2O, 200 | 2-Carboxyphenyl, 50 | 120 | 6 | 48 |
| MgCl2·6H2O, 200 | 2-Carboxyphenyl, 25 | 120 | 6 | 46 |
| MgCl2·6H2O, 200 | 2-Carboxyphenyl, 10 | 120 | 6 | 31 |
| NiCl2·6H2O, 300 | 2-Carboxyphenyl, 100 | 105 | 4 | 24 |
| ZnCl2 + 6H2O, 300 | 2-Carboxyphenyl, 100 | 105 | 4 | 18 |
| CeCl3·7H2O, 300 | 2-Carboxyphenyl, 100 | 120 | 3 | 51 |
| LaCl3·7H2O, 300 | 2-Carboxyphenyl, 100 | 120 | 3 | 49 |
| — | 2-Methoxycarbonylphenyl, 100 | 100 | 6 | 22 |
| MgCl2·6H2O, 200 | 2-Methoxycarbonylphenyl, 100 | 120 | 4 | 57 |
| — | 2-Ethoxycarbonylphenyl, 100 | 100 | 6 | 15 |
| MgCl2·6H2O, 200 | 2-Ethoxycarbonylphenyl, 100 | 120 | 4 | 52 |
Next, we attempted to increase the efficiency of the conversion while maintaining environmental benignancy. To do so, we turned to organocatalysis,13 which had not been employed previously in a biomass conversion process.2,3 We were aware that boronic acids readily form boronate esters with 1,2- and 1,3-diols, and bind to furanose sugars with greater affinity than to pyranose sugars.14 We reasoned that these organoboranes would alter the equilibrium between glucose and fructose (which exist primarily in the pyranose and furanose forms, respectively), and thereby enhance HMF production. The precedents were discouraging, however, as phenylboronic acid had been reported to inhibit the hydrolysis of cellulose and the dehydration of sugar monomers.15,16
Phenylboronic acids have an affinity for carbohydrates that is tunable based on substituents.14 We assessed the ability of twenty derivatives of phenylboronic acid to promote the conversion of glucose to HMF (Tables S2–S4 in the ESI†). Our data corroborated the earlier work with phenylboronic acid itself,15 which is not a catalyst in our hands, but revealed that adding an ortho carboxyl group engenders catalysis, especially in the presence of MgCl2·6H2O (Table 1). Further optimization revealed prospects for catalytic turnover. For example, adding 25 mol% of 2-carboxyphenylboronic acid to the magnesium increases the HMF yield from 29% to 46%.
Water can counter the dehydration of fructose (Fig. 1). Accordingly, we attempted to increase our HMF yields by using anhydrous MgCl2. We found, however, that anhydrous MgCl2 provides lower HMF yields than does the hydrated salt (Table S4†). To determine how much water is beneficial for HMF production, we added water to reaction mixtures containing the anhydrous salt (Table S5 in the ESI†) and found that HMF yields increase steadily up to an H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg(II) ratio of ∼6 (Fig. S1†). Other anhydrous metal chlorides that work synergistically with 2-carboxyphenylboronic also have a similar reliance on water (Table S5†).
Mg(II) ratio of ∼6 (Fig. S1†). Other anhydrous metal chlorides that work synergistically with 2-carboxyphenylboronic also have a similar reliance on water (Table S5†).
Encouraged by the successful conversion of glucose to HMF, we next attempted to convert cellulose to HMF using boronic acids in 1-ethyl-3-methylimidazolium chloride ([EMIM]Cl), an ionic liquid that dissolves cellulose17 and enables a mineral acid to catalyze its hydrolysis.18 We observed that phenylboronic acids with an ortho carboxylic acid or ester promote the conversion of cellulose to HMF (Tables S6 and S7 in the ESI†), and that MgCl2·6H2O is necessary to maximize HMF production (Table 2). The observed yields of HMF (e.g., 41% in 1 h at 105 °C with 2-methoxycarbonylphenyl boronic acid) are comparable to those attainable with a heavy metal.8 Boronic acid/MgCl2·6H2O also promote the conversion of cotton, paper towels, and newspaper (Table 2), which are cellulose-rich components of municipal waste. The HMF yields correlate with the purity of the cellulose in these materials. Thus, HMF can be produced efficiently from cellulosic material in a single, one-pot process that is devoid of heavy metals.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Substrate | Ionic liquid | Acid, wt% | Metal chloride, mol% | Boronic acid, mol% | T (°C) | Time (h) | HMF yield (%) |
| a Substrates were at 5 wt% relative to the ionic liquid. Wt% is relative to the ionic liquid. Mol% and HMF yield (HPLC) are relative to glucose monomers within the substrate, which was assumed to be pure cellulose. | |||||||
| Cellulose | [EMIM]Cl | HCl, 0.61 | — | — | 105 | 2 | 10 |
| Cellulose | [EMIM]Cl | HCl, 0.61 | MgCl2·6H2O, 300 | — | 105 | 3 | 15 |
| Cellulose | [EMIM]Cl | HCl, 0.61 | — | 2-Methoxycarbonylphenyl, 120 | 105 | 2 | 12 |
| Cellulose | [EMIM]Cl | HCl, 0.61 | MgCl2·6H2O, 300 | 2-Methoxycarbonylphenyl, 120 | 105 | 2 | 39 |
| Cellulose | [EMIM]Cl | H2SO4, 0.88 | MgCl2·6H2O, 300 | 2-Methoxycarbonylphenyl, 120 | 105 | 1 | 41 |
| Cellulose | [EMIM]Cl | HCl, 0.61 | — | 2-Ethoxycarbonylphenyl, 160 | 105 | 1 | 21 |
| Cellulose | [EMIM]Cl | HCl, 0.61 | MgCl2·6H2O, 300 | 2-Ethoxycarbonylphenyl, 160 | 105 | 2 | 38 |
| Cellulose | [EMIM]Cl | H2SO4, 0.88 | MgCl2·6H2O, 300 | 2-Ethoxycarbonylphenyl, 160 | 105 | 1 | 36 |
| Cotton | [BMIM]Cl | — | MgCl2·6H2O, 300 | 2-Ethoxycarbonylphenyl, 160 | 105 | 2 | 40 |
| Paper towel | [EMIM]Cl | HCl, 0.61 | MgCl2·6H2O, 300 | 2-Ethoxycarbonylphenyl, 160 | 105 | 2 | 31 |
| Newspaper | [BMIM]Cl | — | MgCl2·6H2O, 300 | 2-Ethoxycarbonylphenyl, 160 | 105 | 2 | 18 |
The high boronate loadings necessary to turnover cellulose rapidly (≤2 h) led us to search for a recovery method that enables boronate recovery. Our initial separation strategy focused on isolating 2-carboxyphenylboronic acid from a reaction mixture using an anion-exchange resin. The reaction mixture was diluted with water, filtered to remove any humins (which are insoluble byproducts from aldol addition and condensation19), extracted with ethyl acetate to remove HMF, and passed through a column of resin. The anionic boronate was retained on the resin and eluted with aqueous NH4HCO3 (1 M) to yield purified boronate, which retained catalytic activity (Fig. S2†). This strategy was, however, inapplicable to the o-esterphenylboronic acids, which have low solubility in water and high solubility in ethyl acetate.
Boronic acid moieties become anionic at high pH and partition completely into an aqueous phase. Hence, we added basic water to reaction mixtures containing 2-ethoxycarbonylphenylboronic acid, filtered to remove any humins, and extracted with ethyl acetate (Fig. S3†). Removing solvent from the organic phase provided HMF. Evaporating water from the aqueous phase recovered the boronic acid and MgCl2. The recovered catalysts provided HMF in comparable yield through four reaction cycles.
Additional data provided insight on the chemical mechanism of our conversion (Fig. 1). Chromium is known to mediate the isomerization of aldose to ketose sugars by a 1,2-hydride shift.10,20 To probe the mechanism of our conversion, we performed two deuterium-labeling experiments. In the first, glucose-2-d was converted into HMF by 2-ethoxycarbonylphenylboronic acid and MgCl2·6H2O. 1H NMR spectroscopy revealed that virtually no deuterium was retained in the HMF product. In the second, unlabeled glucose was converted in the presence of D2O, and a substantial quantity of deuterium was found at C-1 of HMF (Fig. S4†). These results are compatible with a mechanism that avails an enediol intermediate (Fig. 1). A similar mechanism is used by the enzyme phosphoglucose isomerase, which catalyzes the interconversion of glucose 6-phosphate and fructose 6-phosphate.21
Boronate ester-formation is known to be more favorable with fructose than glucose.14 We found that boronic acids also serve by catalyzing fructose dehydration (Table S8†). We suspect that the organocatalyst relies on an ortho carboxyl group because its oxygen can donate electron density into the empty p-orbital of boron, thereby decreasing the strength of the fructose–boronate complex in the nearly water-free medium.14b Finally, we propose that water attenuates the reactivity of Mg(II), allowing its participation in catalysis, but deterring reaction pathways that can lead to humins.22
Concluding remarks
In summary, we have discovered novel reactivity mediated by a simple boronic acid composed of hydrogen, boron, carbon, and oxygen—four of the first eight elements in the periodic table. This organocatalyst mediates the transformation of fructose, glucose, and cellulose into the platform chemical HMF. Although boronate loadings for rapid (≤2 h) conversions are high, this liability is overcome by the facility of its recovery (or, potentially, by its immobilization). This discovery, which is the first to link two highly active areas of research: organocatalysis and biomass conversion, could facilitate the transition from fossil-based fuels and chemicals to a more sustainable biomass-based society.Acknowledgements
We are grateful to Prof. S. S. Stahl and Dr. N. A. McGrath for contributive discussions. This work was supported by the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-FC02-07ER64494), a DOE Bioenergy Research Center. M.J.P. was supported by pre-doctoral fellowship 09PRE2260125 (American Heart Association). This work made use of the National Magnetic Resonance Facility at Madison, which is supported by NIH grants P41RR02301 (BRTP/NCRR) and P41GM66326 (NIGMS). Additional equipment was purchased with funds from the University of Wisconsin, the NIH (RR02781, RR08438), the NSF (DMB-8415048, OIA-9977486, BIR-9214394), and the USDA.Notes and references
- US National Petroleum Council, Facing the Hard Truths about Energy, Washington, DC, 2007 Search PubMed.
- (a) D. Tilman, R. Socolow, J. A. Foley, J. Hill, E. Larson, L. Lynd, S. Pacala, J. Reilly, T. Searchinger, C. Somerville and R. Williams, Science, 2009, 325, 270 CrossRef CAS; (b) C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695–699 CrossRef CAS; (c) R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- (a) J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164–7183 CrossRef CAS; (b) M. E. Zakrewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem. Rev., 2011, 111, 397–417 CrossRef.
- J. Lewkowski, ARKIVOC, 2001, 17–54 Search PubMed.
- (a) S. Zhong, R. Daniel, H. Xu, J. Zhang, D. Turner, M. L. Wyszynski and P. Richards, Energy Fuels, 2010, 24, 2891–2899 CrossRef CAS; (b) G. Tian, R. Daniel, H. Li, H. Xu, S. Shuai and P. Richards, Energy Fuels, 2010, 24, 3898–3905 CrossRef CAS; (c) R. Daniel, G. Tian, H. Xu, M. L. Wyszynski, X. Wu and Z. Huang, Fuel, 2011, 90, 449–458 CrossRef CAS; (d) D. A. Rothamer and J. H. Jennings, Fuel, 2012, 98, 203–212 CrossRef CAS.
- (a) Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef; (b) H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS.
- (a) C. V. McNeff, D. T. Nowlan, L. C. McNeff, B. Yan and R. L. Fedie, Appl. Catal., A, 2010, 384, 65–69 CrossRef CAS; (b) S. Zhao, M. Cheng, J. Li, J. Tian and X. Wang, Chem. Commun., 2011, 47, 2176–2178 RSC.
- (a) Y. Su, H. M. Brown, X. Huang, X. Zhou, J. E. Amonette and C. Z. Zhang, Appl. Catal., A, 2009, 361, 117–122 CrossRef CAS; (b) J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS; (c) B. Kim, J. Jeong, D. Lee, S. Kim, H.-J. Yoon, Y.-S. Lee and J. K. Cho, Green Chem., 2011, 13, 1503–1506 RSC; (d) P. Wang, H. Yu, S. Zhan and S. Wang, Bioresour. Technol., 2011, 102, 4179–4183 CrossRef CAS.
- (a) S. Langrrd, Am. J. Ind. Med., 1990, 17, 189–214 CrossRef; (b) A. Levina and P. A. Lay, Chem. Res. Toxicol., 2008, 21, 563–571 CrossRef CAS; (c) R. Marin, Y. Ahuja and R. N. Bose, J. Am. Chem. Soc., 2010, 132, 10617–10619 CrossRef CAS.
- (a) J. B. Binder, A. V. Cefali, J. J. Blank and R. T. Raines, Energy Environ. Sci., 2010, 3, 765–771 RSC; (b) J. B. Binder, A. V. Cefali, J. J. Blank and R. T. Raines, ChemSusChem, 2010, 3, 1268–1272 CrossRef CAS.
- G. B. Haxel, J. B. Hedrick and G. J. Orris, U.S. Geological Survey, 2002, Fact Sheet 087–002 Search PubMed.
- M. J. Laires, C. P. Monteiro and M. Bicho, Front. Biosci., 2004, 9, 262–276 CrossRef.
- (a) S. C. Pan and B. List, Ernst Schering Found. Symp. Proc., 2007, 1–43 CAS; (b) D. W. C. MacMillan, Nature, 2008, 455, 304–308 CrossRef CAS; (c) E. N. Jacobsen and D. W. C. MacMillan, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20618–20619 CrossRef CAS.
- For examples, see: (a) G. Springsteen and B. H. Wang, Tetrahedron, 2002, 58, 5291–5300 CrossRef CAS; (b) M. Berube, M. Dowlut and D. G. Hall, J. Org. Chem., 2008, 73, 6471–6479 CrossRef CAS; (c) M. G. Chudzinski, Y. Chi and M. S. Taylor, Aust. J. Chem., 2011, 64, 1466–1469 CrossRef CAS; (d) G. A. Ellis, M. J. Palte and R. T. Raines, J. Am. Chem. Soc., 2012, 134, 3631–3634 CrossRef CAS.
- H. Kawamoto, S. Saito and S. Saka, J. Anal. Appl. Pyrolysis, 2008, 82, 78–82 CrossRef CAS.
- Boric acid is known to assist an enzyme-catalyzed transformation of glucose to fructose ( R. Huang, W. Qi, R. Su and Z. He, Chem. Commun., 2010, 46, 1115–1117 RSC ) but has varied effects on the other steps in Fig. 1 (ref. 15; H. Kawamoto, S. Saito and S. Shiro, Carbohydr. Res., 2008, 343, 249–255 CrossRef CAS; T. Ståhlberg, S. Rodriguez-Rodriguez, P. Fristrup and A. Riisager, Chem.–Eur. J., 2011, 17, 1456–1464 CrossRef ). Moreover, the reactivity of boric acid cannot be tuned by chemical modification, nor can boric acid be immobilized readily.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS.
- J. B. Binder and R. T. Raines, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4516–4521 CrossRef CAS.
- S. K. R. Patil and C. R. F. Lund, Energy Fuels, 2011, 25, 4745–4755 CrossRef CAS.
- Y. Román-Leshkov, M. Moliner, J. A. Labinger and M. E. Davis, Angew. Chem., Int. Ed., 2010, 49, 8954–8957 CrossRef.
- (a) Y. J. Topper, J. Biol. Chem., 1957, 225, 419–426 CAS; (b) I. A. Rose and E. L. O'Connell, J. Biol. Chem., 1961, 236, 3086–3092 CAS.
- C. Sievers, I. Musin, T. Marzialetti, M. B. V. Olarte, P. K. Agrawal and C. W. Jones, ChemSusChem, 2009, 2, 665–671 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, Tables S1–S8, and Fig. S1–S4. See DOI: 10.1039/c2sc21403b |
| ‡ Present address for Dr. B. R. Caes: Siegwerk USA Co., 3535 SW 56th Street, Des Moines, IA 50321, USA. |
| This journal is © The Royal Society of Chemistry 2013 |


