Role of aggregate size in the hemolytic and antimicrobial activity of colloidal solutions based on single and gemini surfactants from arginine
L. Tavanoab, M. R. Infantec, M. Abo Riyad, A. Pinazoc, M. P. Vinardellef, M. Mitjansef, M. A. Manresag and L. Perez*c
aDepartment of Pharmaceutical Sciences, University of Calabria, Edificio Polifunzionale, 87036 Arcavacata di Rende, Cosenza, Italy
bDepartment of Engineering Modelling, University of Calabria, Via P. Bucci – Cubo 39C, 87036 Arcavacata di Rende, Cosenza, Italy
cDepartment of Chemical and Surfactants Technology, IQAC-CSIC, Jordi Girona 18-26, 08034 Barcelona, Spain. E-mail: lpmste@cid.csic.es
dChemistry Department, Faculty Of Science, Benha University, Egypt
eDepartment de Fisiologia, Universitat de Barcelona, Joan XXIII s/n, 08028 Barcelona, Spain
fUnidad Asociada al CSIC, Departement de Fisiologia, Universitat de Barcelona, Joan XXIII s/n, 08028 Barcelona, Spain
gDepartment de Microbiologia, Universitat de Barcelona, Joan XXIII s/n, 08028 Barcelona, Spain
First published on 19th October 2012
Abstract
Cationic colloidal systems composed of arginine based surfactants (single or gemini structures) and membrane additive compounds such as DLPC or cholesterol have been characterized by means of size distribution and zeta-potential measurements. The single or monocatenary surfactant (LAM) as well as the gemini with the shortest spacer chain (C6(LA)2) formed micelles, while aqueous solutions of pure gemini surfactants with longer spacers (C9(LA)2 and C12(LA)2) formed very big aggregates. The addition of phospholipids or cholesterol changed drastically the aggregation behaviour. In the case of LAM and C6(LA)2, the incorporation of additives gave rise to the formation of cationic vesicles. For C9(LA)2 and C12(LA)2, this type of additives promoted the formation of smaller aggregates. All the formulations had positive zeta-potential values and in general exhibited high colloidal stability. We also evaluated the hemolysis and the antimicrobial activity of these systems. The capability of disrupting erythrocyte membranes depends on the hydrophobicity of the molecules and the size of aggregates in the solution. Gemini surfactants with short spacer chains are more hemolytic than their single chain homologue, while gemini surfactants with long spacers are much less hemolytic than their single chain counterpart. Moreover, for the same formulation, the hemolysis depends on the initial concentration of the stock solution used to set up the hemolysis/concentration curve. Results show that small aggregates interact easily with these biological membranes. The alkyl spacer chain and the presence of additives also play an important role in the antimicrobial activity, and, in general, the interaction with bacteria and erythrocytes is affected by the same parameters. The physico-chemical and biological characterization of these systems might be important for several biotechnological applications in which cationic vesicular systems are involved.
Introduction
In recent years the concept of drug-delivery has been changing. It is no more a simple formulation that allows the administration of the drug, but a much more complex system based on the use of nanomaterials, which can present special properties. Cationic vesicles based on positively charged surfactants are, among other colloidal systems, one of the most promising, showing great potential.1Cationic liposomes have been successfully employed to interact with negatively charged surfaces or biomolecules such as prokaryotic2 or eukaryotic cells,3 antigenic proteins,4 synthetic polymers,5 latex6 and mineral surfaces.7 At present, there is an unprecedented level of interest in the properties and structures of complexes consisting of DNA mixed with oppositely charged cationic liposomes.8 This interest arises because the complexes mimic natural viruses as chemical carriers of DNA into cells in worldwide human gene therapy clinical trials.9
Other very useful applications of cationic liposomes are the delivery of peptides, proteins or antimicrobial agents.10,11 It has been demonstrated that these vesicles are able to overcome bacterial resistance related to the permeability barrier and enzymatic hydrolysis by a fusion process between the liposomes and bacterial membranes.12 Cationic liposomes were also investigated for their potential targeting ability to the bacterial biofilms produced by skin and oral bacteria.13 Furthermore, it has been demonstrated that the same cationic lipids used in liposome preparations might act by themselves as anti-infective agents.14
Among the classical cationic surfactants, quaternary ammonium compounds (QACs) and bis(QACs) are usually employed to obtain cationic liposomes.15 Usually, they have the ability to interact with bacterial species and cultured mammalian cells. Vesicles based on QACs also have been found to solubilize amphotericin B and other hydrophobic drugs such as miconazol (MCZ) and to transfer DNA into cells through fusion with the cell membrane.16,17
Unfortunately, one of the major problems related to the use of cationic vesicles based on QACs is their high degree of toxicity towards the host.18 In fact, QACs present acute toxicity, poor chemical and biological degradation and hemolytic activity. Thus, they are not suitable for biomedical applications.19
For these reasons, a large variety of synthetic cationic amphiphiles with low toxicity have been synthesized as an interesting alternative to the conventional cationic surfactants.20 An obvious strategy to increase the efficiency of surfactants and improve their environmental properties is to build up structures from renewable materials. In light of this, our group has reported the synthesis of new cationic surfactants based on the amino acid arginine, with different structures (monocatenary, gemini, and glycerolipid), characterized by relevant nontoxic and antimicrobial properties as well as rapid biodegradability.21 In addition, gemini surfactants from arginine are extraordinarily active in reducing surface tension and it has been reported that the single chain derivatives improve transfection efficiency if conjugated to cationic liposome systems.22,23
The physico-chemical and biological properties of liposomal systems obtained from arginine-based surfactants have not yet been studied, so it would be very interesting to learn how colloidal formulations can affect the hemolysis and the antibacterial activity of this class of surfactants.
In this work, we report on the physico-chemical and biological properties of colloidal systems obtained from this class of surfactants. Single-chain arginine based surfactants (LAM) and arginine gemini derivatives (C6(LA)2, C9(LA)2, and C12(LA)2) (Fig. 10) were synthesized and used as pure components or formulated in cationic mixtures with membrane additives (cholesterol or dilauroylphosphatidylcholine (DLPC)). The influence of different parameters including surfactant structure (gemini or monocatenary), spacer chain length and membrane additive type on the physico-chemical properties of the cationic vesicles as well as on the hemolytic and antimicrobial activity has been investigated.
Results and discussion
Vesicle diameter, size distribution as well as charge density are important parameters in liposome applications and can affect the biological activity of liposome formulations. In order to study how these parameters affect the hemolysis and antimicrobial activity of cationic surfactants from arginine, different formulations have been designed: pure surfactant solutions of LAM, C6(LA)2, C9(LA)2 and C12(LA)2 at two different concentrations (4 and 2.5 mM) and mixed surfactant–additive suspensions at two different molar ratios have been prepared. Table 1 presents the composition of every formulation.| Sample number and label | Concentration (mM) | Surfactant amount (n) | Additive amount (n) | Mole ratio surf.–add. |
|---|---|---|---|---|
| a (C6: C6(LA)2; C9: C9(LA)2; C12: C12(LA)2). | ||||
| LAM 1 | 4 | 8 × 10−6 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| LAM 2 | 2.5 | 5 × 10−6 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| LAM/CHOL 1 | 5 | 8 × 10−6 | 2 × 10−6 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| LAM/CHOL 2 | 5 | 5 × 10−6 | 5 × 10−6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| LAM/DLPC 1 | 5 | 8 × 10−6 | 2 × 10−6 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| LAM/DLPC 2 | 5 | 5 × 10−6 | 5 × 10−6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| C6 1 | 4 | 8 × 10−6 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| C6 2 | 2.5 | 5 × 10−6 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| C6/CHOL 1 | 5 | 8 × 10−6 | 2 × 10−6 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| C6/CHOL 2 | 5 | 5 × 10−6 | 5 × 10−6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| C6/DLPC 1 | 5 | 8 × 10−6 | 2 × 10−6 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| C6/DLPC 2 | 5 | 5 × 10−6 | 5 × 10−6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| C9 1 | 4 | 8 × 10−6 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| C9 2 | 2.5 | 5 × 10−6 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| C9/CHOL 1 | 5 | 8 × 10−6 | 2 × 10−6 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| C9/CHOL 2 | 5 | 5 × 10−6 | 5 × 10−6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| C9/DLPC 1 | 5 | 8 × 10−6 | 2 × 10−6 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| C9/DLPC 2 | 5 | 5 × 10−6 | 5 × 10−6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| C12 1 | 4 | 8 × 10−6 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| C12 2 | 2.5 | 5 × 10−6 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| C12/CHOL 1 | 5 | 8 × 10−6 | 2 × 10−6 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| C12/CHOL 2 | 5 | 5 × 10−6 | 5 × 10−6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| C12/DLPC 1 | 5 | 8 × 10−6 | 2 × 10−6 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| C12/DLPC 2 | 5 | 5 × 10−6 | 5 × 10−6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Usually, there is an upper limit to cholesterol incorporation in lipid bilayers and above this limit cholesterol starts to precipitate. It has been reported that for phosphatidylcholine vesicles the maximum cholesterol solubility is about 66%, while for phosphatidylethanolamine the limit is about 51%.24 Taking into account this behavior, two formulations containing cholesterol or DLPC with a surfactant–additive ratio of 8/2 and 5/5 have been prepared.
Visual observation
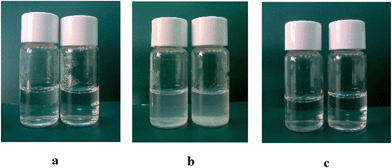
It is known that a change in the turbidity of a surfactant solution can relate to the change in the amount and/or size of the surfactant aggregates.The pure LAM and C6(LA)2 surfactants formed transparent solutions, and this behaviour suggests that these compounds do not form vesicular aggregates (Fig. 1a and 2a). In fact, these surfactants yield typical high-resolution 1H-NMR spectra with Lorentzian band shape corresponding to the presence of small micelles.25
 | ||
| Fig. 1 Pictures of LAM formulations at different concentrations: (a) LAM 1 and LAM 2, (b) LAM/CHOL 1 and LAM/CHOL 2, (c) LAM/DLPC 1 and LAM/DLPC 2. | ||
 | ||
| Fig. 2 Pictures of C6 formulations at different concentrations: (a) C6 1and C6 2, (b) C6/CHOL 1 and C6/CHOL 2, (c) C6/DLPC 1 and C6/DLPC 2. | ||
Solutions of pure C9(LA)2 (Fig. 3a) and C12(LA)2 (figure not shown) appeared as translucent and viscous dispersions without sedimentation after several months. Moreover, the 1H-NMR spectra of C9(LA)2 and C12(LA)2 indicated the presence of larger aggregates. The slow motion of alkyl chains in the vesicles or big aggregates resulted in extremely broad NMR signals with low intensity.26 Previous cryo-TEM studies showed that aqueous solutions of LAM and C6(LA)2 mainly contain classical spherical micelles at the concentrations used in this work. However, different results were obtained for C9(LA)2 and C12(LA)2. For these two surfactants no spheroidal micelles were seen at any of the concentrations examined. Gemini Cn(LA)2 solutions contain cylinder micelles, twisted ribbons, flat ribbons or threadlike micelles and the concentration at which these aggregates appear decreases as the spacer chain increases.27 It has been reported that the formation of these types of aggregates is favored by the presence of chiral head groups and intermolecular H-bonding.28–30
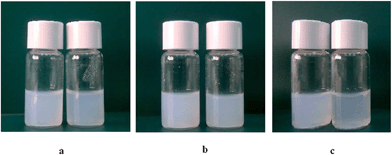 | ||
| Fig. 3 Pictures of C9 formulations at different concentrations: (a) C9 1 and C9 2, (b) C9/CHOL 1 and C9/CHOL 2, (c) C9/DLPC 1 and C9/DLPC 2. | ||
In general, the use of DLPC or cholesterol often helps in the formation of self-closed bilayers.31,32 Some surfactants can not assemble to form vesicles due to their critical packing parameter, i.e. relative space requirements of the hydrophobic and the hydrophilic parts of the amphiphiles. The incorporation of phospholipids or cholesterol into the surfactant aggregates leads to appropriate molecular geometry and hydrophobicity for vesicle formation.33 Cholesterol also changes the fluidity of the hydrophobic chains in the bilayer, thus promoting the formation of surfactant vesicles. In this work, we have prepared aggregates formed by cationic arginine surfactants and cholesterol or DLPC to study how these additives affect the aggregation of these cationic surfactants.
Surfactant formulations with additives showed different appearances. In the case of LAM, the addition of cholesterol gave rise to milky and cloudy dispersions (Fig. 1b). In the case of C6(LA)2, formulations appeared slightly less milky than the ones based on LAM, which suggests the presence of smaller aggregates (Fig. 2b). Some evidence of phase separation has been observed after 1 week for the formulation LAM/CHOL 2.
The addition of 20% DLPC to LAM or C6(LA)2 solutions originates transparent formulations with the same aspect as those of respective pure surfactants (Fig. 1c and 2c). The incorporation of 50% DLPC originates turbid and bluish dispersions with the typical aspect of vesicular formulations for LAM, but in the case of C6(LA)2, transparent solutions were obtained, suggesting that these compounds do not form vesicular aggregates even in the presence of 50% DLPC (Fig. 2c).
For C9(LA)2 and C12(LA)2 surfactants, changes are different for every formulation. Given that both surfactant solutions are milky and cloudy, the introduction of additives did not change in a relevant manner the final aspect of the formulations. The presence of cholesterol gave rise to an increase in sample turbidity, while the formulations containing DLPC seem to contain smaller aggregates since they appear slightly less turbid than those of pure gemini surfactants (Fig. 3b and c).
Size and charge density of the aggregates
All the formulations previously described were characterized by Dynamic Light Scattering measurements to determine particle size and size distribution. Fig. 4 shows the results obtained for some LAM formulations. Visual observations showed only transparent solutions for both concentrations of pure LAM solutions, and the DLS measurement did not show any peak related to vesicles, but only peaks of low intensity, which refer to spherical micelles (Fig. 4c). The low scattering intensity of these solutions could be attributed to the small amount of aggregates in the sample. Notice that the concentration of the LAM 1 solution is near the CMC of this surfactant (4–6 mM), and thus it is expected that few micelles are present in the solution.34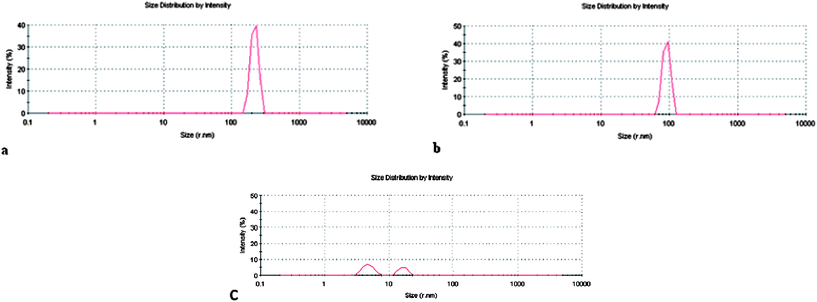 | ||
| Fig. 4 Size distribution profiles of LAM formulations: (a) LAM/CHOL 2, (b) LAM/DLPC 2, and (c) LAM 1. | ||
The inclusion of cholesterol or phospholipids strongly affected the aggregate's size. In particular, the presence of cholesterol at 20% and 50% gave rise to higher aggregates. Fig. 4a shows the narrow peak centered at 200 nm obtained for the LAM/CHOL 2 sample. In the presence of 20% DLPC no peaks related to vesicular systems were found, and it is probable that mixed micelles are present in the solution. In fact, it has been reported that the solubilization of water-insoluble compounds, such as lipids, in the hydrophobic core of micellar aggregates leads to the breakdown of bilayered structures and formation of lipid-surfactant mixed micelles.35 In particular, different studies on the properties of mixed systems composed of cationic gemini surfactants and phospholipids have been conducted, demonstrating that the origin of synergism of phospholipids with amino acid surfactants is based on the reduction of electrostatic repulsions between the ionic head groups of the surfactant due to intercalation of zwitterionic lipids in the mixed micelles.36 The formation of mixed micelles is the basis of the wide application of surfactants in the isolation and purification of membrane proteins,37 in DNA extraction38 and as drug delivery vehicles.39 On the other hand, the incorporation of 50% DLPC gave monodisperse aggregates with a peak centered at 100 nm, which is consistent with the visual observation (Fig. 4b).
The transparent C6 1 and C6 2 samples show only peaks centered at 0.5 and 5 nm, which can be attributed to micelles (Fig. 5a). The CMC of this surfactant is around 0.4–0.6 mM,34 because of which the number of micelles at 4 mM is high and the intensity of the DLS graph is greater than that obtained for the LAM 1 solution. With the introduction of DLPC similar results were obtained. The incorporation of cholesterol promotes the formation of small and big aggregates with a mean hydrodynamic diameter (dH) higher than 1000 nm (Fig. 5b).
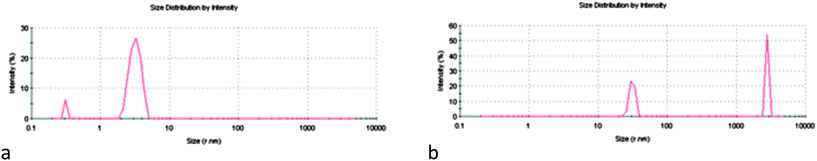 | ||
| Fig. 5 Size distribution profiles of C6(LA)2 based formulations: (a) C6 1, (b) C6/CHOL 1. | ||
The intensity distribution graphs of C9(LA)2 formulations are shown in Fig. 6. Different features can be observed in every graph. The pure C9 1 has two different populations, aggregates with diameters in the range of 100 nm and large aggregates with diameters around 900 nm (Fig. 6a). These large aggregates could correspond to twisted ribbons, flat ribbons, threadlike ribbons or helical aggregates. These aggregates are consistent with the viscosity of the solutions and with previous cryo-TEM and NMR studies.27 Although forming large aggregates, such dispersions are stable for an indefinitely long time (up to 1 year) and a tendency to phase separation was not observed.
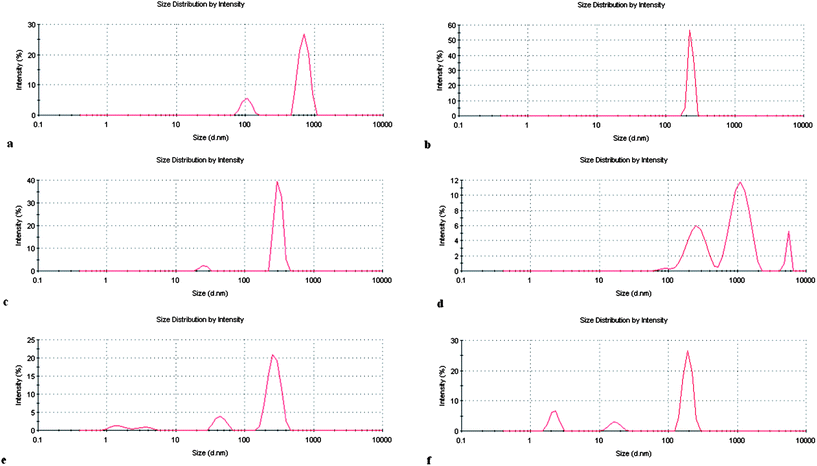 | ||
| Fig. 6 Size distribution profiles of C9 formulations: (a) C9 1; (b) C9 2; (c) C9/CHOL 1; (d) C9/CHOL 2; (e) C9/DLPC 1; (f) C9/DLPC 2. | ||
In order to establish a control on the aggregate's size, this formulation was subjected to extrusion 20 times with an extruser device equipped with a 200 nm pore size polycarbonate membrane. Extrusion of this solution was very difficult; moreover, HPLC analysis showed that some surfactant was retained on the membrane. The extrused formulation also contained large aggregates. It is probable that the big elongated aggregates, long thread-like twisted ribbons or helical aggregates can cross the membrane retaining the original shape and size. The less concentrated C9(LA)2 solution presents only a single population with the average hydrodynamic value centered at 200 nm (Fig. 6b). Perhaps this lower concentration is not enough to promote the formation of the large aggregates observed at higher concentrations. The cryo-TEM studies carried out with C9(LA)2 and C12(LA)2 indicated that no spheroidal micelles were seen at any of the concentrations examined in this work. C9(LA)2 showed ribbons at very low concentrations (0.1 wt%) and when the concentration was increased to 0.7% the ribbons were tightly twisted. Similar results were obtained for C12(LA)2, but in this case aggregates appeared at lower concentrations (0.05%).27 These types of aggregates have also been observed for other amino acid surfactants. At high concentrations (8.0 mM), the z-average dH value of aggregates formed by histidine based surfactants is around 1.0 μm and the optical microscopic images of these solutions reveal the existence of helical and fiber-like aggregates. At lower concentrations, these histidine based surfactants form aggregates with a z-average dH value of 100 nm.40
In addition, we decided to study the influence of different methods of preparation on the physicochemical properties of these formulations. The experiments were performed by using both the sonication method and the thin layer evaporation method (film), and starting from the same surfactant–additive composition, but no important differences in the aggregate's size and distribution were found.
The inclusion of neutral amphoteric vesicle inducing agents in the aggregates formed by this surfactant was found to have a strong influence on their shape and size. The addition of 20% cholesterol to C9(LA)2 solution drastically changes the intensity average size distribution plot. The large aggregates present in the original solution (C9 1 sample) disappear and two populations can be observed with dH values in the range of 30–200 nm (Fig. 6c). The size of these aggregates suggests that the incorporation of cholesterol into the surfactant assemblies promotes the formation of vesicles. The formulation in which 50% cholesterol has been incorporated into the original solution (C9/Chol 2, Fig. 6d) shows very big aggregates. It has been observed that as the concentration of cholesterol increases, the system undergoes a process of phase separation to form cholesterol-rich domains.40 The big aggregates observed in the figure could correspond to cholesterol rich domains. In fact, at this high concentration of additive the dispersion becomes more turbid and some evidence of phase separation has been observed after 4 weeks. Usually, there is an upper limit to cholesterol incorporation in lipid bilayers, above which the system undergoes a process of phase separation to form cholesterol rich domains. It has been observed that for membranes composed of phosphatidylcholine, the maximum cholesterol solubility is about 66%,24 and for vesicles formed by rhamnolipid surfactants, the maximum limit is about 50%.41
The addition of DLPC to the more concentrated C9(LA)2 solution also originates a heterogeneous dispersion with dH values in the range of 40–200 nm (Fig. 6e). Notice that also in this case the large aggregates of the original C9 1 sample have disappeared, probably due to the formation of mixed vesicles with the DLPC. The z-average dH is slightly lower than that of the similar formulation made up of cholesterol. The increase of DLPC concentration decreases the dH values, and in the C9/DLPC 2 formulation there is no evidence of phase separation perhaps because these phospholipids form stable vesicles (Fig. 6f). Table 2 shows the different aggregates present in every LAM and C9(LA)2 formulation.
| LAM 1 | LAM/CHOL 1 | LAM/DLPC 1 |
| Micelles (dH = <30 nm) | Medium aggregates (dH = 100 nm) | Mixed micelles (dH = <30 nm) |
| LAM 2 | LAM/CHOL 2 | LAM/DLPC 2 |
| Micelles (dH = <30 nm) | Medium aggregates (dH = 200 nm) | Mixed aggregates (dH = 100 nm) |
| C9 1 | C9/CHOL 1 | C9/DLPC 1 |
| Medium and big aggregates (dH = 100–900 nm) | Small and medium aggregates (dH = 30–200 nm) | Small and medium aggregates (dH = 40–200 nm) |
| C9 2 | C9/CHOL 2 | C9/DLPC 2 |
| Medium aggregates (dH = 200 nm) | Medium and big aggregates (dH = 300–1000 nm) | Small and medium aggregates (dH = 5–20–200 nm) |
The intensity graphs obtained for the C12-based formulations are similar to those of C9(LA)2 (data not shown). The aqueous solutions of pure surfactants are viscous and the DLS graphs indicate the presence of very big aggregates and in general cholesterol and DLPC also promote the formation of colloidal solutions with smaller aggregates, except for the C12/CHOL 2 formulation.
Zeta-potential
Zeta-potential measurements are a convenient way to characterize the electrostatic properties of vesicles, since the zeta-potential is a measure of the electrical charge close to the surface of the vesicles. For all the formulations studied in this work, large positive zeta-potential values (between 50 and 70 mV) have been obtained. The addition of neutral or amphoteric additives to pure surfactant solutions does not affect significantly the zeta-potential of the aggregates. The inclusion of additives in the cationic aggregates causes the separation of the cationic polar head on the assemblies and consequently decreases the electrostatic repulsion between the cationic charges. This could promote a decrease of the counterions bonded to the aggregates, which explains the similar zeta potential of the different formulations. Similar performance has been reported for dioctadecyldimethylammonium bromide (DODAB)/dipalmitoylphosphatidylcholine (DPPC) vesicles. Upon increasing the percentage of DODAB in the vesicles from 0 to 50% the Z-potential remained practically constant.42 In general, it was observed that the formulations exhibited high colloidal stability and no phase separation or precipitation was observed up to 2 months after preparation. The stability of these suspensions is probably due to the stabilizing effect produced by the repulsive interaction among aggregates with positive surface charges. The positive charge density of the aggregates is large enough to ensure that no coagulation occurs. In fact, one method used to stabilize non-charged vesicles or niosomes is to add a charged molecule to the bilayer to prevent aggregation of niosomes. Phosphatidic acid and stearylamine or cetylpyridinium chloride are commonly used.43Hemolytic activity
Hemolysis by surfactants is a process of great fundamental and practical importance. The mammalian erythrocyte lacks internal organelles and since it is the simplest cellular model obtainable, it is the most popular cell membrane system to study the surfactant–membrane interaction. For all these reasons, it is adopted as a convenient model system.44 Gemini cationic surfactants from amino acids have attracted much interest due to their excellent surface properties (very low CMC values and large activity reducing surface tension) and their antimicrobial and environmental behaviour. However, the influence of the spacer chain on the hemolytic character of these surfactants has been rarely studied. Here we have evaluated the hemolytic behaviour of different gemini surfactant solutions in which we have varied the spacer chain of the gemini, the concentration of the stock solution employed to calculate the HC50 value and the additive used to enhance the formation of mixed vesicles.The experiments to study the hemolytic activity of our formulations were carried out by preparing stock formulations of pure surfactant solutions of LAM, C6(LA)2, C9(LA)2 and C12(LA)2 and mixed surfactant–additive suspensions at two different concentrations (Table 2). Then, the HC50 of all stock formulations was calculated by preparing, from each of these original solutions, samples with decreasing concentrations of surfactant or surfactant–additive mixture. The experiments were carried out on the same day as the sample preparation. The degree of hemolysis induced by surfactants was represented by a surfactant concentration–response curve, in which hemolysis is expressed as a function of the logarithm of surfactant concentration.
Concentration-dependent surfactant effects on erythrocyte membranes are well known and widely described in the literature. Conventional single-chain amphiphiles have the ability to induce shape alterations, vesiculation and hemolysis in human erythrocytes, as well as to protect erythrocytes against hypotonic hemolysis,45 but it is important to compare these membrane effects with those of the corresponding gemini surfactant or lipid–surfactant mixtures. Fig. 7 shows the HC50 values for all formulations based on LAM. The results show that regardless of the initial concentration of the stock formulations, no relevant difference in terms of HC50 was achieved. Pure LAM solutions showed very similar hemolytic activity. When cholesterol is present in the sample, hemolysis was found to be a little lower than that of the corresponding pure LAM solution: HC50 of LAM 1 is 91 μM, while HC50 of LAM/CHOL 1 is 104 μM. This behaviour could be ascribed to the cloudy dispersions obtained in the presence of cholesterol, indicative of the presence of bigger aggregates compared to the ones present in LAM 1 and 2. In fact, as mentioned before, the LAM solutions appear as transparent solutions containing small micelles. Small aggregates seem to be more toxic than bigger ones, perhaps because they interact better and more easily with erythrocytes, causing higher hemolysis.
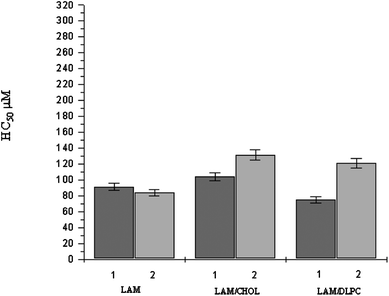 | ||
| Fig. 7 HC50 values of LAM based formulations (mean ± S.E of three experiments). For HC50 of LAM/additive formulation, the value corresponds to the LAM concentration in the sample. | ||
Compared with pure LAM, LAM/DLPC 1 is more hemolytic while LAM/DLPC 2 is less lytic to erythrocytes. Notice that LAM/DLPC 1 contains mainly mixed micelles, whereas LAM/DLPC 2 has large size mixed vesicles. This fact indicates that incorporation of additives can reduce the hemolytic character of cationic surfactants.
These results are in concordance with some reported in the literature showing that the hemolytic activity of surface active agents can be reduced by co-formulation with egg phosphatidylcholine (PC);46 in particular, the combination of dodecyl betainate and PC gives very low hemolytic activity.47 Hemolysis due to diacylglycerol arginine based surfactants can be drastically reduced when this surfactant is included in cationic vesicles formed by phosphatidylglycerol48 and the toxicity of dioctadecyl-dimethyl-ammonium bromide was drastically reduced when it was mixed with cholesterol and phosphatidylcholine.49
In the case of C6(LA)2 and C6(LA)2–additive mixtures, the percentage of hemolysis at the lowest concentration tested (20 μM) was almost 100%. This means that this surfactant is much more hemolytic than its single chain homologue LAM. It has been reported that the C3(LA)2 homologue with three carbon atoms in the spacer chain is also more hemolytic than LAM,50 and gemini surfactants from lysine with spacer chains of six CH2 groups are more hemolytic than the corresponding monocatenary counterparts.51 Similar results have been reported for Bis(Quats) surfactants in which the gemini surfactants are more toxic than the corresponding monomeric ones.52,53 Gemini surfactants are more hydrophobic compounds and it has been described that when the hydrophobicity of the surfactant increased, stronger membrane permeabilization was obtained and the molecules became more hemolytic.54
Fig. 8 and 9 show the HC50 data obtained for C9(LA)2 and C12(LA)2 based formulations. Similar behaviour has been observed for the two gemini surfactants. The most remarkable feature is that the hemolytic activity of these two gemini surfactants is very low in comparison with that obtained for C6(LA)2 and LAM, indicating that the spacer chain length plays an important role in hemolysis. In other words, the erythrocyte-disrupting ability of gemini with short spacers is considerably higher than that of LAM, while the gemini surfactants with longer spacer chains are rather less hemolytic than LAM. This behavior seems to be related to the morphology and size of the aggregates formed by every surfactant. As it has been described, pure LAM and C6(LA)2 form mainly spherical micelles while C9(LA)2 and C12(LA)2 form viscous solutions that contain big aggregates like helicoidal or worm-like micelles. The reasons for this might be related to the conformation of the surfactant in aqueous media. In fact, the high hydrophobicity and flexibility of the spacer in C9(LA)2 and C12(LA)2 allows the spacer chain to penetrate into the aggregated core, reducing the distance between the polar head groups and forming a compact molecule with low surface area. This particular conformation, due to the length of the spacer, can certainly influence the size of aggregates. In this way, C9 and C12 are able to form closely packed structures at lower concentrations than the C6 homologue.27 These results indicate that big aggregates produce less hemolysis.
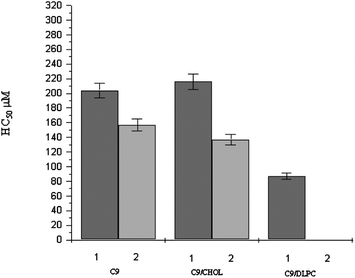 | ||
| Fig. 8 HC50 of C9 and C9 based formulations (mean ± S.E. of three replicates). | ||
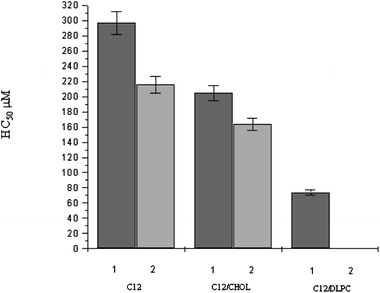 | ||
| Fig. 9 HC50 of C12 and C12 based formulations (mean ± S.E. of three replicates). | ||
Another noteworthy result is that for C9(LA)2 and C12(LA)2 the HC50 depends on the surfactant concentration in the initial stock solution used to determine the hemolytic activity. For example, for C9(LA)2, it can be observed that when the stock solution used is the C9 1 (4 mM), the HC50 is 200 μM, while for the C9-2 stock solution (2.5 mM), the HC50 is slightly lower (170 μM). This has also been observed in the case of gemini/additive formulations. Finally, it is worth pointing out that the addition of cholesterol does not affect the hemolytic activity of these two surfactants, while the addition of DLPC increases their hemolytic behavior.
All these results can be related to the aggregate's size obtained by DLS and confirm that hemolysis increases when the aggregate's size decreases. In fact, it has been observed that in all cases the solutions containing most gemini (C9 1, C9/CHOL 1 and C9/DLPC 1) have bigger aggregates. For example, the C9 1 solutions contain aggregates with dH around 800 nm, while the C9 2 solution contains aggregates of about 200 nm.
A relevant difference in terms of HC50 values was found when phospholipids were added. The presence of 20% DLPC gave an HC50 value of 87 μM and the increase of additive up to 50% gave polydispersed small aggregates with an HC50 value smaller than 20 μM. Notice that solutions containing phospholipids form vesicles with lower dH rather than the aggregates observed in pure gemini or gemini/cholesterol formulations.
According to the bilayer couple hypothesis, amphiphiles induce shape alterations in erythrocytes by being asymmetrically distributed between the bilayer leaflets, thereby expanding one leaflet relative to the other. This transbilayer movement from the outer to the inner leaflet of the membrane is easier for single or gemini amphiphiles with a shorter alkyl chain, because of their small dimensions, while amphiphiles with longer spacers are readily intercalated into the outer membrane leaflet, but cannot translocate to the inner membrane because of their limited mobility.55
Some studies have reported that the hemolytic activity is higher for compounds with more hydrophobic content48 but in some cases this trend is not followed. Fogt et al.56 and Lv et al.18 for example, reported that traditionally for aliphatic chains, single-tailed cationic lipids are more toxic and less efficient than their double-tailed counterparts. Pinnaduwage et al.57 reported that cetyl trimethylammonium bromide (CTAB) was more toxic and less efficient than DOTMA. Moreover, the cytotoxicity of gemini cationic surfactants could be related to the length of the spacer; for example, Floch et al. reported that an increase in the length of the linker segment led to decreased toxicity in cell culture.58 The effect of hydrophobic domains on toxicity has not been adequately addressed to date and many scientists have been trying to give a proper explanation; however, there is still a long way to go.
Fig. 7–9 indicate that the hemolytic activity of these surfactants showed the following trend: C12 < C9 < LAM < C6. These data confirm that it is difficult to predict the hemolytic activity of surfactants and a lot of variables need to be considered. From the results obtained in this work, it can be stated that aggregate size and morphology play an important role in the hemolytic behaviour of these gemini surfactants: the bigger the aggregate's size, the smaller the hemolytic activity of the solutions. It can also be concluded that it is essential to accurately characterize colloidal formulations in order to obtain data that allow correlating results with toxic effects.
It is also worth mentioning that the hemolytic activity of LAM is lower than that of classical monoQuat surfactants with comparable CMC values and the hemolytic activity of C9(LA)2 and C12(LA)2 is much lower than that of bis(Quats) gemini surfactants53 or gemini surfactants based on pyridinium salts.19
Antimicrobial activity
The common antimicrobial agents are extremely irritant and lethal and it is necessary to formulate new types of safe and cost-effective biocidal materials. In this light, cationic surfactants could be good alternatives to traditional antimicrobial drugs because of the possibility to combine their biological properties with their surface features. It has been reported that the formulation of mixtures of cationic lipids, like DODAB, with other neutral lipids could influence the bacterial membrane lipids, modifying the final antibacterial activity.59 In fact, many studies have shown that antimicrobial formulations in the form of vesicles could be successfully used as potential bactericidal materials or in combination with hydrophobic drugs if adequately conjugated.60The arginine-based surfactants appear to be good candidates for the preparation of pharmaceutical formulations, thanks to their physico-chemical and biological properties.61 Within the monocatenary surfactants, the most effective against Gram positive and Gram negative bacteria is LAM, a homologue with 12 carbon atoms in the alkyl chain. The antimicrobial activity of pure Cn(LA)2 surfactants depends on the spacer chain: compounds with short spacers appear to improve the antimicrobial activity of their single chain homologue LAM, while the antibacterial potency decreases for longer spacer chains (C9(LA)2 or C10(LA)2).62 The hemolytic studies carried out in this work suggest that the interaction with biological membranes depends not only on the hydrophobicity of the molecules but also on the type and size of aggregates present in the solution. To confirm this behaviour, the antimicrobial activity was evaluated. Taking into account that the most anomalous results have been obtained for the longer spacer chains (C9(LA)2 and C12(LA)2) the antimicrobial activity has been determined for C9(LA)2 based formulations.
The antimicrobial activity of the formulations was determined in vitro on the basis of the MIC values,63 defined as the lowest concentration of an antimicrobial agent that inhibits the development of visible growth after 24 h of incubation at 37 °C. Stocks of pure surfactant solutions of C9(LA)2 and mixed surfactant–additive suspensions at two different concentrations were prepared as previously reported for hemolysis experiments and then diluted in the concentration range of 0.1–256 μg mL−1. The following bacteria were used: Gram positive bacteria: Bacillus subtilis ATCC 663, Staphylococcus epidermidis ATCC 12228, Micrococcus luteus ATCC 9341; Gram negative bacteria: Escherichia coli ATCC 8739; yeast: Candida albicans ATCC 10213. The MIC values are shown in Table 3.
| C9 1 μg mL−1 | C9 2 μg mL−1 | C9/CHOL 1 μg mL−1 | C9/CHOL 2 μg mL−1 | C9/DLPC 1 μg mL−1 | C9/DLPC 2 μg mL−1 | |
|---|---|---|---|---|---|---|
| Escherichia coli ATCC 8739 | >180 | >112 | >180 | >112 | >180 | 56 |
| Bacillus subtilis ATCC 6633 | >180 | >112 | 180 | >112 | >180 | 28 |
| Staphylococcus epidermidis ATCC 12228 | 90 | 56 | 45 | 28 | >180 | 28 |
| Micrococcus luteus ATCC 9341 | 90 | 56 | 45 | 28 | >180 | 28 |
| Candida albicans ATCC 10231 | >180 | 112 | 90 | >112 | >180 | 56 |
The results of the antimicrobial tests for all the formulations are reported in Table 3. Usually, the Gram-negative bacteria were more resistant than the Gram-positive bacteria to cationic surfactants.64 As expected, our systems showed low activity against Gram-negative bacteria, and only the C9/DLPC 2 formulation was active against Escherichia coli. Gram-negative bacteria exhibit a complex structure consisting of two bilayers: the outer membrane and the inner or cytoplasmic membrane. The outer membrane has a highly asymmetric composition, formed by a polysaccharide layer located in the outer leaflet and, to a large extent, by the zwitterionic phosphatidylethanolamine (PE) in the inner one. The cytoplasmic membrane consists to a large extent of PE and the negatively charged phosphatidylglycerol (PG). In contrast, Gram-positive bacteria have only a cytoplasmic membrane, which consists to a large extent of PG.
Large differences in the MIC values of these formulations have been found; this means that the additive plays an important role in the antibacterial activity. A higher activity was obtained for the C9/DLPC 2 sample, with this being the only formulation active against all microorganisms tested. This high activity confirms the hemolysis results. In fact, as mentioned before, it has not been possible to evaluate the EC50 of this formulation, due to its relevant toxicity against erythrocytes. It was reported that mixtures of more than one surfactant (either similar or different types) provide many advantages related to either the physical or chemical properties over the use of a single surfactant.42 Mixtures of alkyltrimethylammonium bromide surfactants–sodium alkyl methyl ester sulfonate or DODAB–DPPC at an equimolar mixing ratio (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) also have the highest potency against bacteria.65 This could be explained by the close packing of the mixed surfactant molecules at this mixing ratio, which was associated with high hydrophobicity, high adsorption tendency at the interface, smaller aggregate size and hence with disruption of the bacterial membrane.66
5) also have the highest potency against bacteria.65 This could be explained by the close packing of the mixed surfactant molecules at this mixing ratio, which was associated with high hydrophobicity, high adsorption tendency at the interface, smaller aggregate size and hence with disruption of the bacterial membrane.66
In general, for these formulations, the antimicrobial activity as well as the interaction with erythrocyte membranes follows similar trends: (a) the most active formulation is C9/DLPC 2, (b) C9/Chol samples are slightly more active than the corresponding C9 pure solutions and (c) in all cases the antimicrobial activity depends on the C9 concentration of the stock solution used to determine the MIC, the most active being those with a lower quantity of surfactant (for example, the MIC values of C9 1 are superior to those of C9 2).
In general, the antimicrobial activity is attributed largely to the net hydrophobic character and positive surfactant charge. Nevertheless, in all formulations, the active compound is the same C9(LA)2, and the zeta-potential values are comparable. This suggests that as previously described, the size of the aggregates in the solution strongly affects the interaction of this compound with bacteria. These studies indicate that the antimicrobial activity increases as the aggregate's size decreases. The molecular details of the mechanism by which cationic liposomes interact with biological membranes are still poorly understood. It has been reported that micelle-forming quaternary ammonium surfactants react with phospholipid components in the membrane, producing membrane distortion and lytic effects.67 The mechanism of vesicle prepared with bilayer-forming lipids such as DODAB seems to be different. In this case, the bacterial action possibly involves damage to protein function at the bacterial external wall level, where vesicles do adhere without vesicle rupture or cell lysis.68 Adsorption of DODAB cationic aggregates onto bacterial cells changes the sign of the cell surface potential from negative to positive with a clear relationship between positive charge on bacterial cells and cell death. It has also been reported that size, charge and transition temperature (Tm)69 of liposomes control the interaction with cellular membranes. Usually, for a given concentration, smaller aggregates imply more surface area and thus greater activity. Rejman et al.70 also showed that particle size might be an important parameter in the pathway of entry into the cells; moreover, the mechanism by which the particles were internalized was strongly dependent on particle size. These authors found that cells took up particles of size up to 500 nm and no uptake was seen for particles 1 μm in size. Moreover, from 50 to 500 nm the internalization decreases as the size increases. The experimental procedures carried out in this work do not allow us to establish the mechanism of interaction. It can be speculated that in these systems several of the mechanisms described above are present. Notice that in our formulations, prepared with micelle-forming surfactants, there are monomers, micelles and liposomes of very different size.
These data agree with those obtained by Carmona-Ribeiro and Vieira14 who studied the antimicrobial and hemolytic activity of DODAB bilayer fragments (dH = 79 nm and ξ = 41 mV) and DODAB large vesicles (dH = 500–800 nm and ξ = 48 mV) and found that small aggregates penetrate more deeply into the cell surface and consequently have lower MIC and HC50 values. However, different results have been obtained for small and large particles from cationic lipids and polyelectrolytes. In this case, antimicrobial effect was dependent on the amount of positive charge on the particles and independent of particle size.71
From these results, it can be stated that the addition of additives modifies the size of surfactant aggregates and this modification affects the interaction of these surfactants with biological membranes. Moreover, the results show that different variables have to be considered for antimicrobial activity and hemolytic behaviour.
It is noteworthy that using lipid–cationic gemini surfactant mixtures, formulations with low hemolytic activity and moderate antimicrobial activity were obtained. Notice that the gemini cationic surfactants used are easily biodegradable compounds that can be prepared from renewable materials using sustainable procedures.21 Moreover, these formulations show antimicrobial activity at lower concentrations than those at which they show erythrocyte toxicity, so that they can be considered safe formulations for biological applications.
Materials and methods
Materials
Chemicals and reagents were supplied by Sigma Aldrich and used without further purifications. L-Dilauroylphosphatidylcholine (DLPC) and cholesterol (Chol) were obtained from Sigma Chemicals and used as received. Pure Millipore water from a Milli-Q four-bowl system was used for preparing all sample solutions. Mueller-Hinton broth (MHB) was purchased from Difco Laboratories (USA).The Nα-lauroylarginine methyl ester (LAM) and the arginine-based gemini surfactants (CnLA2) were synthesized according to the procedure described previously72 and their physicochemical properties are shown in Table 4. Gemini surfactants are made up of two symmetrical long chain Nα-lauroylarginine residues of 12 carbon atoms linked by amide covalent bonds to an alkylenediamine spacer chain of six, nine or twelve methylene groups (Fig. 10). They were obtained at a purity of 99% by chemical condensation of the single Nα-lauroyl-L-arginine previously protected to the corresponding diamine in the presence of an activating agent. A final deprotection reaction was carried out to obtain the Gemini compounds. More details on the synthetic procedures, purification, NMR and HPLC characterization are given elsewhere.72 LAM was obtained by a two step procedure: (a) methylation of the acid group of the commercial L-arginine and (b) condensation of the lauric acid to the amino group of the arginine methyl ester using an activating agent. Further details regarding the synthesis are reported in the literature.62
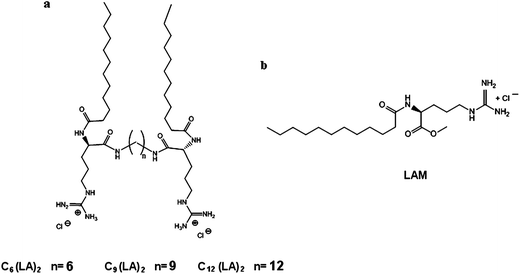 | ||
| Fig. 10 Molecular structure of (a) Cn(LA)2 and (b) LAM. | ||
Elemental analysis of LAM and Cn(LA)2:
Lauroyl arginine methyl ester, LAM. Found: C, 55.58; H, 9.45; N, 13.77. Anal. calcd for C19H39N4O3Cl: C, 56.07; H, 9.66; N, 13.77.
Nα,Nω-bis(Nα-dodecylarginine)α,ω-hexyl-diamide, C6(LA)2. Found: C, 58.03; H, 10.63; N, 16.12. Anal. calcd for C42H86N10O4Cl2: C, 58.33; H, 10.28; N, 16.20.
Nα,Nω-bis(Nα-dodecylarginine)α,ω-nonyl-diamide, C9(LA)2. Found: C, 59.33; H, 10.33; N, 15.22. Anal. calcd for C45H92N10O4Cl2: C, 59.60; H, 10.15; N, 15.50.
Nα, Nω-bis(Nα-dodecylarginine)α,ω-dodecyl-diamide, C12(LA)2. Found: C, 60.32; N, 14.57; H, 10.42. Anal. calcd for C48H98Cl2N10O4: C, 60.67; N, 14.74; H, 10.39.
Sample preparation
Pure cationic surfactant solutions were prepared weighing a defined number of moles (8 × 10−6 or 5 × 10−6) of every compound. Then 2 mL of Milliq water was added and the solution was gently stirred for 5 minutes at room temperature.Mixed cholesterol or phospholipid/cationic surfactant formulations were prepared using the sonication method. Vesicles were prepared starting from different molar ratios between surfactant and additive as reported in Table 1. Then, 2 mL of Milliq water was added and each sample was strongly stirred for 5 minutes and sonicated for 15 minutes at 50 °C. After preparation, the dispersion was left to equilibrate at room temperature overnight before use.
Zeta-potential and size distribution analysis
The Z-potential of the formulations was measured with the laser Doppler electrophoretic mobility measurements using the Zetasizer 2000 (Malvern Instruments Ltd., Malvern, U.K.), at 25 °C. All analysis was done in triplicate. Z-potential values and standard deviations (±S.D.) were elaborated directly from the instrument.The Dynamic Light Scattering technique (DLS) was used to measure the size distribution of all formulations. The DLS unit determining vesicle size is a Malvern Zeta Nanosizer, working at 632.8 nm and 25 °C. The DLS measurements, started 5–10 min after the sample solution, were placed in the DLS optical system to allow the sample to equilibrate at room temperature. The scattering intensity was measured at a 173° angle to the incident beam. This is known as backscatter detection and allows measurements in turbid dispersions, minimizing multiple scattering effects. Depending on the system turbidity, the unit automatically determines the sample thickness under investigation and focuses the beam at a given position from the cell walls. At least 10 runs were performed for each sample. The viscosity value (0.8872 mPa s) and the refractive index (1.33) of water were used for all the measurements. The correlation function obtained for monomodal distributions is different from that obtained for the multimodal distribution. After the correlation function has been measured, this information is used to calculate the size distribution. The software supplied by the manufacturer (Zetasizer v6.20) automatically determines the optimum parameters for the CONTIN73 algorithm for monomodal as well as multimodal distributions in order to produce a size distribution profile.
The time decay of the autocorrelation function of the concentration fluctuations has a characteristic decay rate, Γ, which is proportional to q2(Γ = Dq2), which characterizes a translational diffusion with the mutual diffusion constant, D. The scattering vector, q, is given by the equation
| Q = 4πn/λ0sin(θ/2) |
| D = kT/6πηRh |
Preparation of erythrocyte suspensions
Fresh blood was taken from rats and used on the same day of the experiment. The procedure was approved by the institutional ethics committee on animal experimentation. The erythrocytes were washed three times in a phosphate buffer solution (PBS) containing 123.3 mM NaCl, 22.2 mM Na2HPO4 and 5.6 mM KH2PO4 in distilled water (pH 7.4). The cells were then suspended at a cell density of 8 × 109cells per mL.Hemolysis assay
Hemolysis is the breakdown of red blood cells (erythrocytes) and the release of hemoglobin, occurring in a living organism or in a blood sample. To determine the hemolytic capacity of the colloidal systems prepared in this work, we followed an adaptation of the method described by Pape et al. (1987).74A series of different volumes of the samples (1–10 mg mL−1), ranging from 10 to 80 μL, were placed in polystyrene tubes containing 25 μL of erythrocyte suspension and phosphate buffered saline was added to each tube, to a total volume of 1 mL. Samples were incubated at room temperature while shaking for 10 minutes. Following incubation, the tubes were centrifuged (5 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm). The percentage of hemolysis was determined by comparing the absorbance (540 nm) of the supernatant of the samples with that of the control totally hemolysed with distilled water. Concentration-response curves were determined from hemolysis results (Fig. 11), and the concentration inducing 50% hemolysis (HC50) was calculated from these curves.
000 rpm). The percentage of hemolysis was determined by comparing the absorbance (540 nm) of the supernatant of the samples with that of the control totally hemolysed with distilled water. Concentration-response curves were determined from hemolysis results (Fig. 11), and the concentration inducing 50% hemolysis (HC50) was calculated from these curves.
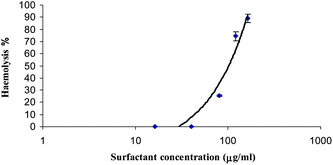 | ||
| Fig. 11 Haemolysis versus logarithm of concentration C, for LAM/CHOL 1 sample. | ||
Antimicrobial activity
Antimicrobial activity was determined in vitro on the basis of MIC values, defined as the lowest concentration of an antimicrobial agent that inhibits the development of visible growth after 24 h of incubation at 37 °C.75The compounds tested were dissolved in MHB in the concentration range of 0.1–256 μg mL−1, and no precipitate was observed at the highest concentration of the surfactant. The MHB was prepared according to the manufacturer's instructions. Then 10 μL of a nutrient broth starter culture of each bacterial strain was added to achieve final inoculums of ca. 5 × 10−4 to 5 × 10−5 CFU mL−1. The cultures were incubated overnight at 37 °C. Nutrient broth medium without the compound served as control. Microorganism growth was determined visually after incubation for 24 h at 37 °C compared with an inoculate culture without antimicrobial compound. The development of turbidity in an inoculated medium is a function of growth. A rise in turbidity reflects an increase in both mass and cell number. The lowest concentration of the antimicrobial agent at which no visible turbidity was observed was taken as the MIC.
Conclusions
Stable colloidal formulations based on arginine-single chain or gemini surfactants were prepared. The size of the aggregates depends on the molecular architecture of the surfactants, and the concentration and type of additives added to the formulation: aggregates of different dimensions and stability can be obtained starting from pure surfactant solutions or surfactant–additive mixtures. Single chain surfactants and Gemini with short spacer chains give rise to solutions with micellar aggregates while Gemini with long spacers form big aggregates as twisted ribbons.Antimicrobial and hemolytic activity is strongly affected by the size of the aggregates; small aggregates penetrate more deeply into the cell surface and consequently have lower MIC and HC50 values. The length of the spacer chain modulates the aggregation type and consequently the hemolytic and antimicrobial activities. Gemini with long spacer chains give rise to big aggregates and have more difficulties in interacting with biological membranes.
The introduction of additives such as cholesterol or DLPC also affects the biological properties of these surfactants. Moreover, the concentration of the stock solution used to carry out the biological tests is another important factor to take into account.
Several factors can be used to modulate the biological activity of these cationic surfactants. In fact, cationic colloidal solutions (mixtures of C9/CHOL 1) have been obtained in this work that are effective antimicrobial solutions with very low haemolytic activity.
The results of this work suggest new possible pharmaceutical devices based on arginine surfactants as a viable alternative to the classical formulations, showing good stability, low hemolytic effects and also a natural antimicrobial activity, which is not provided by conventional vesicles.
Acknowledgements
The authors would like to thank the financial support from Spanish Plan National I+D+I CTQ2009-14151-C02-01, CTQ2009-14151-C02-02, AGAUR 2009 SRG 246, CTQ2010-14897, CTQ2010-21183C02-01 and Unidad Asociada “Interacción de tensioactivos con membranes celulares”. Moreover, the project has been co-funded with support from the Commission European Social Fund and Region of Calabria (Italy) and the contract Estancia de Jóvenes Doctores Extranjeros en España, MEC, SB2010-0129.References
- D. Dwarakanadha-Reddy and D. Swarnalatha, Int. J. PharmTech Res., 2010, 2, 2025–2027 Search PubMed.
- L. M. S. Martins, E. M. Mamizuka and A. M. Carmona-Ribeiro, Langmuir, 1997, 13, 5583–5587 CrossRef CAS.
- A. M. Carmona-Ribeiro, F. Ortis, R. I. Schumaker and M. C. S. Armelin, Langmuir, 1997, 13, 2215–2218 CrossRef CAS.
- L. R. Tsuruta, W. Quintilio, M. H. B. Costa and A. M. Carmona-Ribeiro, J. Lipid Res., 1997, 38, 2003–2011 Search PubMed.
- A. M. Carmona-Ribeiro and B. R. Midmore, Langmuir, 1992, 8, 801–806 CrossRef CAS.
- M. M. Lessa and A. M. Carmona-Ribeiro, J. Colloid Interface Sci., 1996, 182, 166–172 CrossRef CAS.
- R. Rapuano and A. M. Carmona-Ribeiro, J. Colloid Interface Sci., 1997, 193, 104–109 CrossRef CAS.
- J. H. Felgner, R. Kumar, C. N. Sridhar, C. J. Wheeler, Y. J. Tsai, R. Border, P. Ramsey, M. Martin and P. L. Felgner, J. Biol. Chem., 1994, 28, 2550–2561 Search PubMed.
- Liposomes: A practical approach, ed. V. P. Torchilin and V. Weissig, Oxford Univ. Press, Oxford, 2003 Search PubMed.
- Y. T. Ko, C. Falcao and V. P. Torchilin, Mol. Pharmaceutics, 2009, 6, 971–977 CAS.
- Z. Drulis-Kawaa, A. Dorotkiewicz-Jacha, J. Gubernator, G. Gula, T. Bocera and W. Doroszkiewicza, Int. J. Pharm., 2009, 367, 211–219 Search PubMed.
- S. Sachetelli, H. Khalil, T. Chen, C. Beaulac, S. Senechal and J. Lagace, Biochim. Biophys. Acta, 2000, 1463, 254–266 Search PubMed.
- N. M. Sanderson, B. Guo, A. E. Jacob, P. S. Handley, J. G. Cunniffe and M. N. Jones, Biochim. Biophys. Acta, Biomembr., 1996, 1283, 207–214 CrossRef.
- D. B. Vieira and A. M. Carmona-Ribeiro, J. Antimicrob. Chemother., 2006, 58, 760–767 Search PubMed.
- S. Aleandri, M. G. Bonicelli, F. Bordi, S. Casciardi, M. Diociaiuti, L. Giansanti, F. Leonelli, G. Mancini, G. Perrone and S. Sennato, Soft Matter, 2012, 8, 5904–59015 RSC.
- M. T. Campanhã, E. M. Mamizuka and A. M. Carmona-Ribeiro, J. Phys. Chem. B, 2001, 105, 8230–8236 Search PubMed.
- S. M. Sicchierolli, E. M. Mamizuka and A. M. Carmona-Ribeiro, Langmuir, 1995, 11, 2991–2995 Search PubMed.
- H. Lv, S. Zhang, B. Wang, S. Cui and J. Yan, J. Controlled Release, 2006, 114, 100–109 CrossRef CAS.
- A. Shirai, T. Maeda, H. Nagamune, H. Matsuki, S. Kaneshina and H. Kourai, Eur. J. Med. Chem., 2005, 40, 113–123 Search PubMed.
- T. Thorsteinsson, M. Masson, K. G. Kristinsson, M. A. Hjalmarsdottir, H. Hilmarsson and T. Loftsson, J. Med. Chem., 2003, 46, 4173–4181 CrossRef CAS.
- M. R. Infante, L. Pérez, C. Morán, R. Pons and A. Pinazo, Biobased Surfactants and Detergents: Synthesis, Properties, and Applications, ed. D. G. Hayes, D. Kitamoto, D. K. Y. Solaiman and R. D. Ashby, AOCS Press, Champaign, IL USA, 2009 Search PubMed.
- M. Rosa, N. Penacho, S. Simoes, M. C. P. Lima, B. Lindman and M. G. Miguel, Mol. Membr. Biol., 2008, 25, 23–34 Search PubMed.
- J. A. Castillo, A. Pinazo, J. Carilla, M. R. Infante, M. A. Alsina, I. Haro and P. Clapes, Langmuir, 2004, 20, 3379–3387 CrossRef CAS.
- J. Y. Huang, J. T. Bulboltz and G. W. Feigenson, Biochim. Biophys. Acta, 1999, 1417, 89–100 CAS.
- L. Perez, A. Pinazo, M. R. Infante and R. Pons, J. Phys. Chem. B, 2007, 111, 11379–11387 CrossRef CAS.
- H. Wennerström and J. Ulmius, J. Magn. Reson., 1976, 23, 431–435 CAS.
- D. Weihs, D. Danino, A. Pinazo, L. Pérez, E. I. Franses and Y. Talmon, Colloids Surf., A, 2005, 255, 73–78 Search PubMed.
- L. Ziserman, A. Mor, D. Harries and D. Danino, Phys. Rev. Lett., 2011, 106, 238105–238109 CrossRef.
- L. Ziserman, H. Y. Lee, S. R. Raghvan, D. Mor and D. Danino, J. Am. Chem. Soc., 2011, 133, 2511–2517 CrossRef CAS.
- A. L. Barrán-Berdón, M. Muñoz-Úbeda, C. Aicart-Ramos, L. Pérez, M. R. Infante, P. Castro-Hartmann, A. Martín-Molina, E. Aicart and E. Junquera, Soft Matter, 2012, 8, 7368–3380 RSC.
- L. Tavano, R. Muzzalupo, R. Cassano, S. Trombino, T. Ferrarelli and N. Picci, Colloids Surf., B, 2010, 75, 319–322 Search PubMed.
- R. Muzzalupo, L. Tavano, R. Cassano, S. Trombino, T. Ferrarelli and N. Picci, Eur. J. Pharm. Biopharm., 2011, 79, 28–35 Search PubMed.
- I. F. Uchegbu, in Synthetic Surfactant Vesicles: Niosomes and Other Non-phospholipid Vesicular Systems, Harwood Academic, Amsterdam, 2000 Search PubMed.
- A. Pinazo, X. Wen, L. Pérez, M. R. Infante and E. I. Franses, Langmuir, 1999, 15, 3134–3142 CrossRef CAS.
- Surfactant Systems. Their Chemistry, Pharmacy and Biology, ed. D. Attwood and A. T. Florence, Chapman and Hall, London, 1983 Search PubMed.
- C. Vautier-Giongo, M. S. Bakshi, J. Singh, R. Ranganathan, J. Hajdu and B. L. Bales, J. Colloid Interface Sci., 2005, 282, 149–155 CrossRef CAS.
- A. M. Seddon, P. Curnow and P. J. Booth, Biochim. Biophys. Acta, 2004, 1666, 105–117 CAS.
- A. Bonincontro, C. La Mesa, C. Proietti and G. A. Risuleo, Biomacromolecules, 2007, 8, 1824–1829 CrossRef CAS.
- A. S. Narang, D. Delmarre and D. Gao, Int. J. Pharm., 2007, 345, 9–25 CrossRef CAS.
- T. Patra, S. Ghosh and J. Dey, Soft Matter, 2010, 6, 3669–3679 RSC.
- O. Pornusunthorntawee, S. Chavadej and R. Rujiravanit, J. Biosci. Bioeng., 2011, 112, 102–106 Search PubMed.
- C. Sobral, M. Soto and A. M. Carmona-Riberio, Chem. Phys. Lipids, 2008, 152, 38–45 Search PubMed.
- V. B. Junyaprasert, V. Teeranachaideekul and T. Supaperm, AAPS PharmSciTech, 2008, 9, 851–859 Search PubMed.
- F. A. Robertis and E. M. H. Robertis, in Cell Membrane Sunders, London, 1995 Search PubMed.
- M. A. Vives, M. R. Infante, E. Garcia, C. Selve, M. Maugras and M. P. Vinardell, Chem.–Biol. Interact., 1999, 118, 1–18 CrossRef CAS.
- L. A. Gould, A. B. Lansley, M. B. Brown, B. Forbes and G. P. Martin, J. Pharm. Pharmacol., 2000, 52, 1203–1209 Search PubMed.
- D. Lundberg, H. Ljusberg-Wahren, A. Norlin and K. Holmberg, J. Colloid Interface Sci., 2004, 278, 478–487 CrossRef CAS.
- N. Lozano, L. Perez, R. Pons and A. Pinazo, Amino Acids, 2011, 40, 721–729 Search PubMed.
- R. Cortesi, E. Esposito, E. Menegatti, R. Gambari and C. Nastruzzi, Int. J. Pharm., 1996, 139, 69–78 CrossRef CAS.
- M. Mitjans, V. Martinez, P. Clapés, L. Pérez, M. R. Infante and M. P. Vinardell, Pharm. Res., 2003, 20, 1697–1701 Search PubMed.
- A. Colomer, A. Pinazo, M. T. García, M. Mitjans, M. P. Vinardell, M. R. Infante, V. Mártinez and L. Pérez, Langmuir, 2012, 28, 5900–5912 Search PubMed.
- N. Nagamune, T. Maeda, K. Ohkura, K. Yamamoto, M. Nakajima and H. Kourai, Toxicol. in Vitro, 2000, 14, 139–147 CrossRef CAS.
- M. Dubnickova, M. Bobrowska-Hägerstrand, T. Söderström, A. Iglic and H. Hägerstrand, Acta Biochim. Pol., 2000, 47, 651–660 CAS.
- M. Lukac, J. Mojzis, G. Molzisova, M. Mrva, F. Ondriska, J. Valentova, I. Lacko, M. Bukovsky, F. Devinsky and J. Karlovska, Eur. J. Med. Chem., 2009, 44, 4970–4977 Search PubMed.
- M. P. Sheetz and S. J. Singer, Proc. Natl. Acad. Sci. U. S. A., 1974, 71, 4457–4461 CrossRef CAS.
- A. Fogt, H. Hagerstrand and B. Isoma, Chem.-Biol. Interact., 1995, 94, 147–155 Search PubMed.
- P. Pinnaduwage, L. Schmitt and L. Huang, Biochim. Biophys. Acta, 1989, 98, 533–537 Search PubMed.
- V. Floch, S. Loisel, E. Guenin, A. C. Herve, J. C. Clement, J. J. Yaouanc, H. des Abbayes and C. Ferec, J. Med. Chem., 2000, 30, 4617–4628 Search PubMed.
- N. Lincopan and A. M. Carmona-Ribeiro, J. Antimicrob. Chemother., 2006, 58, 66–75 Search PubMed.
- M. T. Campanhã, E. M. Mamizuka and A. M. Carmona-Ribeiro, J. Lipid Res., 1999, 40, 1495–1500 Search PubMed.
- L. Perez, M. T. García, I. Ribosa, P. Vinardell, A. Manresa and M. R. Infante, Environ. Toxicol. Chem., 2002, 21, 1279–1285 CAS.
- M. R. Infante, L. Pérez and A. Pinazo, in Novel Cationic Surfactants from Arginine, Novel Surfactants, Preparation, Applications and Biodegradability, Surfactant Science Series, ed. K. Holmberg, Marcel Dekker, 2003 Search PubMed.
- J. P. Anhalt and J. A. Washington, in Manual of Clinical Microbiology, ed. P. R. Murray, ASM Press, Washington, DC, 6th edn, 1995 Search PubMed.
- T. S. Franklin and G. A. Snow, Biochemistry of Antimicrobial Action, Chapman and Hall, New York, 3rd edn, 1981 Search PubMed.
- S. P. Wong, W. H. Lim, S. F. Cheng and C. H. Chuah, Colloids Surf., B, 2012, 89, 48–52 Search PubMed.
- A. N. Nabel, A. A. Ismail and M. T. J. Salah, J. Surfactants Deterg., 2010, 13, 503–511 Search PubMed.
- J. P. S. Cabral, Can. J. Microbiol., 1992, 38, 115 Search PubMed.
- L. M. S. Martins, E. M. Mamizuka and A. M. Carmona-Ribeiro, Langmuir, 1997, 13, 5583–5587 CrossRef CAS.
- C. Bombelli, A. Stringaro, S. Borocci, G. Bozzuto, M. Colone, L. Giansanti, R. Sgambato, L. Toccaceli, G. Mancini and A. Molinari, Mol. Pharmaceutics, 2010, 7, 130–137 CAS.
- J. Rejman, V. Oberle, I. S. Zuhorn and D. Hoekstra, Biochem. J., 2004, 377, 159–169 CrossRef CAS.
- L. D. Melo, E. M. Mamizuka and A. M. Carmona-Ribeiro, Langmuir, 2010, 26, 12300–12306 CrossRef CAS.
- L. Pérez, J. L. Torres, A. Manresa, C. Solans and M. R. Infante, Langmuir, 1996, 12, 5296–5301 CrossRef CAS.
- S. W. Provencher, Comput. Phys. Commun., 1982, 27, 229–242 CrossRef.
- W. J. Pape, U. Pfannenbecker and U. Hoppe, Validation of the red blood cell test system as in vitro assay for rapid screening of irritation potential of surfactants, Mol. Toxicol., 1987, 1, 525–536 Search PubMed.
- R. N. Jones, A. L. Barry, T. L. Gavan and J. A. Washington, in Manual of Clinical Microbiology, ed. E. H. Lennette, A. Ballows, W. J. Hauser and H. J. Shadomy, American Society for Microbiology, Washington, DC, 4th edn, 1985 Search PubMed.
| This journal is © The Royal Society of Chemistry 2013 |
