pH-triggered self-assembly of biocompatible histamine-functionalized triblock copolymers†
Pontus
Lundberg
a,
Nathaniel A.
Lynd
a,
Yuning
Zhang
b,
Xianghui
Zeng
b,
Daniel V.
Krogstad
a,
Tim
Paffen
a,
Michael
Malkoch
c,
Andreas M.
Nyström
b and
Craig J.
Hawker
*ad
aMaterials Research Laboratory, University of California, Santa Barbara, CA 93106, USA. E-mail: hawker@mrl.ucsb.edu
bSwedish Medical Nanoscience Center, Department of Neuroscience, Karolinska Institutet, Retzius väg 8, Stockholm SE-171 77, Sweden
cDepartment of Fibre and Polymer Technology, KTH Royal Institute of Technology, School of Chemical Science and Engineering, Teknikringen 56-58, Stockholm SE-100 44, Sweden
dDepartment of Chemistry and Biochemistry and Materials Department, University of California, Santa Barbara, CA 93106, USA
First published on 15th October 2012
Abstract
Histamine functionalized poly(allyl glycidyl ether)-b-poly(ethylene glycol)-b-poly(allyl glycidyl ether) (PAGE-PEO-PAGE) triblock copolymers represent a new class of physically cross-linked, pH-responsive hydrogels with significant potential for biomedical applications. These telechelic triblock copolymers exhibited abrupt and reversible hydrogelation above pH 7.0 due to a hydrophilic/hydrophobic transition of the histamine units to form a network of hydrophobic domains bridged by a hydrophilic PEO matrix. These hydrophobic domains displayed improved ordering upon increasing pH and self-assembled into a body centered cubic lattice at pH 8.0, while at lower concentrations formed well-defined micelles. Significantly, all materials were found to be non-toxic when evaluated on three different cell lines and suggests a range of medical and biomedical applications.
Introduction
Hydrogels have become an increasingly important class of materials for biomedicine, pharmaceutics, coatings and cosmetics.1,2 Their utility is derived from their high water-content, yet solid-like mechanical properties derived from the presence of a three-dimensional network. The network can be formed by either covalent bonds or through physical interactions such as hydrophobic, electrostatic or hydrogen bonding.1–4For many applications, it is desirable to have gels that respond to external stimuli, i.e. smart gels.5 Smart gels can be tailored to be responsive to a change in the surrounding environment, such as temperature, pH, ionic strength, or light.2,5–9 Of these external stimuli, pH-responsive gels are of particular interest for biomedical applications since different intra- and extracellular compartments maintain varying levels of acidity or alkalinity. A highly relevant example is the difference in pH between healthy tissue and tumor tissue. In blood and extracellular fluids of healthy tissue the pH is 7.4, whereas intracellular components such as endosomes and lysosomes have lower pH.10 Similarly, the pH of extracellular fluids in tumor tissues is lower, ca. 6.0, due to the elevated levels of metabolic byproducts such as lactic acid produced by cancer cells.11,12 For biomedical applications, such as in chemotherapeutic drug delivery, it is desirable to exploit these differences in pH by tailoring materials that respond dramatically and specifically within a narrow physiologically relevant pH range, e.g., pH 6.0 to 7.4.
Due to the potential of polymer-based therapeutics that exploit these differences in pH, an extensive amount of effort has been directed toward developing pH-responsive materials. In one example, Thomas et al. prepared copolymers of 2-ethylacrylic acid and methyl methacrylate which allowed the pKa to be systematically increased to 6.5, compared to 5.7 for pure poly(2-ethylacrylic acid).13 Philippova et al. used a similar strategy to tune the swelling of hydrogels by copolymerizing acrylic acid, N,N′-methylenebis(acrylamide), and n-alkyl acrylates,14 while single component systems like 2-(diisopropylamino)ethyl methacrylate (DPA), which has a pKa between 6 and 7, have also been developed.15,16 However, a major drawback to many of these systems is toxicity, for example the DPA-based polymer can release 2-(diisopropylamino)ethanol upon hydrolytic degradation which is highly toxic.17 Looking to nature for inspiration, researchers have addressed this toxicity issue by using natural, non-toxic building blocks. Our attention was therefore directed to developing materials based on the amino acid histidine and its derivative histamine, which are both non-toxic and have ideal pKa's of ca. 6.5.18 At neutral or basic pH, e.g. in blood, the imidazole group is uncharged and hydrophobic; whereas in acidic environments, such as in the endosome or extracellular sites of tumors, the imidazole is charged and hydrophilic. As an example, poly(histidine)-b-poly(ethylene oxide) was shown to exhibit pH-dependent micellization, and promote endosomal escape of the carrier system to achieve higher efficiency in delivering chemotherapeutic drugs, such as paclitaxel.18,19
While covalently crosslinked hydrogels have been widely used in various coating applications, the need for degradation and versatile in- and ex situ processing has focused attention on the development of physically crosslinked hydrogels. As an effective and versatile strategy for the preparation of bio-compatible hydrogels it was decided to combine histidine-based building blocks with the self-assembly of telechelic ABA-type triblock-copolymers where the mid-block (B) is hydrophilic and physical association between the outer blocks (A) forms physical cross-links.20 Our group has recently developed a modular approach to the synthesis of ionic hydrogels based on poly(allyl glycidyl ether)-b-poly(ethylene oxide)-b-poly(allyl glycidyl ether) (PAGE-PEO-PAGE). The key step in this strategy is functionalization of the allyl groups using thiol–ene chemistry,3 which has proven to be an extremely versatile and effective method for polymer functionalization.21–25 In this study, a histamine-functional thiol was synthesized, reacted onto a PAGE-PEO-PAGE triblock-copolymer through thiol–ene chemistry and the structure/properties of the resulting hydrogels investigated for a range of polymer concentrations and pH values (Fig. 1). At polymer concentrations of 10 wt%, small angle X-ray scattering (SAXS) and rheometry were used to investigate the pH-dependent gel formation. At lower concentrations, the triblock copolymers formed pH-responsive micelles which were investigated using cryo-TEM and DLS.
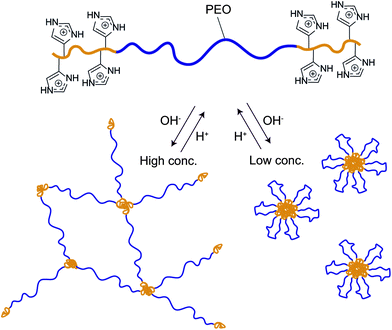 | ||
| Fig. 1 Schematic strategy of the pH-responsive ABA triblock copolymer and its self-assembly into gels and micelles upon changes in pH. | ||
Experimental
Materials
The synthesis of PAGE-b-PEO-b-PAGE with 26 wt% PAGE at a molecular weight of 27 kg mol−1 (PAGE3.5k-b-PEO20k-b-PAGE3.5k) has been reported previously.3 Histamine (≥97.0%), γ-thiobutyrolactone (98%), 2,2-dimethoxy-2-phenylacetophenone (99%) (DMPA), bromothymol blue (BTB) solution in ethanol, pyrene (p.a.), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetra sodium bromide (MTT), and Dulbecco's modified Eagle's medium (DMEM) were purchased from Sigma-Aldrich and used without further purification. Acetonitrile (HPLC) and methanol (ACS) were purchased from Fisher Scientific. Dialysis membranes with MWCO 3500 Da were purchased from Spectrum labs. 100 mM buffer solutions where prepared by mixing NaH2PO4·H2O (ACS, EMD chemicals) and Na2HPO4·7H2O (ACS, EMD chemicals) with deionized water in appropriate amounts to reach the targeted pH. Fetal bovine serum (FBS) and penicillin/streptomycin solution were obtained from Hyclone Laboratories. MDA-MB-231, and MCF-7 cells were purchased from the American Type Culture Collection (ATCC). RAW 264.7 (mouse leukemic monocyte macrophage cell line, originating from ATCC) was obtained from the laboratory of Prof Richter-Dahlfors at the Swedish Medical Nanoscience Center, Department of Neuroscience Karolinska Institutet, Stockholm Sweden.Instrumentation
Rheological experiments were performed on a Rheometrics Scientific ARES II rheometer with parallel-plate geometry (25 mm or 50 mm diameter). Frequency sweeps were performed using 2% strain for gel-like materials and 6% for liquid-like materials. Strain sweeps were performed at a frequency of 1 Hz and all frequency sweeps were performed within the linear viscoelastic regime. All experiments were performed at room temperature (21–24 °C).Small angle X-ray scattering (SAXS) was performed at beamline DND-CAT line 5 at the Advanced Photon Source, Argonne National Laboratory. The samples were packed into 1 mm diameter quartz capillaries through the use of a centrifuge. The samples were then flame sealed and the top was covered with epoxy in order to prevent drying.
Dynamic light scattering experiments were performed on a DynaPro NanoStar from Wyatt. Reported values are average values of 20 acquisitions.
Fluorescence spectroscopy was performed on Cary Eclipse from Varian using an excitation wavelength of 332 nm and emission spectra were collected between 350 and 420 nm.
Samples for cryogenic transmission electron microscopy (cryo-TEM) were prepared at a polymer concentration of 1 mg ml−1. The samples were then vitrified using the environmentally controlled FEI Mark IV Vitrobot (24 °C, 100% humidity). A 3.5 μl droplet was placed on a glow discharged copper grid coated with a lacey Formvar support film. The samples were blotted once before being plunged into liquid nitrogen cooled liquid ethane. The samples were placed in a Gatan cryoholder and were kept below −170 °C throughout imaging. The samples were imaged at 200 kV with an FEI T20 TEM using low dose imaging mode. Images were recorded digitally using a Gatan Ultrascan 1000 CCD camera and analyzed using Gatan Digital Micrograph and ImageJ software. Using the ellipse tool in ImageJ, the size of 100 particles in each picture was measured and recorded.
Methods
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH2–), 2.49 (t, 2H, J = 10 Hz, –CH2–SH), 2.79 (t, 2H, J = 15 Hz, imidazole–CH2–), 3.44 (t, 2H, J = 15 Hz, –CH2–NH–C(
O)–CH2–), 2.49 (t, 2H, J = 10 Hz, –CH2–SH), 2.79 (t, 2H, J = 15 Hz, imidazole–CH2–), 3.44 (t, 2H, J = 15 Hz, –CH2–NH–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–), 6.86 (s, 1H, –NH–CH
O)–), 6.86 (s, 1H, –NH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C<), 7.60 (s, 1H, –NH–CH
C<), 7.60 (s, 1H, –NH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). 13C-NMR δ = 23.06, 26.39, 29.89, 34.11, 38.90, 134.65, 173.94. FT-IR ν (cm−1) = 3230, 3130, 2980, 2895, 1640, 1575, 1495, 1420, 1360, 1300, 1253, 1225, 1195, 1090, 1060, 929, 889, 833, 736, and 714. Mass spectrometry ESI/TOF found 236.0811 Da [M + Na+], calculated 236.0834 Da [M + Na+].
N–). 13C-NMR δ = 23.06, 26.39, 29.89, 34.11, 38.90, 134.65, 173.94. FT-IR ν (cm−1) = 3230, 3130, 2980, 2895, 1640, 1575, 1495, 1420, 1360, 1300, 1253, 1225, 1195, 1090, 1060, 929, 889, 833, 736, and 714. Mass spectrometry ESI/TOF found 236.0811 Da [M + Na+], calculated 236.0834 Da [M + Na+].
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH2–CH2–CH2–S–), 2.31 (t, J = 15 Hz, –C(
O)–CH2–CH2–CH2–S–), 2.31 (t, J = 15 Hz, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH2–), 2.52 (t, J = 15 Hz, –C(
O)–CH2–), 2.52 (t, J = 15 Hz, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH2–CH2–CH2–S–), 2.60 (t, J = 15 Hz –S–CH2–CH2–CH2–O–), 2.79 (t, J = 15 Hz, imidazole–CH2–), 3.44 (t, J = 15 Hz, –CH2–NH–C(
O)–CH2–CH2–CH2–S–), 2.60 (t, J = 15 Hz –S–CH2–CH2–CH2–O–), 2.79 (t, J = 15 Hz, imidazole–CH2–), 3.44 (t, J = 15 Hz, –CH2–NH–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–), 3.49–3.71 (m, backbone), 6.86 (s, –NH–CH
O)–), 3.49–3.71 (m, backbone), 6.86 (s, –NH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C<), 7.61 (s, –NH–CH
C<), 7.61 (s, –NH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). 13C-NMR δ = 28.44, 29.240, 30.77, 32.28, 33.58, 41.696, 72.30, 72.54, 72.70, 72.86, 73.02, 73.84, 81.46, 119.25, 137.43, 176.51. FT-IR ν (cm−1) = 3418, 3275, 3121, 2880, 2695, 2659, 1644, 1554, 1466, 1454, 1359, 1342, 1279, 1241, 1145, 1100, 1060, 962, 841, 755, 707, 663. FT-Raman ν (cm−1) = 3110, 2912, 2885, 1644, 1567, 1478, 1442, 1363, 1281, 1235, 1142, 1060, 1029, 844, 649, 364, 278.
N–). 13C-NMR δ = 28.44, 29.240, 30.77, 32.28, 33.58, 41.696, 72.30, 72.54, 72.70, 72.86, 73.02, 73.84, 81.46, 119.25, 137.43, 176.51. FT-IR ν (cm−1) = 3418, 3275, 3121, 2880, 2695, 2659, 1644, 1554, 1466, 1454, 1359, 1342, 1279, 1241, 1145, 1100, 1060, 962, 841, 755, 707, 663. FT-Raman ν (cm−1) = 3110, 2912, 2885, 1644, 1567, 1478, 1442, 1363, 1281, 1235, 1142, 1060, 1029, 844, 649, 364, 278.
Results and discussion
The imidazole group of histidine is of specific interest as a pH-responsive unit due to its bio-compatibility as well as the physiologically relevant pH range (6.0–7.4) for protonation. For attachment to our previously developed alkene functionalized polyether platform, a thiol-functional imidazole was initially targeted in tandem with orthogonal thiol–ene chemistry. In developing this synthesis strategy, we were inspired by the well-known reaction between amines and homocysteine, which has been used in small molecule chemistry27 as well as for protein modification.28 Hence, histamine and γ-thiobutyrolactone were chosen as the starting materials and reacted by simply mixing the two compounds in acetonitrile and heating (Scheme 1). Upon mixing at room temperature, histamine remains insoluble; however, once the temperature reaches 95 °C, the histamine dissolves and product formation can be seen as precipitation of a yellow solid. After allowing the reaction to proceed overnight, it was cooled to ambient temperature and the product (Hist-SH) isolated by filtration (95%). Functionalization of the PAGE-PEO-PAGE triblock-copolymer was then achieved through photochemical, thiol–ene chemistry with quantitative conversion being achieved using 1.5 equivalents of thiol-functional histamine per allyl group (Scheme 1). After purification by dialysis in methanol, the functionalized triblock-copolymer (PHGE-b-PEO-b-PHGE) was isolated in 91% yield with 1H-NMR spectroscopy showing the disappearance of peaks corresponding to the allyl group and appearance of unique resonances for the thio-histamine unit (peaks a′, b′, h′, and g′) (Fig. 2). Removal of the excess histamine and degradation products from the initiator was monitored by 1H-NMR, which also confirmed >95% of the histamine in the sample was attached to the polymer. Additionally, FT-IR and FT-Raman confirmed the quantitative incorporation of histamine units (see Fig. ESI 1 and 2†). As expected, the functionalized PHGE-b-PEO-b-PHGE triblock copolymer was found to have poor solubility in most organic solvents and basic aqueous solutions; however, methanol and acidic aqueous solutions readily dissolved the product.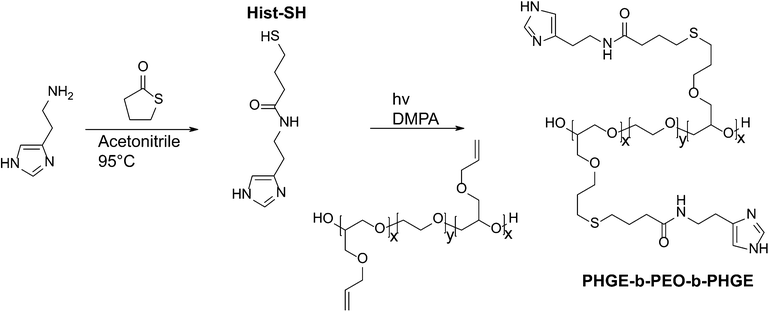 | ||
| Scheme 1 Synthetic route to Hist-SH and PHGE-b-PEO-b-PHGE. | ||
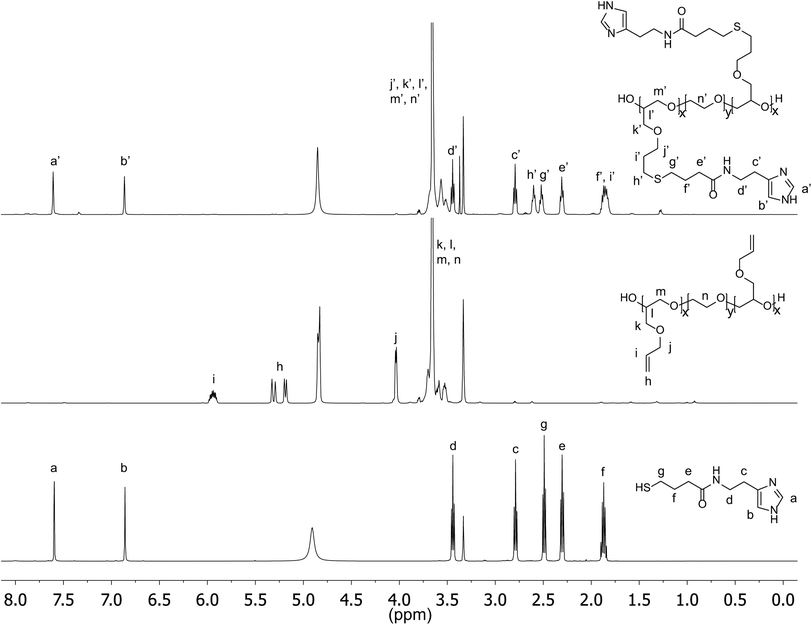 | ||
| Fig. 2 1H-NMR of Hist-SH (bottom), PAGE3.5k-b-PEO20k-b-PAGE3.5k (middle), and PHGE-b-PEO-b-PHGE (top). | ||
In order to characterize the pH-responsiveness of PHGE-b-PEO-b-PHGE, potentiometric titration was performed (Fig. ESI 3†) and compared with a NaCl control solution. Significantly, a buffering region from pH 3 to 8 is observed and this buffering agrees well with other polymeric materials having an imidazole as the only pH-responsive moiety.19,29 Within the pH range of 3 to 8, the histamine units transition between a protonated hydrophilic state and a deprotonated hydrophobic state which is in agreement with the design of the triblock copolymer architecture of PHGE-b-PEO-b-PHGE and should result in pH-triggered self-assembly into gels or micelles.
The ability to control gel formation with changes in pH was initially demonstrated through encapsulation of the pH-indicator, bromothymol blue (BTB). To a 20 wt% solution of PHGE-b-PEO-b-PHGE in 1 M HCl, was added BTB to give a yellow colored solution (indicating acidic pH), addition of 1 M NaOH then resulted in the instantaneous formation of a green/blue colored gel (indicating neutral pH). Cycling between a green/blue gel and yellow solution could then be achieved by simply adding acid or base with no change in physical properties after repeated cycling. These results suggest that the histamines indeed switch between hydrophilic and a hydrophobic state as expected. To further explore gelation and the viscoelastic properties of these gels, a set of 10 wt% samples were prepared in buffer solutions from pH 8.0 to 5.0 and as can be seen in Fig. 3, a distinct transition from a free-standing gel to liquids of decreasing viscosity is observed on changing pH. Of particular note is the sharp change in properties between pH values of 6.6 and 7.4, which is within the range of pH-differences that is expected when moving from healthy tissue to cancerous or inflammatory tissue in the human body.10–12 The mechanical properties of these gels were then probed with a series of rheological experiments which revealed viscoelastic properties consistent with gels at pH 8.0 and pH 7.4 (Fig. ESI 4†). For the pH 8.0 sample, a crossover is observed at 0.25 Hz corresponding to a relaxation time of 0.65 s, while for the pH 7.4 sample, this transition is observed at 1.9 Hz and leads to a relaxation time of 0.08 s.30 Significantly, at pH 8, a G′ of 1.1 kPa was observed for the 10 wt% PHGE-b-PEO-b-PHGE gel, which is comparable to previous reports on pH responsive ABA gelators and further demonstrates the utility of this structural platform (Fig. 4).9,16,31 On lowering the pH to 7.0, no crossover frequency can be observed and the material behaves as a viscous liquid. Additional decrease in pH results in further reduction of both G′ and G′′.
 | ||
| Fig. 3 Photo of 10 wt% gels/solutions of PHGE-b-PEO-b-PHGE at different pH. | ||
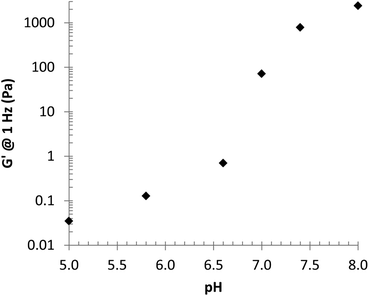 | ||
| Fig. 4 Storage modulus (G′) at 1 Hz vs. pH. G′ values extracted from Fig. ESI 4.† | ||
Further analysis of the rheological properties reveal that the dynamic viscosity also drops as the pH is lowered (Fig. ESI 6†) indicating that protonation of the imidazole groups reduces hydrophobic interactions and the resulting aggregate size. However, lowering the pH below 5.8 did not result in a further reduction in the viscosity. This suggests that although some imidazole units were still charge neutral, as supported by the titrations, there were too few to interact with each other in a significant manner. The relationship between gel formation and mesostructural differences underlying changes in viscoelasticity with pH was then examined by small angle X-ray scattering. Increasing the pH revealed several trends (Fig. 5): the domain spacing increased from 20 nm at pH 6.6 to 26 nm at pH 8.0 with microphase-separation and long-range structural ordering increasing with pH.32 From the q* value of the gel at pH 8.0, the unit cell distance was calculated to be 37 nm (a = 23/2πq*−1) based on a cubic lattice with BCC symmetry and the full reversibility associated with this assembly demonstrates the robustness of the histamine units as a structural directing unit.
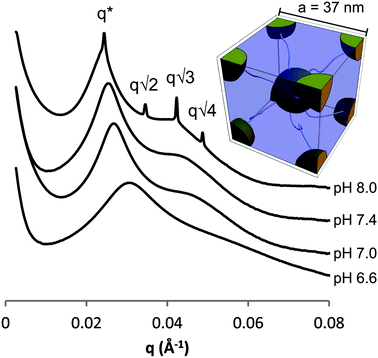 | ||
| Fig. 5 SAXS measurements of 10 wt% gels/solutions of PHGE-b-PEO-b-PHGE at different pH with inset of BCC unit cell. | ||
The power of combining an amphiphilic triblock-copolymer architecture with the reversible association of the histamine units can also be appreciated through the formation of flower-like micelles at low concentration.33 As demonstrated previously, polymer micelles based on triblock copolymers are of specific interest when compared to traditional diblock copolymer micelles for use in therapeutics34 as they benefit from the enhanced permeability and retention effect (EPR) and increased biocompatibility.35 In order to explore the micelle formation of PHGE-b-PEO-b-PHGE, low concentration (0.1 wt%) solutions were prepared at pH 5.0 to 8.0. Significantly, micelles were observed by DLS and cryo-TEM throughout the entire pH-range; however, their distribution differed greatly (see ESI 7–12†). At pH 8.0 and 7.4, narrow size distributions were observed (mean intensity radius of 40 nm and 34 nm respectively), whereas a broad range of particle sizes were observed at lower pH. This difference can also be clearly seen on analysis by cryo-TEM (Fig. 6). Further investigation of the micelles was performed by determining the CMC at different pH values using a fluorescent probe technique (Fig. ESI 13†) with the CMC (ca. 50 μg ml−1) being independent of pH. These results suggest that micellization is not primarily driven by the change in protonation of the imidazole group, and instead, is driven by the permanent hydrophobic spacer between the backbone and the imidazole unit.
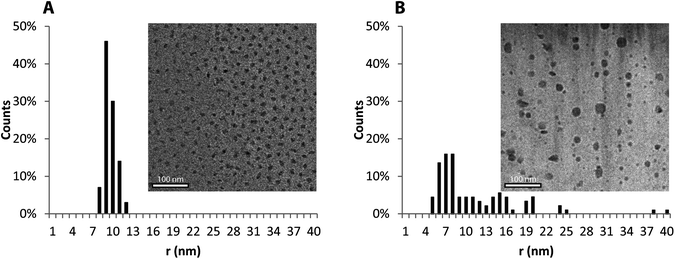 | ||
| Fig. 6 Cryo-TEM image of a micelle solution at pH 7.4 and a histogram showing the distribution of particle sizes (A). Cryo-TEM image of a micelle solution at pH 5.0 and a histogram showing the distribution of particle sizes (B). | ||
A key design feature of these pH-responsive hydrogels for in vivo applications is the biocompatibility of the building blocks used in their construction. Toxicological evaluation was therefore performed by incubating the triblock copolymers with three different cell lines at three different pH values (6.7, 7.0 and 7.4) (Fig. 7). Two well-known breast cancer cell lines MCF-7 and MDA-MB231, and one macrophage-like cell line RAW 264.7 were selected with the macrophages being a good model for toxicity testing as they are an instrumental part of the innate immune system; clearing foreign bodies, such as nanoparticles from the blood stream, which may evoke an immune reaction.36 The viability of cells was then evaluated using MTT staining after 48 and 72 h. Significantly, in the tested range of concentrations (0.01 μg ml−1 to 100 μg ml−1), all cell lines displayed excellent viability indicating that the triblock copolymer assemblies have little or no toxicity over several orders of magnitude change in concentration. However, we can see a small reduction in the cell viability for the intermediate concentration of 1 μg ml−1 at pH 7, indicating perhaps that the self-assembly processes can have an effect on cell viability. Further understanding of this effect requires a detailed investigation of the self-assembly process in complex media containing proteins.
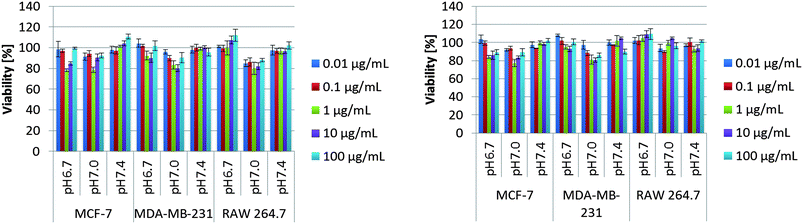 | ||
| Fig. 7 MTT-assay of PHGE-b-PEO-b-PHGE after 48 h (left) and 72 h (right) using three different cell lines varying the concentration of polymer and pH. | ||
Conclusions
Among responsive hydrogels, pH-responsive gels are of particular interest as the human body displays significant variation in pH depending on tissue, intracellular compartment, and health status. Following a modular approach, a thiol-functional histamine unit (Hist-SH) with a pKa of ∼6.5, which is ideally suited for in vivo applications, was synthesized and used to functionalize PAGE-b-PEO-b-PAGE. The responsive nature of the triblock copolymer, PHGE-b-PEO-b-PHGE, allows free standing gels to be readily formed at pH >7 and at concentrations as low as 7 wt%. Interestingly, SAXS characterization of the 10 wt% gels showed that at pH 8.0, the hydrophobic domains order onto a BCC lattice while at low concentrations (0.1 wt%) micelles could be formed. The CMC of these micelles was independent of pH; however, the micellar size and size distribution was highly dependent on pH. Toxicological screening against three different cell lines showed that the material was non-toxic, which further supports the potential of in vivo use of the triblock material. These results suggest that the strategy of combining the responsiveness of histamine repeat units with the biocompatibility of functional polyethers may be of great utility for drug delivery applications with a passive targeting strategy and pH as an active trigger.Acknowledgements
This work was partially supported by the National Institutes of Health as a Program of Excellence in Nanotechnology (HHSN268201000046C) (NAL and CJH) and the National Science Foundation through the MRSEC program (DMR-1121053 – PL, NAL, TP and CJH). PL would like to acknowledge the Wenner-Gren and Bengt Lundqvist foundations for financial support. AMN acknowledges financial support from STINT, Karolinska Institutet, Jeanssons Foundation, Åke Wibergs Foundation, Eva and Axel Wallströms Foundation, Percy Falks Fundation, Carl Bennet AB, The Swedish Research Council (VR) 2009-3259 (together with MM) and 2011-3720, The Swedish Medical Nanoscience Center and The Swedish Governmental Agency for Innovation Systems (Vinnova).Notes and references
- Y. Ting, H. Long, M. Malkoch, E. K. Gamstedt, L. Berglund and A. Hult, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4044–4054 Search PubMed.
- N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Adv. Mater., 2006, 18, 1345–1360 CrossRef CAS.
- J. N. Hunt, K. E. Feldman, N. A. Lynd, J. Deek, L. M. Campos, J. M. Spruell, B. M. Hernandez, E. J. Kramer and C. J. Hawker, Adv. Mater., 2011, 23, 2327–2331 CrossRef CAS.
- M. Lemmers, J. Sprakel, I. K. Voets, J. van der Gucht and M. A. C. Stuart, Angew. Chem., Int. Ed., 2010, 49, 708–711 CAS.
- W. Zhu, A. Nese and K. Matyjaszewski, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1942–1952 Search PubMed.
- S. K. Ahn, R. M. Kasi, S. C. Kim, N. Sharma and Y. X. Zhou, Soft Matter, 2008, 4, 1151–1157 RSC.
- Y. Qiu and K. Park, Adv. Drug Delivery Rev., 2001, 53, 321–339 CrossRef CAS.
- C. Tsitsilianis, Soft Matter, 2010, 6, 2372–2388 RSC.
- J. Madsen and S. P. Armes, Soft Matter, 2012, 8, 592–605 RSC.
- A. Roos and W. F. Boron, Physiol. Rev., 1981, 61, 296–434 CAS.
- S. A. Glynn and D. Albanes, Nutr. Cancer, 1994, 22, 101–119 Search PubMed.
- I. F. Tannock and D. Rotin, Cancer Res., 1989, 49, 4373–4384 CAS.
- J. L. Thomas, H. You and D. A. Tirrell, J. Am. Chem. Soc., 1995, 117, 2949–2950 CrossRef CAS.
- O. E. Philippova, D. Hourdet, R. Audebert and A. R. Khokhlov, Macromolecules, 1997, 30, 8278–8285 CrossRef CAS.
- V. Castelletto, I. W. Hamley, Y. H. Ma, X. Bories-Azeau, S. P. Armes and A. L. Lewis, Langmuir, 2004, 20, 4306–4309 CrossRef CAS.
- Y. H. Ma, Y. Q. Tang, N. C. Billingham, S. P. Armes and A. L. Lewis, Biomacromolecules, 2003, 4, 864–868 CrossRef CAS.
- 2-(Diisopropylamino)ethanol; MSDS no. 471488 [Online], Sigma-Aldrich, Saint Louis, Jan 19, 2012, http://www.sigmaaldrich.com/catalog/product/aldrich/471488, accessed 27 April, 2012.
- J. S. Park, T. H. Han, K. Y. Lee, S. S. Han, J. J. Hwang, D. H. Moon, S. Y. Kim and Y. W. Cho, J. Controlled Release, 2006, 115, 37–45 CrossRef CAS.
- E. S. Lee, H. J. Shin, K. Na and Y. H. Bae, J. Controlled Release, 2003, 90, 363–374 CrossRef CAS.
- J. Kops, J. H. Truelsen, M. Lei and S. P. Armes, Abstr. Pap. Am. Chem. Soc., 2002, 224, U444 Search PubMed.
- B. F. Lee, M. J. Kade, J. A. Chute, N. Gupta, L. M. Campos, G. H. Fredrickson, E. J. Kramer, N. A. Lynd and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4498–4504 CrossRef CAS.
- M. J. Kade, D. J. Burke and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 743–750 CrossRef CAS.
- A. B. Lowe, Polym. Chem., 2010, 1, 17–36 RSC.
- B. S. Sumerlin and A. P. Vogt, Macromolecules, 2010, 43, 1–13 CrossRef CAS.
- R. K. Iha, K. L. Wooley, A. M. Nystrom, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620–5686 CrossRef CAS.
- K. Kalyanasundaram and J. K. Thomas, J. Am. Chem. Soc., 1977, 99, 2039–2044 CrossRef CAS.
- R. Benesch and R. E. Benesch, Proc. Natl. Acad. Sci. U. S. A., 1958, 44, 848–853 Search PubMed.
- H. Jakubowski and R. Glowacki, in Advances in Clinical Chemistry, ed. G. S. Makowski, Elsevier Academic Press Inc., San Diego, 2011, vol. 55, pp. 81–103 Search PubMed.
- S. M. Ramirez, J. M. Layman, P. Bissel and T. E. Long, Macromolecules, 2009, 42, 8010–8012 Search PubMed.
- K. Nishinari, Some Thoughts on the Definition of a Gel in Gels: Structures, Properties and Functions, Springer, Berlin/Heidelberg, 2009, vol. 136, pp. 87–94 Search PubMed.
- M. Guvendiren, P. B. Messersmith and K. R. Shull, Biomacromolecules, 2008, 9, 122–128 CrossRef CAS.
- Samples at pH 5.0 and 5.8 could unfortunately not be measured as the sample-tubes had broke during transport.
- I. W. Hamley, The Physics of Block Copolymers, Oxford University Press, Oxford, 1998 Search PubMed.
- R. Duncan, Nat. Rev. Drug Discovery, 2003, 2, 347–360 CrossRef CAS.
- Y. Matsumura and H. Maeda, Cancer Res., 1986, 46, 6387–6392 CAS.
- Z. Wu, X. Zeng, Y. Zhang, N. Feliu, P. Lundberg, B. Fadeel, M. Malkoch and A. M. Nystrom, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 217–226 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional characterization of the material, gels and micelles including FT-IR, Raman, potentiometric titrations, rheology, DLS and CMC measurements can be found. See DOI: 10.1039/c2sm26996a |
| This journal is © The Royal Society of Chemistry 2013 |
