Cellulose nanofiber–graphene all solid-state flexible supercapacitors†
Kezheng
Gao
,
Ziqiang
Shao
*,
Jia
Li
,
Xi
Wang
,
Xiaoqing
Peng
,
Wenjun
Wang
and
Feijun
Wang
School of Materials Science and Engineering, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: shaoziqiang@263.net; Fax: +86 10-68941797-609; Tel: +86 10-68941797-601
First published on 15th November 2012
Abstract
Cellulose nanofibers are selected as nano-spacers, electrolyte nano-reservoirs and hierarchical nanostructure makers of CNF–RGO hybrid aerogel. The CNF–RGO hybrid aerogel based flexible supercapacitor exhibits high capacitance (207 F g−1). Taking its higher capacitance, low cost and environmentally friendly nature, they have great potential for use in flexible supercapacitors.
All-solid-state flexible supercapacitors, the most promising flexible power source with long cycle lives and high power densities, have attracted wide attention because they could be applied in many fields, such as flexible personal consumer electronics.1–9 Currently, graphene-based porous carbon materials (such as RGO aerogel) are widely used for the electrode material of supercapacitors. However, due to unavoidable re-stacking of graphene nanosheets (the solid walls of RGO aerogel have the structural characteristics of graphite), the electrolyte ion diffusion becomes difficult or even impossible in the densely stacked structure of RGO nanosheets, and the specific surface area of graphene-based materials is significantly lower than the theoretical value (2600 m2 g−1). Therefore, the performance of graphene-based supercapacitors is seriously degraded.10–20 It is very important for improving the performance of graphene-based supercapacitors to effectively prevent the aggregation of graphene nanosheets in an assembled bulk form (as much as possible to allow the RGO to behave as graphene rather than graphite), decrease the distance of ion diffusion, improve the wettability of graphene with electrolytes, and enhance the utilization of mesopores.21,22 One-dimensional cellulose nanofibers (CNFs) possess an appropriate geometric structure (at least a few microns long and 3–4 nm wide) and hydrophilic characteristics (dried CNFs are easy to re-swell in aqueous electrolyte).23 Furthermore, CNFs also have some excellent properties, such as low density, low cost, environmentally friendly nature, and so on. Therefore, it may be a good decision to choose CNFs as nano-spacers, aqueous electrolyte nano-reservoirs, and the hierarchical nanostructure maker of graphene-based electrode materials for all-solid-state flexible supercapacitors compared with other species (such as nanoparticles, polymers, and so on) in our view.
In this communication, we report a novel CNF–RGO hybrid aerogel as the electrode material of all-solid-state flexible supercapacitors. Our results indicate that all-solid-state flexible CNF–RGO hybrid aerogel supercapacitors possess outstanding performance.
GO nanosheets and CNFs are prepared according to the literature methodology reported by Isogai and Tung respectively.24,25 The detailed characterization of the GO nanosheets and the CNFs is provided in the ESI.† CNF–GO hybrid hydrogels are prepared by acidizing the homogeneous solution of CNFs, GO nanosheets and VC–Na with hydrochloric acid vapor. Then, the CNF–RGO hybrid hydrogels can form after GO–CNFs hybrid hydrogels are stored at 80 °C for 24 h. The CNF–RGO hybrid aerogels are prepared from CNF–RGO hybrid hydrogels by supercritical CO2 drying. For CNF–RGO hybrid hydrogels (with the solid content unchanged), the effectiveness of CNFs as nano-spacers of RGO can be studied by rheology: as shown in Fig. 1a, all the G′ values are always one order of magnitude higher than the G′′ values of each sample over the entire range of frequencies. These indicate that elastic response is predominant and the CNF–RGO hybrid hydrogels have a permanent network. Furthermore, the G′ value of CNF–RGO (20%) hybrid hydrogel at 10 rad s−1 is about 8.73 kPa, which is significantly lower than pure RGO hydrogel (630 kPa, reported by Zhang26), but much higher than pure CNFs hydrogel (about 389 Pa). These phenomena indicate that the mechanical strength of RGO hydrogel via π–π stacking interactions is much higher than the CNF hydrogel via hydrogen bonds, and the nano-spacers (CNFs) can effectively reduce π–π stacking interactions between the graphene nanosheets, which will lead to the π–π stacking interactions at cross-linking sites being replaced by hydrogen bonds. Besides, the G′ value of CNF–RGO (20%) hybrid hydrogel at 10 rad s−1 is much higher than CNF–RGO (10%) hybrid hydrogel (928 Pa), and significantly lower than CNF–RGO (30%) hybrid hydrogel (45.3 kPa). These rheological data seem to imply that 20 wt% RGO content may be a knee point. At this point, the aggregation of RGO nanosheets can be prevented to a large extent (maintaining the characteristics of graphene nanosheets). At the same time, they perhaps connect each other to form a network structure to some extent.
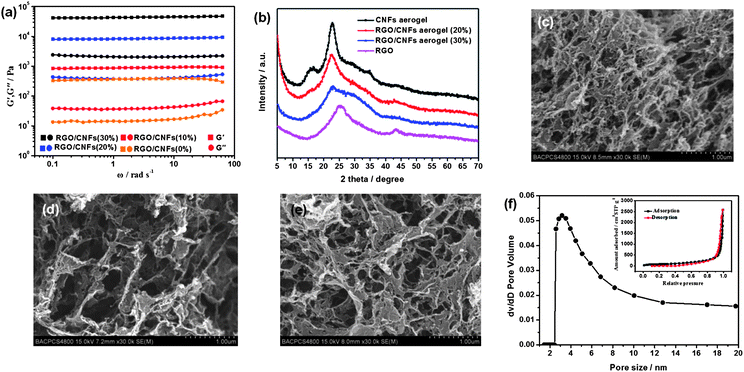 | ||
| Fig. 1 (a) Dynamic rheological behavior of the CNF-based hydrogels. (b) XRD patterns of CNFs aerogel, RGO–CNFs (20%) hybrid aerogel, RGO–CNFs (30%) hybrid aerogel, and RGO film. SEM images of (c) RGO–CNFs (10%) hybrid aerogel, (d) RGO–CNFs (20%) hybrid aerogel, and (e) RGO–CNFs (30%) hybrid aerogel. (f) BJH pore distribution of RGO–CNFs (20%) hybrid aerogel, the inset is nitrogen adsorption/desorption isotherms. | ||
Fig. 1b shows X-ray diffraction (XRD) patterns of RGO film, CNF–RGO (20%) hybrid aerogel, CNF–RGO (30%) hybrid aerogel, and CNF aerogel. The diffractogram of the CNF–RGO (20%) hybrid aerogel show two peaks at around 2θ = 15.6° and 22.5° (the diffraction peak at 25.5° for the RGO film is not detected), which is very similar to the diffractogram of typical cellulose I crystalline structure.27 However, the diffractogram of CNF–RGO (30%) hybrid aerogel show the characteristics of RGO film to some extent. These results indicate that aggregation of graphene nanosheets can be effectively prevented by the CNFs at 20 wt% RGO content. The morphology and hierarchical nanostructure of CNF–RGO hybrid aerogels are characterized by SEM observations (Fig. 1c–e). All the SEM images show a 3D porous web-like structure. However, the morphology of the CNF–RGO hybrid aerogel changes significantly with increasing RGO content. The 3D porous network of CNF–RGO (10%) hybrid aerogel seems to be formed mainly by CNFs, and the graphene nanosheets uniformly disperse in the 3D porous network. The CNF–RGO (30%) hybrid aerogel became more compact (compared with the CNF–RGO (20%) hybrid aerogel) probably due to enhanced π–π stacking, the CNFs cannot effectively prevent restacking of RGO nanosheets at 30 wt% RGO content. Therefore, the morphology of CNF–RGO (30%) hybrid aerogel shows the characteristics of pure RGO aerogel largely. For CNF–RGO (20%) hybrid aerogel, both RGO nanosheets and CNFs are inter-dispersed uniformly and form a 3D porous web-like structure, the pore diameter of which ranged from submicrometer to micrometer scale. Generally, the solid walls of the traditional 3D graphene network (RGO aerogel), which formed via dense packing of graphene nanosheets using π–π stacking, have the structural characteristics of graphite.28–30 Therefore, only surface graphene nanosheets instead of all the graphene nanosheets contribute to the electrochemical performance. The pore walls of CNF–RGO (20%) hybrid aerogel have rougher and more wrinkled textures than traditional RGO aerogel.26 This phenomenon indicates that RGO in solid walls of CNF–RGO (20%) hybrid aerogel not only have characteristics of individual graphene nanosheets, but also connected to each other (which could favour electron transport). Therefore, it is probably a good decision to select CNF–RGO (20%) hybrid aerogel as an electrode material of supercapacitors. Therefore, the pore size distribution and surface chemistry of CNF–RGO (20%) hybrid aerogel are further characterized.
The pore size distribution of CNF–RGO (20%) hybrid aerogel, estimated by the Barret–Joyner–Halenda (BJH) method, is shown in Fig. 1f. The curve of pore size distribution shows that pore diameters are in the mesoporous range (2–50 nm). A notable single peak appears at around 3.5 nm. In addition, the small micropores (<2 nm) of CNF–RGO (20%) hybrid aerogel disappear. This phenomenon indicates that the CNFs with 3–4 nm width and a few microns long are not only very effective nano-spacers of graphene nanosheets, but also excellent makers of mesopores. According to the SEM image and the pore size distribution of CNF–RGO (20%) hybrid aerogel, we have reason to believe that mesopores mainly come from the hierarchical nanostructure of the solid walls.
X-ray photoelectron spectroscopy (XPS) is used for characterizing the surface chemistry of CNF–RGO (20%) hybrid aerogel. Fig. 2a and b shows the high-resolution XPS C1s spectra of CNF aerogel and CNF–RGO (20%) hybrid aerogel. The curves are fitted considering the following contributions: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C (284.4 eV), C–O (286.6 eV), C
C/C–C (284.4 eV), C–O (286.6 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.4 eV), O–C
O (288.4 eV), O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (289.8 eV).31 The C
O (289.8 eV).31 The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C peak intensity of CNF–RGO (20%) hybrid aerogel increase obviously compared with CNF aerogel. This indicates that GO nanosheets are effectively reduced by VC–Na.32 In addition, there is still a large number of oxygen containing functional groups, which mainly derived from CNFs in the CNF–RGO (20%) hybrid aerogel. These hydrophilic CNFs can be used as electrolyte nano-reservoirs, which can effectively improve the utilization of the mesopores, and effectively reduces the ion transport distance. More importantly, CNF–RGO (20%) hybrid aerogel should have an excellent re-swell performance in aqueous electrolyte due to hydrophilic CNFs. Owing to the existence of CNFs, the CNF–RGO (20%) hybrid aerogel also exhibits high flexibility and ductility. This aerogel can be easily compressed into sheets by hand without cracking (Fig. S6†). The porous structure of RGO–CNF (20%) hybrid aerogel can be preserved largely in the RGO–CNF (20%) hybrid aerogel film (Fig. S7†). However, the morphology of RGO–CNF (20%) hybrid film exhibits a compact layer-by-layer stacking structure. The electrical conductivity of the RGO–CNF (20%) hybrid aerogel film, which measured by a four-probe method, is about 100 S m−1.
C/C–C peak intensity of CNF–RGO (20%) hybrid aerogel increase obviously compared with CNF aerogel. This indicates that GO nanosheets are effectively reduced by VC–Na.32 In addition, there is still a large number of oxygen containing functional groups, which mainly derived from CNFs in the CNF–RGO (20%) hybrid aerogel. These hydrophilic CNFs can be used as electrolyte nano-reservoirs, which can effectively improve the utilization of the mesopores, and effectively reduces the ion transport distance. More importantly, CNF–RGO (20%) hybrid aerogel should have an excellent re-swell performance in aqueous electrolyte due to hydrophilic CNFs. Owing to the existence of CNFs, the CNF–RGO (20%) hybrid aerogel also exhibits high flexibility and ductility. This aerogel can be easily compressed into sheets by hand without cracking (Fig. S6†). The porous structure of RGO–CNF (20%) hybrid aerogel can be preserved largely in the RGO–CNF (20%) hybrid aerogel film (Fig. S7†). However, the morphology of RGO–CNF (20%) hybrid film exhibits a compact layer-by-layer stacking structure. The electrical conductivity of the RGO–CNF (20%) hybrid aerogel film, which measured by a four-probe method, is about 100 S m−1.
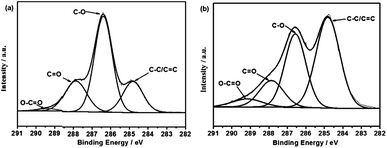 | ||
| Fig. 2 C1s XPS spectra of (a) CNF aerogel, (b) RGO–CNF (20%) hybrid aerogel. | ||
The CNF–RGO (20%) hybrid aerogel film is used to fabricate all-solid-state flexible supercapacitors without need for any other binders and electroactive additives (Fig. S8†). The H2SO4–polyvinyl (PVA) gel is chosen as the electrolyte and it also performs the function of a separator.33 Electrochemical performance of the CNF–RGO hybrid aerogel film based all-solid-state flexible supercapacitors (A-SCs) is measured at room temperature (Fig. S6†). For comparison, the CNF–RGO (20%) film based all-solid-state flexible supercapacitor (F-SCs) is also evaluated. Fig. 3a presents the cyclic voltammetry (CV) curves of A-SCs at scan rates of 5–100 mV s−1 with a potential window ranging from 0 V to 1 V. The symmetric and rectangular CV curves indicate the typical electrical double layer capacitive behavior with very rapid current response on voltage reversal. The CV curve still remains nearly rectangular without apparent distortion even at a scan rate of 100 mV s−1. However, the CV curve of F-SCs shows apparent distortion at a scan rate of 50 mV s−1 (Fig. S9†). This indicates that A-SCs exhibit lower resistance of ion transport and higher electrochemical performance than F-SCs. The specific electrode capacitance Cg of A-SCs is about 207 F g−1 (based on the mass of RGO) at a scan rate of 5 mV s−1. In addition, the specific electrode capacitance of F-SCs is only 188 F g−1 at the same scan rate. Compared with the values (120 F g−1, at a scan rate of 1 mV s−1) reported by Weng,34 the specific electrode capacitance of A-SCs was significantly enhanced by more than 72%. Furthermore, the performance of A-SCs was even better than many graphene-based electrode materials measured in liquid electrolytes (Table S1†). The excellent electrochemical performance of A-SCs can be ascribed to the aggregation of graphene nanosheets being effectively prevented by CNFs, and the superior hydrophilic characteristic of CNFs. Therefore, CNFs can significantly improve the accessible specific surface area and enhance utilization of mesopores of CNF–RGO electrode material. Area capacitance is an important indicator for all-solid-state flexible supercapacitors, and the area capacitance of A-SCs is up to 158 mF cm−2. This value is significantly higher than recently reported values (Table S2†). Galvanostatic charge–discharge curves of the A-SCs at different current densities are shown in Fig. 3b. All charge–discharge curves show an ideal linear profile, and all of the charge curves are perfect symmetrical with their corresponding discharge curve within the potential window. This indicates that the A-SCs have excellent capacitive performance (mainly attributed to pure electric double layer capacitance), and rapid current–voltage response. The specific electrode capacitances are calculated from the discharge curves, and shown in Fig. 3c. A-SCs have a specific capacitance of 203 F g−1 at a current density of 0.7 mA cm−2. When the current density is increased from 0.7 mA cm−2 to 11.2 mA cm−2, the A-SCs' still maintained 66% of the initial capacitance (134 F g−1), which indicates that A-SCs have a good capacitance retention capability due to the formation of a highly open, continuous pore structure (CNFs are used as hierarchical nanostructure maker), and then the diffusion of ions is much facilitated. Electrochemical stability in the bent state is an important property for all-solid-state flexible supercapacitors. The CV curves of A-SCs (5 mV s−1) in normal and bent (180°) states are shown in Fig. 3d. The change of CV curve in the bent state seems to be subtle, which indicates that the bending has almost no effect on electrochemical performance of A-SCs, and its specific electrode capacitance remained at about 207 F g−1. More importantly, the shape of the CV curve does not change obviously after withstanding 100 bending cycles (Fig. S10†), which indicates that the supercapacitor performance is almost unffected by bending cycles.
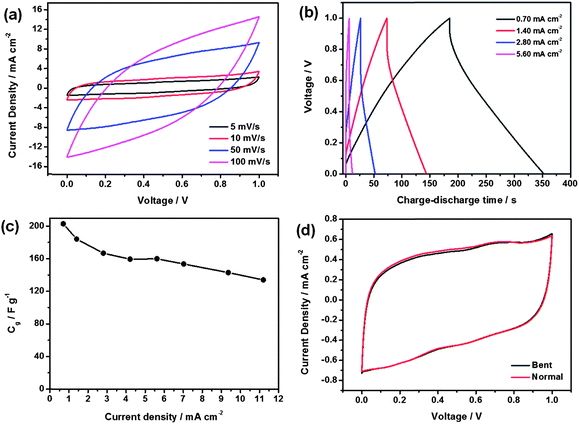 | ||
| Fig. 3 (a) Typical CV curves of A-SCs device at different scan rates. (b) Typical galvanostatic charge–discharge curves of the A-SCs at different current densities. (c) The specific electrode capacitance of A-SC devices as a function of the current density. (d) Comparison of CV curves at 5 mV s−1 for A-SCs tested in normal and bent states. | ||
Electrochemical impedance spectroscopy (EIS) is a very effective method to study in-depth the electrochemical performance of supercapacitors. Fig. 4a shows the Nyquist plots of the A-SCs and F-SCs using a sinusoidal signal of 5 mV in the frequency range from 1 Hz to 100 kHz under open circuit potential. The equivalent circuit is shown in the inset of Fig. 4a, where Rs is the electrolyte resistance, Cdl is double-layer capacitance, Rct is charge transfer resistance at the electrode/electrolyte interface, Wo is the Warburg impedance of the ionic diffusion, Cc and Rc are capacitance and resistance of the electrodes. At high frequency, the equivalent series resistance (which is described as Rs) value of A-SCs is about 32 Ω, obtained from the intercept of the Nyquist plot with the real axis. The absence of a semicircle at high frequencies is probably due to the charge transfer resistance (Rct) which is very small and the charge is stored primarily via a non-Faradaic process. At medium frequencies, Nyquist plots exhibit a Warburg-type line with a slope of about 45° (which is described as Wo). The projected length of the Warburg line on the real axis can be used for assessment of the ion diffusion process in porous electrodes.35,36 The Warburg-type line of A-SCs was significantly shorter than F-SCs, suggesting the faster ion diffusion in the A-SCs. The fast ion diffusion in the A-SCs is due to the novel hierarchical nanostructure, in which CNFs play an important role. The CNFs enclosed in the mesopores can be used as aqueous electrolyte nano-reservoirs, which can not only effectively improve the accessible surface area of RGO with aqueous electrolyte but also significantly reduce the ion diffusion distance from aqueous electrolyte nano-reservoirs to RGO nanosheets. At low frequencies, Nyquist plots of A-SCs and F-SCs are almost vertical lines showing ideal capacitive behavior. However, the curves are not ideal vertical lines, this may be attributed to the different distributions of pore size.37 The knee frequencies of the A-SCs and F-SCs are 464 Hz and 6.8 Hz, respectively. Generally speaking, below this frequency, the supercapacitors show pure capacitive behavior and most of the stored energy is available. Therefore, the A-SCs have better rate performance than F-SCs, which agrees with the CV results. A-SCs have a maximum areal energy of 20 μW h cm−2 (about 6 W h kg−1) at an areal power of 15.5 mW cm−2, which is much higher than recently Weng reported (15 μW h cm−2).34 This indicates that CNF–RGO hybrid aerogel is a promising electrode material of all-solid-state flexible supercapacitors. The electrochemical cycle stability of A-SCs is evaluated using galvanostatic charge–discharge at a current density of 3.4 mA cm−2 (Fig. 4b). The device capacitance still retains about 99.1% of the initial capacity after 5000 charge–discharge cycles, which indicates that A-SCs have superior cycle stability.
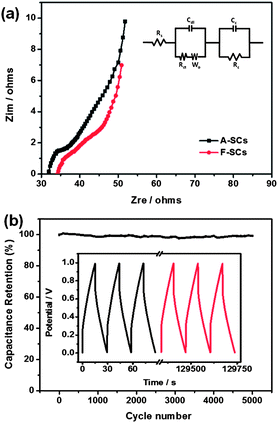 | ||
| Fig. 4 (a) Nyquist impedance plots of the A-SCs and F-SCs. (b) Cycling stability of A-SCs over 5000 cycles, the inset is the galvanostatic charge–discharge curve of the A-SCs. | ||
In summary, one-dimensional cellulose nanofibers are selected as nano-spacers, aqueous electrolyte nano-reservoirs, and the hierarchical nanostructure maker of CNF–RGO hybrid aerogels. All-solid-state flexible supercapacitors using CNF–RGO hybrid aerogel film as electrode material exhibit excellent performance: the areal capacitance, maximum areal power, and areal energy density are 158 mF cm−2 (207 F g−1), 15.5 mW cm−2, and 20 μW h cm−2, respectively. The flexible supercapacitor also exhibited excellent cyclic stability. Our work provides a novel method using a hydrophilic 1D nanomaterial to realize the full potential of graphene in an assembled bulk form. Therefore, the CNF–RGO hybrid aerogel has great potential as an electrode material for flexible supercapacitors. We think that this kind of hybrid aerogel can also be used as an electrode material for biomedical due to the excellent biocompatibility of CNFs.
Acknowledgements
This work was financially supported by the Natural Science Foundation of Beijing, and Science and Technology Key Project of Beijing Municipal Education Commission.Notes and references
- Z. Niu, W. Zhou, J. Chen, G. Feng, H. Li, W. Ma, J. Li, H. Dong, Y. Ren, D. Zhao and S. Xie, Energy Environ. Sci., 2011, 4, 1440–1446 CAS.
- Y. J. Kang, S.-J. Chun, S.-S. Lee, B.-Y. Kim, J. H. Kim, H. Chung, S.-Y. Lee and W. Kim, ACS Nano, 2012, 6, 6400–6406 CrossRef CAS.
- L. Bao and X. Li, Adv. Mater., 2012, 24, 3246–3252 CrossRef CAS.
- M. Kaempgen, C. K. Chan, J. Ma, Y. Cui and G. Gruner, Nano Lett., 2009, 9, 1872–1876 CrossRef CAS.
- L. Yuan, X. Xiao, T. Ding, J. Zhong, X. Zhang, Y. Shen, B. Hu, Y. Huang, J. Zhou and Z. L. Wang, Angew. Chem., Int. Ed., 2012, 51, 4934–4938 CrossRef CAS.
- L. Yuan, Y. Tao, J. Chen, J. Dai, T. Song, M. Ruan, Z. Ma, L. Gong, K. Liu, X. Zhang, X. Hu, J. Zhou and Z. L. Wang, Adv. Funct. Mater., 2011, 21, 2150–2154 CrossRef CAS.
- Y. Qi and M. C. McAlpine, Energy Environ. Sci., 2010, 3, 1275–1285 CAS.
- G. Zheng, L. Hu, H. Wu, X. Xie and Y. Cui, Energy Environ. Sci., 2011, 4, 3368–3373 CAS.
- D. Tobjork and R. Osterbacka, Adv. Mater., 2011, 23, 1935–1961 CrossRef.
- F. Liu, S. Song, D. Xue and H. Zhang, Adv. Mater., 2012, 24, 1089–1094 CrossRef CAS.
- Q. Wu, Y. Xu, Z. Yao, A. Liu and G. Shi, ACS Nano, 2010, 4, 1963–1970 CrossRef CAS.
- B. G. Choi, J. Hong, W. H. Hong, P. T. Hammond and H. Park, ACS Nano, 2011, 5, 7205–7213 CrossRef CAS.
- L. Yuan, X.-H. Lu, X. Xiao, T. Zhai, J. Dai, F. Zhang, B. Hu, X. Wang, L. Gong, J. Chen, C. Hu, Y. Tong, J. Zhou and Z. L. Wang, ACS Nano, 2012, 6, 656–661 CrossRef CAS.
- X. Zhang, Z. Sui, B. Xu, S. Yue, Y. Luo, W. Zhan and B. Liu, J. Mater. Chem., 2011, 21, 6494–6497 RSC.
- L. L. Zhang, R. Zhou and X. S. Zhao, J. Mater. Chem., 2010, 20, 5983–5992 RSC.
- B. Xu, S. Yue, Z. Sui, X. Zhang, S. Hou, G. Cao and Y. Yang, Energy Environ. Sci., 2011, 4, 2826–2830 CAS.
- M. Pumera, Energy Environ. Sci., 2011, 4, 668–674 CAS.
- L. L. Zhang, X. Zhao, M. D. Stoller, Y. Zhu, H. Ji, S. Murali, Y. Wu, S. Perales, B. Clevenger and R. S. Ruoff, Nano Lett., 2012, 12, 1806–1812 CrossRef CAS.
- K. Zhang, L. Mao, L. L. Zhang, H. S. O. Chan, X. S. Zhao and J. Wu, J. Mater. Chem., 2011, 21, 7302–7307 RSC.
- Y. Sun, Q. Wu and G. Shi, Phys. Chem. Chem. Phys., 2011, 13, 17249–17254 RSC.
- X. Yang, J. Zhu, L. Qiu and D. Li, Adv. Mater., 2011, 23, 2833–2838 CrossRef CAS.
- F. B. Su, C. K. Poh, J. S. Chen, G. W. Xu, D. Wang, Q. Li, J. Y. Lin and X. W. Lou, Energy Environ. Sci., 2011, 4, 717–724 CAS.
- A. Isogai, T. Saito and H. Fukuzumi, Nanoscale, 2011, 3, 71–85 RSC.
- T. Saito, Y. Nishiyama, J.-L. Putaux, M. Vignon and A. Isogai, Biomacromolecules, 2006, 7, 1687–1691 CrossRef CAS.
- V. C. Tung, M. J. Allen, Y. Yang and R. B. Kaner, Nat. Nanotechnol., 2009, 4, 25–29 CrossRef CAS.
- Z. Sui, X. Zhang, Y. Lei and Y. Luo, Carbon, 2011, 49, 4314–4321 CrossRef CAS.
- Y. Okita, T. Saito and A. Isogai, Biomacromolecules, 2010, 11, 1696–1700 CrossRef CAS.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS.
- H. C. Schniepp, J. L. Li, M. J. McAllister, H. Sai, M. Herrera-Alonso, D. H. Adamson, R. K. Prud'homme, R. Car, D. A. Saville and I. A. Aksay, J. Phys. Chem. B, 2006, 110, 8535–8539 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- J. Zhang, H. Yang, G. Shen, P. Cheng, J. Zhang and S. Guo, Chem. Commun., 2010, 46, 1112–1114 RSC.
- A. V. Murugan, T. Muraliganth and A. Manthiram, Chem. Mater., 2009, 21, 5004–5006 CrossRef CAS.
- C. Meng, C. Liu, L. Chen, C. Hu and S. Fan, Nano Lett., 2010, 10, 4025–4031 CrossRef CAS.
- Z. Weng, Y. Su, D.-W. Wang, F. Li, J. Du and H.-M. Cheng, Adv. Energy Mater., 2011, 1, 917–922 CrossRef CAS.
- G. P. Pandey, S. A. Hashmi and Y. Kumar, J. Electrochem. Soc., 2010, 157, A105–A114 CrossRef CAS.
- Y. J. Kim, I. Y. Jang, K. C. Park, Y. C. Jung, T. Oka, S. Iinou, Y. Komori, T. Kozutsumi, T. Hashiba, Y. A. Kim and M. Endo, Electrochim. Acta, 2010, 55, 5624–5628 CrossRef CAS.
- B. Xu, F. Wu, S. Chen, C. Zhang, G. Cao and Y. Yang, Electrochim. Acta, 2007, 52, 4595–4598 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, TEM images of cellulose nanofibers and GO nanosheets, IR spectra of cellulose nanofibers, XRD spectra of cellulose nanofibers and GO nanosheets, CV curves for F-SCs, and photographs of A-SCs and CNF–RGO (20%) aerogel film, SEM image of RGO–CNFs (20%) hybrid aerogel film and RGO–CNFs (20%) hybrid film, CV curves of A-SCs measured before and after 100 bending cycles at 10 mV s−1. See DOI: 10.1039/c2ta00386d |
| This journal is © The Royal Society of Chemistry 2013 |
