Robust, electro-conductive, self-healing superamphiphobic fabric prepared by one-step vapour-phase polymerisation of poly(3,4-ethylenedioxythiophene) in the presence of fluorinated decyl polyhedral oligomeric silsesquioxane and fluorinated alkyl silane†
Hongxia
Wang
a,
Hua
Zhou
a,
Adrian
Gestos
a,
Jian
Fang
a,
Haitao
Niu
a,
Jie
Ding
b and
Tong
Lin
*a
aInstitute for Frontier Materials, Deakin University, Geelong, VIC 3216, Australia. E-mail: tong.lin@deakin.edu.au
bHuman Protection and Performance Division, Defence Science and Technology Organisation, Melbourne, VIC 3207, Australia
First published on 18th October 2012
Abstract
A robust, electrically conductive, superamphiphobic fabric was prepared by vapour-phase polymerisation of 3,4-ethylenedioxythiophene (EDOT) on fabric in the presence of fluorinated decyl polyhedral oligomeric silsesquioxane (FD-POSS) and a fluorinated alkyl silane (FAS). The coated fabric had contact angles of 169° and 156° respectively to water and hexadecane, and a surface resistance in the range of 0.8–1.2 kΩ □−1. The incorporation of FD-POSS and FAS into the PEDOT layer showed a very small influence on the conductivity but improved the washing and abrasion stability considerably. The coated fabric can withstand at least 500 cycles of standard laundry and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of abrasion without apparently changing the superamphiphobicity, while the conductivity only had a small reduction after the washing and abrasion. More interestingly, the coating had a self-healing ability to auto-repair from chemical damages to restore the liquid repellency.
000 cycles of abrasion without apparently changing the superamphiphobicity, while the conductivity only had a small reduction after the washing and abrasion. More interestingly, the coating had a self-healing ability to auto-repair from chemical damages to restore the liquid repellency.
Introduction
Smart or intelligent textiles are being widely developed as a new generation of textiles with advanced enforcement of wearers' physiology, health and safety.1,2 They are expected to have sensing, actuating, data processing, communicating, energy supplying and/or other functions, which are currently accomplished chiefly by incorporation of hard electronics into textiles. As such, electrically conductive textiles are prerequisite to functioning smart textiles,3 and their quality also determines the durability, launderability, reusability and fibrous performances of smart textiles.A range of techniques have been developed to impart fibres/textiles with electrical conductivity, including co-weaving of metal wires with synthetic fibres into the fibrous matrix4 or deposition of metals,5,6 conductive carbons (e.g. carbon nanotubes7 and graphene8) or conducting polymers9–12 on the fibre surface. Conducting polymers, such as polypyrrole, polyaniline and polythiophene, have become a popular choice of conductive coating materials because they are easy to prepare, lightweight, flexible, biocompatible, and can be potentially tailored to have a sensing or actuating function.13,14 Polypyrrole and polyaniline have been reported as textile coatings widely throughout the literature.15–18 Poly(3,4-ethylenedioxythiophene) (PEDOT) is being increasingly investigated as a textile coating19,20 because of its good stability and high conductivity.21
Recently, superhydrophobicity or superoleophobicity has been added to conducting polymers with the purpose of creating multi-functional materials. Superhydrophobic surfaces have a contact angle greater than 150° to water. They show self-cleaning, anti-contaminating and anti-sticking functions. Recent studies have indicated that superhydrophobic surfaces can be used to protect electronic devices22 which are useful for development of smart textiles. Similarly, superoleophobic surfaces have a contact angle greater than 150° to oil fluids. They have great potential in antifouling from hazardous chemicals and biological contaminants.23 When a surface is both superhydrophobic and superoleophobic, which is referred to as a “superamphiphobic” surface,24 its liquid repellency is enhanced considerably.
Electrically conductive superhydrophobic surfaces have been fabricated by several methods, such as direct electrochemical polymerisation of conducting polymers on metal substrates,25 self-assembly of conductive nanomaterials26 and template-assisted solution polymerisation.27 Conductive superoleophobic coatings are mainly prepared by electrochemical polymerisation methods using fluorinated monomers.28–30 However, all these techniques are based on hard conductive substrates. Conductive superhydrophobic/superoleophobic fabrics have received little attention until very recently.31
Most superhydrophobic/superoleophobic coatings have problems with poor durability.32 Repeated washing or mechanical abrasion makes them lose their surface liquid repellency. To improve the durability, several strategies have been developed, including crosslinking the coating layer,33–35 creating multi-scaled roughness on the substrate,36 establishing chemical bonds between the coating and the substrate,37 introducing a bio-inspired self-healing function,38,39 or endowing the coating with an elastomeric nanocomposite structure.40 However, work on the durability of electrically conductive superhydrophobic/superoleophobic coatings has not been reported in the research literature.
In our previous study, we have prepared an electrically conductive, superamphiphobic fabric by direct vapour-phase polymerisation of polypyrrole in the presence of a fluorinated alkyl silane (FAS) on fabric.31 The coating is patternable and it can even work as an electrical connector in a fluid-contaminated environment. Separately, we have also developed a durable superamphiphobic fabric, simply by applying a hydrolysed FAS containing fluorinated decyl polyhedral oligomeric silsesquioxane (FD-POSS) onto fabrics.41 The coating was not only durable against repeated washing and abrasion, but also had a bio-mimicking, self-healing ability.
In our present study, we found that when PEDOT was vapour-phase polymerised onto fabrics, the incorporation of FD-POSS and FAS into the coating layer during the polymerisation process endowed the conductive coating with a durable superamphiphobic surface. The addition of FD-POSS and FAS into the PEDOT coating layer showed little influence on the conductivity. More interestingly, the coating had a self-healing ability to auto-repair from chemical damages. In this paper, for the first time, we report on the preparation of this novel, conductive, super-repellent fabric and its durable performance. A plain weave polyester fabric was used as the fabric substrate.
Experimental
Materials
3,4-Ethylenedioxythiophene (EDOT), FeCl3·6H2O and ethanol were purchased from Aldrich and used as received. Triethoxy(tridecafluorooctyl)silane (FAS) (Dynasylan F 8261) was supplied by Degussa. Commercial polyester (plain weave, 168 g m−2) was used as the substrate. FD-POSS was synthesised using a previously described method.42Coating process
Fabric substrates were dipped in a FeCl3·6H2O (2%, w/v)–acetone solution for 5 minutes, and then removed and allowed to dry at room temperature for 30 minutes. The FeCl3 coated fabric was then put into a small chamber filled with saturated EDOT vapour at 60 °C for 30 minutes to carry out the polymerisation reaction. The fabric was finally rinsed with ethanol followed by water to remove any side products and extra reactants from the fabrics.To incorporate FD-POSS and FAS into the PEDOT coating layer, FD-POSS (0.1 g) was firstly dissolved in FAS (0.5 g). The homogeneous FD-POSS/FAS solution was then dispersed into the aforementioned FeCl3·6H2O–acetone solution (10 ml). The same procedure was used to apply the solution onto fabrics and carry out the polymerisation reaction as for the PEDOT only coating. For comparison, a coating containing FAS with no FD-POSS was also prepared in a similar manner. However, the insolubility of FD-POSS without FAS in acetone prevented the formation of a PEDOT/FD-POSS control coating. As a result, only a PEDOT/FAS comparison coating was produced.
Characterisations
A field emission electron microscope (FESEM, Leo 1530, Gemini/Zeiss, Oberkochen, Germany) was used to image the samples. Fourier Transform Infrared (FTIR) spectra were measured using a FTIR spectrometer (Bruker Optics, Ettlingen, Germany) in ATR mode. X-ray photoelectron spectra (XPS) were collected on a VG ESCALAB 220-iXL XPS spectrometer with a monochromated Al Kα source (1486.6 eV) using samples of ca. 3 mm2 in size. The X-ray beam incidence angle is 0° with respect to the surface normal, which corresponds to a sampling depth of ca. 10 nm. The obtained XPS spectra were analysed by the CasaXPS software. A Cypher atomic force microscope (AFM) (Asylum Research) was used to measure surface roughness. Contact angles were measured by a commercial contact angle meter (KSV CAM101 Instruments Ltd) using 13 μL fluid droplets. The conductivity of the coated fabric was characterised using a standard two-probe method for measurement of surface resistance.43Plasma treatment
The treated fabrics were subjected to a vacuum plasma treatment (gas source: air) for 5 minutes. Such plasma treatment can make the surface completely hydrophilic (contact angle 0°).Washing durability test
The washing durability of the treated fabric was examined by washing the coated fabrics in a washing machine according to the method specified in the Australian Standard (AS 2001.1.4). Such a standard wash procedure is equivalent to five cycles of home machine launderings. For convenience, we use the equivalent number of home machine launderings in this paper.Abrasion resistance test
The abrasion resistance was tested using the Martindale method, according to Standard ASTM D4966. The test was performed under a commercial Martindale abrasion tester (I. D. M. Instrument Design and Maintenance). The fabric sample was mounted on a dynamic disk which was brought into contact with an abradant underneath. The abradant was mounted on a separate motionless disk. Pressure was applied by adding weights onto the upper shaft. During testing, the dynamic disk was rotated on its axis, at the same time as following a circular path across the abradant surface. Untreated fabric was used as the abradant. During the test, 12 kPa of loading pressure was employed, which is typically used to evaluate coated fabrics for heavy duty upholstery usages.Results and discussion
The chemical structures of the monomer EDOT, FD-POSS and FAS are presented in Fig. 1a. Fig. 1b schematically illustrates the coating procedure. After coating treatment, the fabric turned black (Fig. 1c), indicating the successful polymerisation of PEDOT.44,45Fig. 1d shows the SEM image of the PEDOT/FD-POSS/FAS coated fibres, which looks similar to the uncoated ones (see the SEM image of uncoated fibre in the ESI†). The TEM image shown in the inset of Fig. 1d indicates the formation of a thin conformal coating with a thickness around 80 nm after the coating treatment.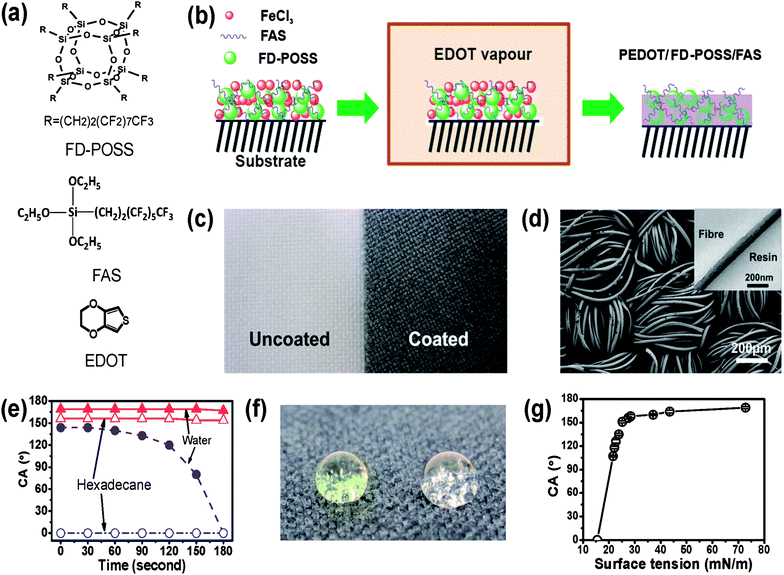 | ||
| Fig. 1 (a) Chemical structures of FD-POSS, FAS and EDOT, (b) illustration of vapour-phase polymerisation to form PEDOT/FD-POSS/FAS coating on fabrics, (c) photo of polyester fabric before (left) and after (right) coating treatment, (d) SEM image of the polyester fabric after coating with PEDOT/FD-POSS/FAS (inset is a cross-sectional TEM image of a PEDOT/FD-POSS/FAS coated fibre), (e) contact angle of the coated fabric changing over time from initial fluid–fabric contact, (f) coloured water (yellow) and clear hexadecane drops on the PEDOT/FD-POSS/FAS coated fabric (the surface tension of water and hexadecane is 72.80 and 27.47 mN m−1, at 20 °C), and (g) dependency of the contact angle on the surface tension of fluids. | ||
Without FD-POSS and FAS, the fabric coated with only PEDOT showed a low liquid repellency. Although the initial water contact angle of the PEDOT coated fabric can be as large as 144°, water droplets could not stay stable on the coated fabric. Within 3 minutes, the droplets spread into the fabric matrix (Fig. 1e). In contrast, the PEDOT/FD-POSS/FAS treatment provided the fabric with a persistent fluid-resistant coating (Fig. 1e and f), with a contact angle (CA) of 169° and 156° to water and hexadecane, respectively. A series of liquid fluids were used to test the dependency of the contact angle on the surface tension. It was revealed that a liquid would have a contact angle greater than 150° when its surface tension was above 27 mN m−1 (Fig. 1g), a typical characteristic of superamphiphobicity.31
XPS and FTIR were used to examine the chemical components of the coated fabrics. When FAS and FD-POSS were incorporated into the coating layer, the elements fluorine and silicon appeared in the XPS spectra, indicating the presence of FD-POSS or FAS in the PEDOT layer. The atomic ratio of F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si for the PEDOT/FD-POSS/FAS coating was slightly higher than that of PEDOT/FAS (ESI†). The FTIR spectra confirmed the formation of PEDOT and the presence of FD-POSS or FAS in coating layers when they were present during the polymerisation reaction (ESI†).
Si for the PEDOT/FD-POSS/FAS coating was slightly higher than that of PEDOT/FAS (ESI†). The FTIR spectra confirmed the formation of PEDOT and the presence of FD-POSS or FAS in coating layers when they were present during the polymerisation reaction (ESI†).
The fibre surface roughness was measured by AFM imaging. The surface became slightly rougher after the PEDOT/FD-POSS/FAS coating (ESI†). Based on these results, it was reasonably assumed that the excellent liquid-repellency should come from the rough fibrous structure, combined with the fluoro-functionalised surface effect.
The surface conductivity of fabrics was measured using a standard method (AATCC 76-1995). Before coating treatment, the polyester fabric had a very large surface resistance due to its non-conductive nature. When the fabric was coated with PEDOT/FD-POSS/FAS, the surface resistance became 1.0 ± 0.2 KΩ □−1. In comparison, the fabrics coated with PEDOT alone or PEDOT/FAS showed a surface resistance of 0.6 ± 0.15 KΩ □−1. The slightly decreased conductivity suggests that FD-POSS has a very small influence on the conductivity of the PEDOT coating. Previous studies using polypyrrole as the conducting polymer in a fabric coating also showed little change in surface resistance upon addition of FAS to the coating.31
The abrasion durability was measured using the Martindale method (ASTM D4966). As expected, the contact angle decreased with increasing abrasion cycles for both PEDOT/FD-POSS/FAS and PEDOT/FAS coated fabrics (Fig. 2a). After long cycles of abrasion, the coated fabric became lighter in appearance (ESI†). The PEDOT/FD-POSS/FAS coating showed enhanced abrasion durability over the PEDOT/FAS coating. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of abrasion, the PEDOT/FD-POSS/FAS coated fabric still retained its superamphiphobicity. For the PEDOT/FAS coated fabric, the contact angle to hexadecane became 0° after 8000 abrasion cycles, and the water contact angle dropped to only 137°. Hexadecane and water drops on the coated fabric after the respective cycles of abrasion are shown in Fig. 2b and c.
000 cycles of abrasion, the PEDOT/FD-POSS/FAS coated fabric still retained its superamphiphobicity. For the PEDOT/FAS coated fabric, the contact angle to hexadecane became 0° after 8000 abrasion cycles, and the water contact angle dropped to only 137°. Hexadecane and water drops on the coated fabric after the respective cycles of abrasion are shown in Fig. 2b and c.
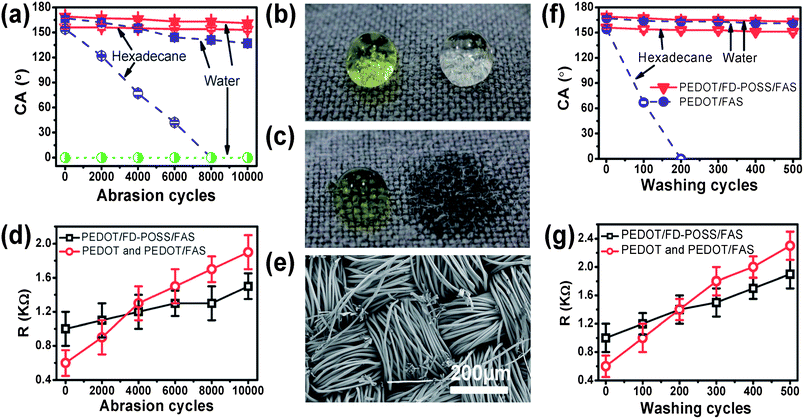 | ||
Fig. 2 (a) Effect of abrasion cycles on the liquid contact angle of coated fabrics, (b) and (c) photo of water (yellow) and clear hexadecane drops on (b) PEDOT/FD-POSS/FAS (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 abrasion cycles) and (c) PEDOT/FAS (8000 abrasion cycles) coated polyester fabrics after the abrasion test, (d) effect of abrasion cycles on the surface resistance of coated fabrics, (e) SEM image of PEDOT/FD-POSS/FAS coated fabric after 10 000 abrasion cycles) and (c) PEDOT/FAS (8000 abrasion cycles) coated polyester fabrics after the abrasion test, (d) effect of abrasion cycles on the surface resistance of coated fabrics, (e) SEM image of PEDOT/FD-POSS/FAS coated fabric after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 abrasion cycles, and (f) and (g) effect of washing cycles on (f) contact angle and (g) surface resistance of coated fabrics. 000 abrasion cycles, and (f) and (g) effect of washing cycles on (f) contact angle and (g) surface resistance of coated fabrics. | ||
The effect of abrasion cycles on the surface conductivity was similar to their effect on the contact angle. As shown in Fig. 2d, the surface resistance increases with increasing the abrasion cycles for all PEDOT coated fabrics regardless of whether the coating contains FD-POSS or FAS additive. The surface resistance of the PEDOT/FD-POSS/FAS coated fabric changed at a lower rate compared to the PEDOT/FAS fabric. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 abrasion cycles, the average surface resistance of the PEDOT/FD-POSS/FAS coated fabric increased only from 1.0 to 1.5 kΩ □−1, while a larger resistance increase was observed for the PEDOT/FAS coated fabric, from 0.6 to 1.9 kΩ □−1.
000 abrasion cycles, the average surface resistance of the PEDOT/FD-POSS/FAS coated fabric increased only from 1.0 to 1.5 kΩ □−1, while a larger resistance increase was observed for the PEDOT/FAS coated fabric, from 0.6 to 1.9 kΩ □−1.
For comparison, the polyester fabric coated only by PEDOT was also subjected to the same abrasion treatment. While the wettability of the PEDOT coated fabric was unchanged by the abrasion, the surface resistance increased with increasing abrasion cycle. It was interesting to note that the surface resistance of the PEDOT coated fabric changed in almost the same way as that of the PEDOT/FAS coated fabrics (ESI†). This suggests that FD-POSS plays a key role in improving the abrasion durability of the PEDOT coating and the addition of FAS to PEDOT does not improve the abrasion durability.
Fig. 2e shows the SEM image of the PEDOT/FD-POSS/FAS coated fabric after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 abrasion cycles. A few fibres appeared frayed and broken in the image, despite the retention of the fabric's overall superamphiphobicity and only a slight increase in the surface resistance. This suggests that the fibre coating is not significantly damaged by the abrasion. The PEDOT/FAS and PEDOT coated fabrics showed a similar result (see the SEM images in the ESI†).
000 abrasion cycles. A few fibres appeared frayed and broken in the image, despite the retention of the fabric's overall superamphiphobicity and only a slight increase in the surface resistance. This suggests that the fibre coating is not significantly damaged by the abrasion. The PEDOT/FAS and PEDOT coated fabrics showed a similar result (see the SEM images in the ESI†).
Fig. 2f and g show the effect of repeated washing on the contact angle and surface resistance. With increasing laundry cycles, the PEDOT/FD-POSS/FAS coated fabric had a small decrease in both water and hexadecane contact angles. After 500 cycles of washing, the superamphiphobic property was retained. However, for the PEDOT/FAS coated fabric, although superhydrophobicity was retained throughout the whole 500 laundry cycles, the superoleophobicity was lost after just a few cycles of washing, and the fabric became completely oleophilic to hexadecane (contact angle 0°) after only 200 washing cycles. This was presumably due to the removal of FAS molecules from the coating layer during washing. The detergent in water may facilitate the leaching FAS molecules off the coating layer. Since the FD-POSS molecule has a large size, it is relatively hard for it to diffuse into water from the polymer layer.
It was also noted that the surface resistance increased with increasing washing cycles. After 500 washing cycles, the average surface resistance of the PEDOT/FD-POSS/FAS coated fabric increased from 1.0 kΩ □−1 to 1.9 kΩ □−1. A larger resistance increase was found on the PEDOT/FAS coated fabric which increased from 0.6 kΩ □−1 to 2.3 kΩ □−1. For the fabric coated with PEDOT only, the change in the surface resistance followed the same trend as that of the PEDOT/FAS coated fabric (ESI†).
More interestingly, we found that the coated fabric showed a self-healing property that can auto-repair from chemical damages and restore the surface liquid repellency. Here, the surface of the coated fabric was deliberately damaged by a vacuum plasma treatment using air as the gas source. After plasma treatment for a few minutes, both PEDOT/FD-POSS/FAS and PEDOT/FAS coated fabrics became hydrophilic and oleophilic with a contact angle of 0° to both water and hexadecane (Fig. 3a). When the plasma-treated fabrics were then heated to 135 °C for 5 minutes, the fabrics completely restored their superamphiphobic property (Fig. 3b). The self-healing also took place at room temperature but took considerably longer, on a scale of 24 hours (ESI†). However, the plasma treatment showed no influence on the surface resistance.
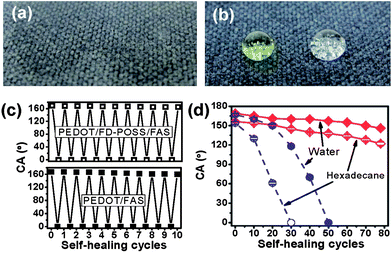 | ||
| Fig. 3 Water and hexadecane drops on a PEDOT/FD-POSS/FAS treated polyester fabric: (a) after plasma treatment and (b) after plasma and heat treatment, (c) water contact angle of the coated fabric in the first 10 cycles of plasma and heat treatment, and (d) change of water and hexadecane contact angles with plasma- and-heat treatment cycles for PEDOT/FD-POSS/FAS and PEDOT/FAS coated fabrics. | ||
This self-healing was repeatable, as shown by the effect of the first 10 cycles of plasma and heat treatments on the water contact angle of coated fabrics, which decreases slightly over the plasma and heat treatment cycles (Fig. 3c). For the PEDOT/FD-POSS/FAS coated fabric, the water contact angle changed from 169° to 165° after the first 10 cycles of plasma and heat treatment, while for the PEDOT/FAS coated fabric it changed from 167° to 160°.
The effects of longer-term plasma and heat cycles on the water and hexadecane contact angles are presented in Fig. 3d. The PEDOT/FD-POSS/FAS coated fabric retained its superhydrophobicity up to 70 cycles of plasma and heat treatment, while its superoleophobicity was retained up to only 20 plasma and heat cycles. Although the superamphiphobicity of the PEDOT/FD-POSS/FAS coating was lost, the amphiphobicity of the coating was retained up to the testing limit of 78 plasma and heat cycles. This retention of amphiphobicity of the PEDOT/FD-POSS/FAS coating was in contrast to the complete wetting of the PEDOT/FAS coating that was achievable after just 50 plasma and heat cycles for water and 30 cycles for hexadecane.
The self-healing is attributed to the molecular migration of surface functional groups so that the bulk surface free energy is minimised, and this can typically be sped up by increasing temperature.41 The self-healing ability of the coating with added FD-POSS was clearly more robust and longer lasting than that without FD-POSS. This is presumably because of the highly concentrated fluoroalkyl groups in the FD-POSS molecule and its nearly spherical molecular structure. As a result of molecular rotation and movement, the polar groups introduced by the air plasma treatment tended to be hidden inside the coating layer, and more fluorinated alkyl chains were exposed to the surface, lowering the surface free energy.
Conclusion
A durable superamphiphobic conductive fabric has been prepared by one-step vapour-phase polymerisation of EDOT with the presence of FD-POSS and FAS. The addition of FD-POSS and FAS to the PEDOT showed little influence on the surface resistance, but it can impart the PEDOT coating with not only durable liquid repellency but also self-healing ability to auto-repair from chemical damages. FD-POSS was found to play an important role in enhancing the washing and abrasion durability and self-healing function of the coating. This novel, electrically conductive superamphiphobic coating may be useful for development of durable smart fabrics for various applications.Acknowledgements
Funding support from the Australia Research Council under the Discovery Project scheme and Deakin University under the Central Research Grant scheme is acknowledged.Notes and references
- P. Gould, Mater. Today, 2003, 6, 38–43 CrossRef CAS.
- L. V. Langenhove, C. Hertleer and A. Schwarz, in Intelligent Textiles and Clothing for Ballistic and NBC Protection Technology at the Cutting Edge, ed. P. Kiekens and S. Jayaraman, Springer, Dordrecht, The Netherlands, 2012 Search PubMed.
- L. Wang, X. Wang and T. Lin, in Smart Textile Coatings and Laminates, ed. W. C. Smith, Woodhead Publishing, 2010, pp. 155–188 Search PubMed.
- H.-C. Chen, K.-C. Lee and J.-H. Lin, Composites, Part A, 2004, 35, 1249–1256 Search PubMed.
- S. M. Bidoki, D. McGorman, D. M. Lewis, M. Clark, G. Horler and R. E. Miles, AATCC Rev., 2005, 5, 11–14 Search PubMed.
- G. Cho and A. Han, International Journal of Fashion Design, Technology and Education, 2011, 4, 83–89 Search PubMed.
- L. Hu, M. Pasta, F. L. Mantia, L. Cui, S. Jeong, H. D. Deshazer, J. W. Choi, S. M. Han and Y. Cui, Nano Lett., 2010, 10, 708–714 CrossRef CAS.
- G. Yu, L. Hu, M. Vosgueritchian, H. Wang, X. Xie, J. R. McDonough, X. Cui, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 2905–2911 CrossRef CAS.
- T. Lin, L. Wang, X. Wang and A. Kaynak, Thin Solid Films, 2005, 479, 77–82 Search PubMed.
- G. Wallace, T. Campbell and P. Innis, Fibers Polym., 2007, 8, 135–142 Search PubMed.
- S. Kathirvelu, L. D'Souza and B. Dhurai, Synth. Fibres, 2008, 37, 9–14 Search PubMed.
- O. Ala and Q. Fan, Res. J. Text. Apparel, 2009, 13, 51–68 Search PubMed.
- P. C. Innis, S. E. Moulton and G. G. Wallace, in Handbook of Conducting Polymers, ed. T. A. Skotheim and J. R. Reynolds, Marcel Dekker, New York, 3rd edn, 2007, vol. 2, pp. 11–33 Search PubMed.
- Y. Z. Long, M.-M. Li, C.-Z. Gu, M.-X. Wan, J.-L. Duvail, Z.-W. Liu and Z.-Y. Fan, Prog. Polym. Sci., 2011, 36, 1415–1442 CrossRef CAS.
- N. V. Bhat, D. T. Seshadri and S. Radhakrishnan, Text. Res. J., 2004, 74, 155–166 Search PubMed.
- J. Avloni, M. Ouyang, L. Florio, A. R. Henn and A. Sparavigna, J. Thermoplast. Compos. Mater., 2007, 20, 241–254 Search PubMed.
- J. Molina, A. I. del Rio, J. Bonastre and F. Cases, Eur. Polym. J., 2009, 45, 1302–1315 Search PubMed.
- B. Yue, C. Wang, X. Ding and G. G. Wallace, Electrochim. Acta, 2012, 68, 18–24 Search PubMed.
- D. Knittel and E. Schollmeyer, Synth. Met., 2009, 159, 1433–1437 Search PubMed.
- K. H. Hong, K. W. Oh and T. J. Kang, J. Appl. Polym. Sci., 2005, 97, 1326–1332 CrossRef CAS.
- R. Yue and J. Xu, Synth. Met., 2012, 162, 912–917 CrossRef CAS.
- S. Lee, W. Kim and K. Yong, Adv. Mater., 2011, 23, 4398–4402 CrossRef CAS.
- D. Satam, H. J. Lee and E. Wilusz, AATCC Rev., 2010, 10, 59–63 Search PubMed.
- Q. Xie, J. Xu, L. Feng, L. Jiang, W. Tang, X. Luo and C. C. Han, Adv. Mater., 2004, 16, 302–305 CrossRef CAS.
- L. Xu, W. Chen, A. Mulchandani and Y. Yan, Angew. Chem., Int. Ed., 2005, 44, 6009–6012 CrossRef CAS.
- Y. Zhu, D. Hu, M. X. Wan, L. Jiang and Y. Wei, Adv. Mater., 2007, 19, 2092–2096 CrossRef CAS.
- N. R. Chiou, C. Lu, J. Guan, L. J. Lee and A. J. Epstein, Nat. Nanotechnol., 2007, 2, 354–357 CrossRef CAS.
- T. Darmanin, F. Guittard, S. Amigoni, E. Tafin de Givenchy, X. Noblin, R. Kofman and F. Celestini, Soft Matter, 2011, 7, 1053–1057 RSC.
- T. Darmanin and F. d. r. Guittard, J. Am. Chem. Soc., 2009, 131, 7928–7933 CrossRef CAS.
- A. Zenerino, T. Darmanin, E. Taffin de Givenchy, S. Amigoni and F. Guittard, Langmuir, 2010, 26, 13545–13549 CrossRef CAS.
- H. Wang, Y. Xue and T. Lin, Soft Matter, 2011, 7, 8158–8161 RSC.
- T. Verho, C. Bower, P. Andrew, S. Franssila, O. Ikkala and R. H. A. Ras, Adv. Mater., 2011, 23, 673–678 CrossRef CAS.
- J. T. Han, Y. Zheng, J. H. Cho, X. Xu and K. Cho, J. Phys. Chem. B, 2005, 109, 20773–20778 CrossRef CAS.
- X. Deng, L. Mammen, Y. Zhao, P. Lellig, K. Müllen, C. Li, H.-J. Butt and D. Vollmer, Adv. Mater., 2011, 23, 2962–2965 CrossRef CAS.
- Y. Zhao, Z. Xu, X. Wang and T. Lin, Langmuir, 2012, 28, 6328–6335 Search PubMed.
- J. Zimmermann, F. A. Reifler, G. Fortunato, L.-C. Gerhardt and S. Seeger, Adv. Funct. Mater., 2008, 18, 3662–3669 CrossRef.
- B. Deng, R. Cai, Y. Yu, H. Jiang, C. Wang, J. Li, L. Li, M. Yu, J. Li, L. Xie, Q. Huang and C. Fan, Adv. Mater., 2010, 22, 5473–5477 CrossRef CAS.
- Y. Li, L. Li and J. Sun, Angew. Chem., Int. Ed., 2010, 49, 6129–6133 CrossRef CAS.
- X. Wang, X. Liu, F. Zhou and W. Liu, Chem. Commun., 2011, 47, 2324–2326 RSC.
- H. Zhou, H. Wang, H. Niu, A. Gestos, X. Wang and T. Lin, Adv. Mater., 2012, 24, 2409–2412 Search PubMed.
- H. Wang, Y. Xue, J. Ding, L. Feng, X. Wang and T. Lin, Angew. Chem., Int. Ed., 2011, 50, 11433–11436 CrossRef CAS.
- J. M. Mabry, A. Vij, S. T. Iacono and B. D. Viers, Angew. Chem., 2008, 120, 4205–4208 CrossRef.
- AATCC, in AATCC Technical Manual, AATCC, 1996, vol. AATCC Test Method 76-1995, pp. 100–101 Search PubMed.
- J. Kim, E. Kim, Y. Won, H. Lee and K. Suh, Synth. Met., 2003, 139, 485–489 Search PubMed.
- B. Winther-Jensen and K. West, Macromolecules, 2004, 37, 4538–4543 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM and AFM images, FTIR and XPS spectra, contact angle and surface resistance data. See DOI: 10.1039/c2sm26871j |
| This journal is © The Royal Society of Chemistry 2013 |
