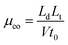Mussel-inspired polydopamine-assisted hydroxyapatite as the stationary phase for capillary electrochromatography
Juan
Zhang
,
Wenpeng
Zhang
,
Tao
Bao
and
Zilin
Chen
*
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University), Ministry of Education, Wuhan University School of Pharmaceutical Sciences, Wuhan, 430071, China. E-mail: chenzl@whu.edu.cn; Fax: +86-27-68759850; Tel: +86-27-68759893
First published on 5th November 2013
Abstract
A novel capillary with hydroxyapatite (HAP) as the stationary phase was prepared for open-tubular capillary electrochromatography (OT-CEC). To immobilize HAP, a mussel-inspired polydopamine method was utilized to modify the capillary firstly, generating a polydopamine layer; and then a layer of HAP would be formed on the polydopamine layer by a biomineralization process, to produce a HAP-modified capillary (HAP@capillary). Scanning electron microscopy (SEM), energy dispersive X-ray spectrometry (EDS) and X-ray photoelectron spectroscopy (XPS) provided evidence of nanostructured HAP grown on the surface of the capillary wall. The electroosmotic flow (EOF) characteristic of the HAP@capillary was investigated by varying the percentage of acetonitrile and pH value of the buffer with thiourea as a marker, and a pH-dependent EOF from anode to cathode was observed. The HAP@capillary exhibits high column efficiency for methylbenzene, up to 151![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138 plates per meter. Different kinds of compounds including alkylbenzenes, phenols and amines have been successfully separated by the HAP@capillary in CEC mode. The HAP@capillary also possessed good separation ability in capillary liquid chromatography (CLC) mode because of the relatively large ratio of HAP in the capillary; however, the separation efficiency was not as good as that in CEC mode. The reproducibilities of the HAP@capillary were evaluated, and the relative standard deviations were found to be lower than 5%.
138 plates per meter. Different kinds of compounds including alkylbenzenes, phenols and amines have been successfully separated by the HAP@capillary in CEC mode. The HAP@capillary also possessed good separation ability in capillary liquid chromatography (CLC) mode because of the relatively large ratio of HAP in the capillary; however, the separation efficiency was not as good as that in CEC mode. The reproducibilities of the HAP@capillary were evaluated, and the relative standard deviations were found to be lower than 5%.
Introduction
Hydroxyapatite (HAP: Ca10(PO4)6(OH)2) is the main mineral component of natural bone tissues and teeth.1,2 Due to its outstanding biocompatibility, osteoconductivity and bioactivity, it has been considered to be a promising biomaterial.3–5 Therefore, various nanostructured HAP with different morphologies and HAP-based composite materials have been prepared,6–10 and have been applied in various areas.11–18HAP can be synthesized by hydrothermal,19 mechanochemical,20 sol–gel,21 precipitation22,23 and template-assisted biomineralization24 methods. HAP prepared by those methods has different morphologies including nanorods, plate-like nanocrystals, nanoparticles and three-dimensional (3-D) structures which have great influence on its chemical and biological properties. Nanostructured HAP with high specific surface area, adsorptivity, biocompatibility and manifold interactions has been applied in chromatographic science. HAP used as a chromatographic medium was first reported by Tiselius25 in 1956. HAP was coated on zirconic-magnesia microspheres via a biomimetic technique by incubating the substrates in supersaturated calcification solution, which was successfully applied in the separation of five model proteins with high performance liquid chromatography (HPLC).26
Capillary electrochromatography (CEC)27–29 is a powerful analytical technique which combines high efficiency of capillary electrophoresis (CE) and high selectivity of HPLC. It provides fast and efficient separation with much lower consumption of samples and solvents than that of HPLC. Packed column, monolithic column and open-tubular column are the commonly used columns in CEC. Among these columns, the main advantages of the open-tubular capillary column are the ease of preparation, the absence of frit formation and particle packing, the absence of bubble formation, short conditioning times and simple instrumental handling.30–33 Although HAP has been used in liquid chromatography, there are few reports about its application in micro-scaled chromatographic separation, which is regarded as more efficient than conventional liquid chromatography. However, it is difficult to modify and immobilize HAP in the capillary of micrometer size, because HAP is an inorganic material which lacks the reactive site to interact with the silanol groups of the capillary. Recently, Krenkova et al.34 prepared a poly(2-hydroxyethylmethacrylate-co-ethylene dimethacrylate) capillary monolith incorporated with commercially available HAP nanoparticles and used for capillary liquid chromatography (CLC). It was the first report about HAP used in micro- and nanoscale separation in a capillary. However, HAP nanoparticles were immobilized by incorporation in the monolithic column, which could result in poor dispersion of HAP in the prepolymerization solution, and the loading capacity and permeability of the column would be limited as a result.
Polydopamine, a functional polymeric mimic of the mussel adhesive protein as a binding agent for coating various substrates, including inorganic or organic materials, was first reported by the Messersmith group.35 The robust polydopamine surface can offer reaction sites for the formation of a secondary ad-layer.36 The polydopamine method has been widely exploited in various fields, including biomineralization,10,37 surface-initiated polymerization,38 surface modification,39 and functionalization of nanomaterials.38,40,41 Recently, a new HAP crystallization method has also been developed on the basis of the polydopamine process,37 which could be applied to different kinds of substances. Inspired by this universal biomineralization route, along with our successful attempts of polydopamine modification inside micro-tubes in our previous studies,42,43 it is also promising to fabricate biomineralized-HAP in a capillary for CEC.
In this work, we prepared a novel open-tubular capillary column using mussel-inspired polydopamine-assisted biomineralization for immobilization of HAP on the inner wall of the capillary for CEC. Polydopamine coating on the inner wall of the capillary facilitated the mobilization and formation of nanostructured HAP crystals. The formation of biominerals on the polydopamine-coated capillary followed a layer-by-layer growth mode by strong interactions between polydopamine and biominerals. The general morphology and surface properties of this column were characterized by scanning electron microscopy (SEM), energy dispersive X-ray spectrometry (EDS) and X-ray photoelectron spectroscopy (XPS). The chromatographic performance of the HAP coated capillary column was systematically evaluated. The separation behaviors of the column towards acid, basic and neutral compounds were examined. To our knowledge, it is the first time that HAP is immobilized in the capillary for capillary electrochromatography.
Experimental
Chemicals and materials
Dopamine hydrochloride was purchased from Sigma-Aldrich (MO, USA). Sodium phosphate dibasic dodecahydrate (Na2HPO4·12H2O) was purchased from Luoyang Chemical Reagent Plant (Henan, China). Sodium chloride (NaCl), potassium chloride (KCl), sodium sulfate anhydrous (Na2SO4), calcium chloride anhydrous (CaCl2), benzene, methylbenzene, pyridine and aniline were obtained from Sinopharm Chemical Reagent Company (Shanghai, China). Magnesium chloride anhydrous (MgCl2) and sodium bicarbonate (NaHCO3) were obtained from Shenshi Chemical Reagent and Instrumental Company (Wuhan, China). Ethylbenzene, n-propylbenzene, n-butylbenzene, phenol, 4-chloro-3-methylphenol, 2-naphthol, 2,4-dimethyl phenol, and N,N-dimethylaniline were purchased from Aladdin (Shanghai, China). All above chemicals are of analytical grade. Acetonitrile was of HPLC grade and purchased from Tedia (OH, USA). Deionized water used was purified with a Milli-Q system (MA, USA). Fused-silica capillaries of 50 μm i.d. × 375 μm o.d. were purchased from Ruifeng Chromatographic Devices (Yongnian, Hebei, China).Apparatus
All experiments were carried out on an Agilent 7100 CE system (Waldbronn, Germany) equipped with an autosampler, a diode array detector and a temperature controlled column compartment. A chromatographic workstation (Chemistry Station, USA) was used for data acquisition and treatment. Surface characteristics and elemental analysis of the HAP open-tubular capillary column were performed on a scanning electron microscope (SEM) and an energy dispersive X-ray spectrometer (EDS) (FEI quanta 200, Netherland), respectively. X-ray photoelectron spectroscopy (XPS) was performed on a KRATOS XSAM800 XPS (Manchester, UK), with monochromated Mg Kα radiation (hν = 1253.6 eV) by FRR mode, the acceleration voltage for X-ray was 12 kV and the vacuum was maintained at 6 × 10−7 Pa.Sample solutions and mobile phase preparation
The standard stock solutions of 1.0 mg mL−1 of all the analytes were prepared by dissolving analytes in acetonitrile individually and were stored at 4 °C in a refrigerator. The mobile phase was prepared by mixing 10 mM Na2HPO4 with different pH values with an appropriate amount of acetonitrile. The pH value of the phosphate solution was adjusted to 3.0–9.0 using phosphoric acid solutions. All these solutions were filtered by using 0.45 μm membrane filters before use.Preparation of HAP@capillary
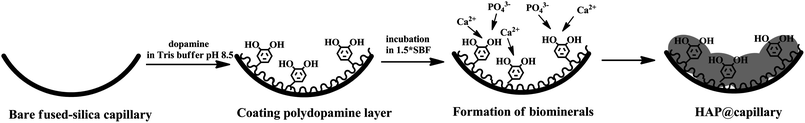
The procedure for preparing a HAP-coated open-tubular column is schematically shown in Fig. 1. As shown in Fig. 1, dopamine can spontaneously polymerize on the surface of the capillary to form a polydopamine coating under alkaline conditions. The catecholamine moieties that are abundant in polydopamine are responsible for the nucleation of HAP by concentrating Ca2+ ions at the interface through electrostatic interactions and the HAP formation was by co-precipitation of calcium and phosphate ions. The concrete procedures were described as follows. Briefly, the bare fused-silica capillary with 60.5 cm total length and 52 cm effective length prior to its first use was preconditioned by flushing with 1 M NaOH for 2 h, H2O for 30 min in sequence, and dried by purging nitrogen gas for further use. 10 mg dopamine hydrochloride was dissolved in 5 mL of 10 mM Tris–HCl buffer (pH = 8.5). Then the resultant solution was continually injected into the pretreated capillary with a syringe pump for 20 h at room temperature for polydopamine modification. Subsequently, the polydopamine coated capillary was rinsed with water for several minutes to avoid residuary dopamine getting physically attached to the tube surface and then dried with the nitrogen stream. The above procedures were repeated to increase the coating thickness. Afterwards, the polydopamine coated capillary was rinsed with freshly prepared 1.5× simulated body fluid (SBF) for 5 min and sealed on both ends with silicone. The composition of 1.5× SBF was as follows: Na+, 213.0; K+, 7.5; Mg2+, 2.25; Ca2+, 3.75; Cl−, 221.7; HCO3−, 6.3; HPO42−, 1.5; SO42−, 0.75 mM. The preparation process of 1.5× SBF was stated in detail as follows. NaCl (354.5 mg), NaHCO3 (15.9 mg), KCl (16.8 mg), Na2HPO4·12H2O (16.1 mg), MgCl2 (6.4 mg), CaCl2 (12.5 mg), and Na2SO4 (3.2 mg) were weighed accurately and dissolved in deionized water in sequence to obtain 30 mL transparent 1.5× SBF. Then, the capillary was put into the water bath and incubated at 37 °C to grow HAP crystals for 2 weeks. Finally, the capillary was taken out and flushed with deionized water. Prior to use, it was equilibrated with running buffer for 20 min until a stable current and baseline were achieved.Results and discussion
Characterization of HAP@capillary
The general morphology of this column was characterized by scanning electron microscopy. As shown in Fig. 2A, the bare fused-silica capillary has a smooth inner surface. When nanostructured HAP crystals were grown on the inner wall of the capillary which had been coated with polydopamine, the inner surface of the capillary became rough and irregular, which is shown in Fig. 2B. In order to further demonstrate the formation of HAP nanocrystals, EDS and XPS were applied to characterize the surface properties. Since it is difficult to obtain EDS and XPS analysis on the inner wall of the capillary, in this experiment fused silica capillary columns were replaced by a microscope cover glass whose character is the same as the fused silica capillary column. Except for that, the modification processes on the microscope cover glass were the same as those used for the modification of the inner wall of the capillary. The EDS is shown in Fig. 3A. The existence of Ca and P elements illustrates the formation of HAP. Since the depth of the test sample by EDS was from dozens of nanometers to several micrometers in diameter, it was vulnerable to the interference of the matrix. Therefore, the XPS experiment was used to further verify the existence of HAP nanocrystals on the inner wall of the capillary. As shown in Fig. 3B, the surface exhibits O 1s, N 1s, Ca 2p, C 1s and P 2p peaks at 526, 402, 348, 310 and 134 eV, respectively. The data further demonstrate the formation of nanostructured HAP crystals.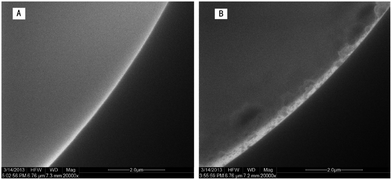 | ||
| Fig. 2 SEM images of the bare fused-silica capillary wall (A) and the capillary wall grown with nanostructured HAP (B). | ||
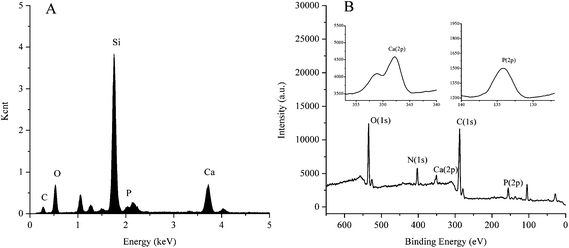 | ||
| Fig. 3 Energy dispersive X-ray spectrometry spectra (A) and X-ray photoelectron spectroscopy spectra (B). | ||
Influence of organic solvents and pH on the EOF mobility
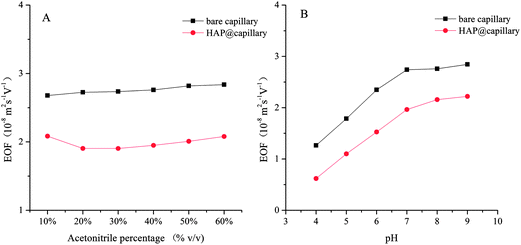
In OT-CEC, the transport of the mobile phase through the capillary is achieved by electroosmotic flow (EOF). The EOF mobility was investigated with thiourea as the natural marker and calculated as follows:where Ld and Lt are the effective length (52 cm) and total length of the capillary (60.5 cm), t0 is the migration time of thiourea, and V is the voltage applied across the capillary (20 kV). Fig. 4 shows the influence of acetonitrile percentage and buffer pH on the EOF mobility of the bare capillary column and the HAP@capillary. The HAP@capillary shows lower EOF mobility than the bare capillary as can be seen in Fig. 4A. The possible reasons are that the capillary wall was covered with the polydopamine-assisted HAP whose surface might carry relatively more negative charged OH− and PO43− than positive charged Ca2+, as a result, the EOF direction of the HAP@capillary was the same as the bare column and the EOF mobility was not dramatically decreased. The influence of the organic modifier on the EOF mainly depends on two factors, dielectric constant and viscosity of the organic modifier. As revealed in Fig. 4A, the acetonitrile percentage has little influence on the EOF mobility of the bare column as well as the HAP@capillary, which is the result of these two factors. Fig. 4B shows the influence of the pH value on the EOF mobility of the bare column and HAP@capillary which was carried out by using the buffer with different pH values ranging from 4 to 9. The EOF mobility of the bare column increased remarkably with the pH value increased from 4 to 7 due to the dissociation of silanol groups and increased slightly with pH above 7, which resulted from the complete dissociation of silanol groups. The results also indicated that the HAP@capillary exhibited a pH-dependent electroosmotic flow from anode to cathode and the trend of EOF change on the HAP@capillary was similar to that of the bare column. The EOF mobility on the HAP@capillary was lower than that on the bare column. The inner surface of the fused-silica capillary was modified by polydopamine-HAP non-covalently, so the charge of the surface is mainly affected by the dissociation of silanol groups of the capillary. The phenolic hydroxyl groups of the polydopamine layer may also contribute a little to the charge of the surface. As some of the silanol groups were covered by the stationary phase, the EOF mobility on the HAP@capillary was lower than that on the bare column as a result.
The influence of repetition times of polydopamine coating on separation ability
Dopamine can spontaneously polymerize on the surface of the capillary to form a robust polydopamine coating under alkaline conditions. The thickness of the polydopamine coating on the inner wall of the capillary is important for nucleus formation of CaP, which has great influence on the separation ability. The thickness of the polydopamine coating is related to the coating time and coating repetition times. Although the dopamine polymerization started very soon, the coating process was carried out for 20 h to ensure complete polymerization. The influence of repetition times of polydopamine coating on the formation of HAP for separation ability was also investigated. The results shown in Fig. 5 demonstrate that the separation ability was enhanced as well as the longer retention time of analytes with the repetition times of polydopamine coating. The reason might be that the more layers of polydopamine coating there are, the more compact and uniform the HAP crystals are. The compact and uniform HAP crystals on the surface of the capillary might have strong interactions with analytes causing the excellent separation. When increasing the repetition times to three times, the prepared HAP capillary column was easy to block. Therefore, the capillary used in the separation was coated with polydopamine twice.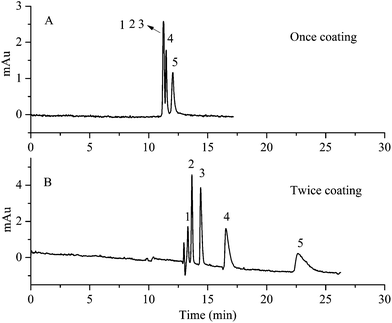 | ||
| Fig. 5 The influence of repetition times of polydopamine coating on separation ability. (A) Once coating. (B) Twice coating. Experimental conditions: acetonitrile–10 mM Na2HPO4 (10/90, v/v), pH 7.0. All other separation conditions are the same as mentioned in Fig. 3. Peak identification: 1, benzene; 2, methylbenzene; 3, ethylbenzene; 4, n-propylbenzene; 5, n-butylbenzene. | ||
The influence of acetonitrile content on separation
Fig. 6 shows the influence of acetonitrile content on the separation of five substituted benzene on the HAP@capillary. It can be found that the five substituted benzene achieved baseline separation at 10% acetonitrile in 10 mM Na2HPO4 at pH 7. The resolution among these five compounds decreased with the increase of acetonitrile content. For example, the resolution between benzene and methylbenzene decreased from 1.62 at 10% acetonitrile to 0.65 at 20% acetonitrile. At 30% acetonitrile, peaks of benzene, methylbenzene and ethylbenzene overlapped completely. These experimental results can be attributed to the fact that with the increase of acetonitrile contents, the eluting power of the mobile phase was enhanced and the increased acetonitrile content weakened the interactions between analytes and stationary phase, which reduced the separation ability. The surface of HAP was comprised of functional groups of positively charged pairs of calcium ions and clusters of negatively charged phosphate groups. The separation mechanism of five substituted benzene on the HAP@capillary might involve the charge-induced dipole–dipole interaction and the cation–π interactions between the positively charged Ca2+ on HAP and the electron-rich π-system of five substituted benzene.44 The electron density of the benzene ring on the five substituted benzene is n-butylbenzene > n-propylbenzene > ethylbenzene > methylbenzene > benzene. As a result, the interaction between HAP and the five substituted benzene is also assisted by the electron density. The retention of benzene was the weakest and was firstly eluted out of the capillary column, while n-butylbenzene was lastly eluted out of the HAP@capillary. Since the basal plane of the nanostructured HAP crystal with high density of OH− was negatively charged and the prismatic plane with rich Ca2+ was positively charged, the electrostatic interaction between HAP nanocrystals and electron-rich π-system of five substituted benzene might be involved in the mechanism. Moreover, the HAP crystals were nano-sized with high surface area and porous structure, and the absorption might contribute to the separation of five substituted benzene.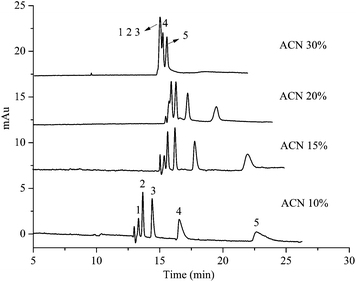 | ||
| Fig. 6 The influence of acetonitrile content on the separation of five substituted benzene on the HAP@capillary. Experimental conditions: acetonitrile–10 mM Na2HPO4 (pH 7.0). All other separation conditions are the same as mentioned in Fig. 4. Peak identifications are identical to Fig. 5. | ||
In order to test whether the baseline separation was mainly attributed to the interaction between the five compounds and polydopamine, or the compounds and HAP particles, the comparison of separation ability on the HAP@capillary and on the polydopamine coated capillary column (polydopamine@capillary) is shown in Fig. 7. The preparation procedure of the polydopamine@capillary was similar to that in Section 2.4 except for 1.5× SBF incubation. Simply put, the preconditioned capillary was modified with polydopamine twice and washed with deionized water, and then the polydopamine@capillary was obtained. As revealed in Fig. 7, the five substituted benzene could be well separated on the HAP@capillary, but not on the polydopamine@capillary under the same separation conditions. However, we can observe three peaks on the polydopamine@capillary which illustrate its separation performance to some extent. The reason might be that polydopamine contains the catechol and quinine functional groups which can form π–π interactions and hydrogen bonding interactions. Therefore, polydopamine could contribute to the separation, but HAP plays a more important role in CEC separations.
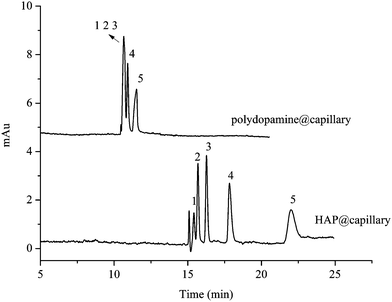 | ||
| Fig. 7 Comparison of separation ability on the polydopamine@column and HAP@capillary. Experimental conditions: acetonitrile–10 mM Na2HPO4 (15/85, v/v), pH 7.0. All other separation conditions are the same as mentioned in Fig. 4. Peak identifications are identical to Fig. 5. | ||
The influence of pH value on separation
Since the EOF mobility on the HAP@capillary showed a pH-dependent tendency, the pH value had great influence on the migration time which further affected the separation ability. It was essential to investigate the influence of pH value on the migration time and resolution which is shown in Fig. 8. The EOF mobility decreased with the pH value decreased from 7 to 4, so the migration time was increased dramatically, which resulted in the increased resolution in general among those five substituted benzene. The reason might be that the long migration time employed a suitable environment for the interactions between HAP and five substituted benzene. The migration time and resolution changed slightly at pH 7 and 8. When the HAP@capillary was used in a strong acidic environment for a long time, the column efficiency and separation ability were significantly decreased. The possible reason is that the HAP might dissolve under strong acidic conditions.45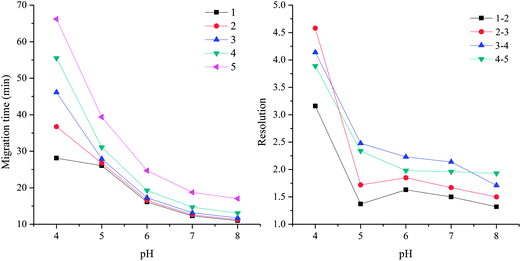 | ||
| Fig. 8 The influence of pH value on the migration time and resolution of five substituted benzene on the HAP@capillary. Experimental conditions: acetonitrile–10 mM Na2HPO4 (10/90, v/v). All other separation conditions are the same as mentioned in Fig. 4. Peak identifications are identical to Fig. 5. | ||
Comparison of OT-CLC and OT-CEC on HAP@capillary
The separation performance of the HAP@capillary by open-tubular capillary liquid chromatography (OT-CLC) mode was also studied to compare with that by OT-CEC mode. Fig. 9 shows the chromatograms of the separation of five substituted benzene by OT-CEC and OT-CLC modes, respectively. It can be observed that five substituted benzene can obtain baseline separation by OT-CEC mode, while only four substituted benzene can be well separated by OT-CLC mode, which illustrated the better separation efficiency of OT-CEC than that of OT-CLC. By OT-CEC mode, the shape of peaks is much narrower which corresponds to higher numbers of theoretical plates. For example, the number of theoretical plates of methylbenzene (N2 = 151![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138) by OT-CEC is seven times larger than that by OT-CLC (N2 = 20
138) by OT-CEC is seven times larger than that by OT-CLC (N2 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 398). The enhanced separation efficiency and resolution of the HAP@capillary by OT-CEC mode probably resulted from the “plug-like” flow profile of EOF in which the band broadening was significantly reduced. As can be seen in Fig. 6, the peak shape was affected by the acetonitrile content in running buffer. Although the peak shape under the condition of 10% acetonitrile in Fig. 6 has a little tailing by OT-CEC, the number of theoretical plates per meter for benzene (N = 109
398). The enhanced separation efficiency and resolution of the HAP@capillary by OT-CEC mode probably resulted from the “plug-like” flow profile of EOF in which the band broadening was significantly reduced. As can be seen in Fig. 6, the peak shape was affected by the acetonitrile content in running buffer. Although the peak shape under the condition of 10% acetonitrile in Fig. 6 has a little tailing by OT-CEC, the number of theoretical plates per meter for benzene (N = 109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950) was obtained, which was comparative to that by other open-tubular columns, for example, the graphene modified capillary (N = 54
950) was obtained, which was comparative to that by other open-tubular columns, for example, the graphene modified capillary (N = 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292 for benzene)46 and C10-cationic latex particle coated capillary (N = 96
292 for benzene)46 and C10-cationic latex particle coated capillary (N = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 056 for benzene).47 A larger number of theoretical plates on the HAP@capillary can be obtained when the content of acetonitrile increased to 15%, where the number of theoretical plates was 167
056 for benzene).47 A larger number of theoretical plates on the HAP@capillary can be obtained when the content of acetonitrile increased to 15%, where the number of theoretical plates was 167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 736 for benzene. The separation ability for the homologs of benzene and polycyclic aromatic hydrocarbons on the HAP@capillary was better than that on the graphene coated open tubular column.32 The successful separation of substituted benzene by OT-CLC further confirms the existence of HAP grown and covered on the inner wall of the capillary. Meanwhile, there was no denying that the strong cation–π interaction between HAP and analytes contributed a lot to the successful separation by OT-CLC. The experimental results show the feasibility of both OT-CEC and OT-CLC, while better chromatography performance was obtained by OT-CEC.
736 for benzene. The separation ability for the homologs of benzene and polycyclic aromatic hydrocarbons on the HAP@capillary was better than that on the graphene coated open tubular column.32 The successful separation of substituted benzene by OT-CLC further confirms the existence of HAP grown and covered on the inner wall of the capillary. Meanwhile, there was no denying that the strong cation–π interaction between HAP and analytes contributed a lot to the successful separation by OT-CLC. The experimental results show the feasibility of both OT-CEC and OT-CLC, while better chromatography performance was obtained by OT-CEC.
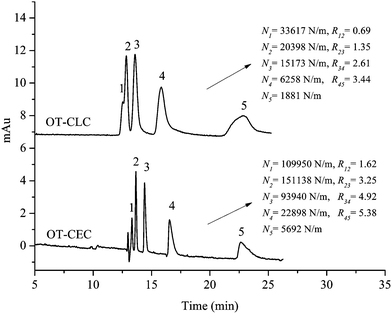 | ||
| Fig. 9 The comparison of separation performance of five substituted benzene on the HAP@capillary by OT-CEC with 20 kV and OT-CLC with 60 mbar inlet pressure. Experimental conditions: acetonitrile–10 mM Na2HPO4 (10/90, v/v), pH 7.0 for OT-CEC and OT-CLC; N, the number of theoretical plates of substituted benzene; R12, resolution between benzene (1) and methylbenzene (2). All other separation conditions are the same as mentioned in Fig. 4. Peak identifications are identical to Fig. 5. | ||
Separation efficiency of HAP@capillary towards neutral, basic and acidic compounds
The HAP@capillary was also applied for the separation of other neutral compounds, such as polycyclic aromatic hydrocarbons (PAHs). PAHs containing multiple benzene rings were electron-rich compounds which can form cation–π interactions with the positively charged Ca2+ on HAP. Moreover, the adsorption effect of porous nanostructured HAP crystals towards strong hydrophobic PAH compounds could also contribute to the separation. As shown in Fig. 10, four PAHs were well separated in a 10 mM phosphate buffer at pH 7.0 containing 20% acetonitrile on the HAP@capillary. As can be seen, the peaks in the electrochromatogram are not symmetric, which may be attributed to the strong affinity of HAP to electron-rich compounds. The heterogeneous surface of nanostructured HAP crystals might also result in the tailing of PAH peaks.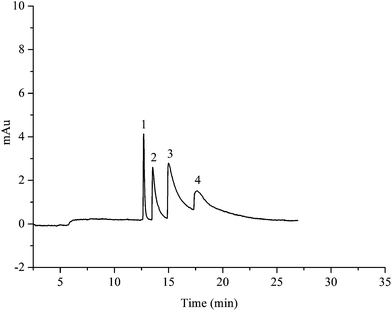 | ||
| Fig. 10 Electrochromatogram of the separation of four PAHs. Experimental conditions: acetonitrile–10 mM Na2HPO4 (25/75, v/v), pH 7.0; detection wave: 254 nm. All other separation conditions are the same as mentioned in Fig. 4. Peak identification: 1, naphthalene; 2, fluorene; 3, phenanthrene; 4, fluoranthene. | ||
The basic compounds and acidic compounds were also separated on the HAP@capillary. As shown in Fig. 11A, three basic compounds including aniline, pyridine, and N,N-dimethylaniline could be separated on the HAP@capillary. Since aniline, pyridine, and N,N-dimethylaniline are all neutral at pH 7 because of the fact that the pKa of their conjugate acids is 4.63, 5.19, and 5.15, respectively, they could not be separated by the differences in the migration mobility on the bare fused-silica capillary, therefore, there was only one peak on the bare fused-silica capillary. However, there were three peaks on the HAP@capillary which revealed that the retention interaction of the HAP stationary phase plays an important role in separation performance. These three basic compounds were not baseline separated on the HAP@capillary, which might be caused by the strong hydrogen bond interactions between basic compounds and the HAP.
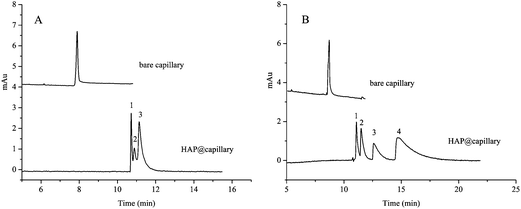 | ||
| Fig. 11 Electrochromatogram of the separation of three basic compounds (A) and four acidic compounds (B). Experimental conditions: acetonitrile–10 mM Na2HPO4 (5/95, v/v), pH 7.0; detection wave: 210 nm (A) and 254 nm (B). All other separation conditions are the same as mentioned in Fig. 4. Peak identification: (A) 1, pyridine; 2, aniline; 3, N,N-dimethylaniline; (B) 1, phenol; 2, 2,4-dimethylphenol; 3, 4-chloro-3-methylphenol; 4, 2-naphthol. | ||
Four acidic compounds which could not be separated on the bare capillary were well separated on the HAP@capillary, which is illustrated in Fig. 11B. The pKa of four acidic compounds including phenol, 2,4-dimethylphenol, 4-chloro-3-methylphenol, and 2-naphthol is 10, 10.6, 9.55, and 9.51. These four acidic compounds cannot be separated on the bare column because they are neutral at pH 7 and there are no differences in the migration mobility of analytes, which was forced by EOF. However, they can be well separated on the HAP@capillary under the same conditions. In these four acidic compounds, the substituent groups on the benzene ring determine the electron density of the benzene ring and the compound polarity, which influence the interactions between HAP and analytes, such as cation–π interaction and hydrophobic interaction. The difference of interactions between different compounds results in the successful separation. The reproducibility of four phenolic compounds was investigated by the relative standard deviations (RSDs). The RSDs of migration time and peak area were in the range of 1.1–2.5% and 2.4–3.5%, respectively.
Stability of HAP@capillary
The stability of the HAP@capillary is also an important indicator of the usability of a column. The reproducibility was evaluated on the basis of the relative standard deviations (RSDs) of migration time and peak area obtained from five substituted benzene for five replicate analyses under OT-CEC. The intra-day and inter-day RSDs shown in Table 1 were all below 5%, which indicated that the HAP@capillary had a good reproducibility. Little change in the separation efficiency was observed after 60 consecutive runs on the HAP@capillary. Moreover, the columns can still maintain the separation performance without significant deviation of retention times and deterioration of peak shapes after repeated analysis 120 times within two months. All these results demonstrate that the HAP@capillary possesses good stability.| Compounds | Time (RSD%) | Peak area (RSD%) | ||
|---|---|---|---|---|
| Intra-day (n = 5) | Inter-day (n = 5) | Intra-day (n = 5) | Inter-day (n = 5) | |
| Benzene | 1.62 | 2.46 | 2.21 | 3.82 |
| Methylbenzene | 1.63 | 2.46 | 1.17 | 4.03 |
| Ethylbenzene | 1.62 | 2.50 | 1.73 | 3.31 |
| n-Propylbenzene | 1.63 | 2.48 | 2.12 | 4.84 |
| n-Butylbenzene | 1.28 | 2.88 | 1.13 | 3.75 |
Conclusion
A novel HAP based capillary for CEC was prepared by mussel-inspired biomineralization for the first time. Mussel-inspired polydopamine acted as the binding agent and assisted the formation of nanostructured HAP crystals. The preparation procedure was simple, economical and environmentally friendly. Experimental results show that the separation ability of this column is influenced by the repetition times of polydopamine coating. The HAP@capillary also exhibits good separation efficiency towards neutral, basic and acidic compounds. The results show the good reproducibility of less than 5% RSD of retention time and peak area, excellent stability with total 120 runs in two-month usage. Our work demonstrates that the polydopamine assisted HAP nanostructure can be used as the stationary phase for OT-CEC, and the HAP@capillary is a promising separation medium for separating certain kinds of compounds due to its good separation efficiency.Acknowledgements
This work was supported by the National Scientific Foundation of China (Grant nos: 21375101, 90817103, 30973672), the Doctoral Fund of Ministry of Education of China (no. 20110141110024), Hubei Provincial Scientific Foundation (no. 2011CDB475) and the Fundamental Research Funds for the Central Universities.References
- Q. Q. Hoang, F. Sicheri, A. J. Howard and D. S. C. Yang, Nature, 2003, 425, 977–980 CrossRef CAS PubMed.
- K. Yamagishi, K. Onuma, T. Suzuki, F. Okada, J. Tagami, M. Otsuki and P. Senawangse, Nature, 2005, 433, 819 CrossRef CAS PubMed.
- Y. Cai and R. Tang, J. Mater. Chem., 2008, 18, 3775–3787 RSC.
- S. V. Dorozhkin and M. Epple, Angew. Chem., Int. Ed., 2002, 41, 3130–3146 CrossRef CAS.
- S. Kim and C. B. Park, Adv. Funct. Mater., 2013, 23, 10–25 CrossRef CAS.
- F. Chen, Q. L. Tang, Y. J. Zhu, K. W. Wang, M. L. Zhang, W. Y. Zhai and J. Chang, Acta Biomater., 2010, 6, 3013–3020 CrossRef CAS PubMed.
- M. Jevtić, M. Mitrić, S. Škapin, B. Jančar, N. Ignjatović and D. Uskoković, Cryst. Growth Des., 2008, 8, 2217–2222 Search PubMed.
- M. Wu, Q. Wang, X. Liu and H. Liu, Carbon, 2013, 51, 335–345 CrossRef CAS PubMed.
- H. Liu, P. Xi, G. Xie, Y. Shi, F. Hou, L. Huang, F. Chen, Z. Zeng, C. Shao and J. Wang, J. Phys. Chem. C, 2012, 116, 3334–3341 CAS.
- M. Lee, S. H. Ku, J. Ryu and C. B. Park, J. Mater. Chem., 2010, 20, 8848–8853 RSC.
- L. C. Palmer, C. J. Newcomb, S. R. Kaltz, E. D. Spoerke and S. I. Stupp, Chem. Rev., 2008, 108, 4754–4783 CrossRef CAS PubMed.
- K. S. Vecchio, X. Zhang, J. B. Massie, M. Wang and C. W. Kim, Acta Biomater., 2007, 3, 910–918 CrossRef CAS PubMed.
- A. Almirall, G. Larrecq, J. A. Delgado, S. Martínez, J. A. Planell and M. P. Ginebra, Biomaterials, 2004, 25, 3671–3680 CrossRef CAS PubMed.
- L. Wang, K. J. Jeong, H. H. Chiang, D. Zurakowski, I. Behlau, J. Chodosh, C. H. Dohlman, R. Langer and D. S. Kohane, Invest. Ophthalmol. Visual Sci., 2011, 52, 7392–7399 CAS.
- M. Y. Ma, Y. J. Zhu, L. Li and S. W. Cao, J. Mater. Chem., 2008, 18, 2722–2727 RSC.
- S. Dasgupta, S. S. Banerjee, A. Bandyopadhyay and S. Bose, Langmuir, 2010, 26, 4958–4964 CrossRef CAS PubMed.
- Z. Li, Z. Liu, M. Yin, X. Yang, Q. Yuan, J. Ren and X. Qu, Biomacromolecules, 2012, 13, 4257–4263 CAS.
- X. Y. Zhao, Y. J. Zhu, F. Chen, B. Q. Lu and J. Wu, CrystEngComm, 2013, 15, 206–212 RSC.
- J. D. Chen, Y. J. Wang, K. Wei, S. H. Zhang and X. T. Shi, Biomaterials, 2007, 28, 2275–2280 CrossRef CAS PubMed.
- W. L. Suchanek, K. Byrappa, P. Shuk, R. E. Riman, V. F. Janas and K. S. TenHuisen, Biomaterials, 2004, 25, 4647–4657 CrossRef CAS PubMed.
- A. Bigi, E. Boanini and K. Rubini, J. Solid State Chem., 2004, 177, 3092–3098 CrossRef CAS PubMed.
- W. He, P. Kjellin, F. Currie, P. Handa, C. S. Knee, J. Bielecki, L. R. Wallenberg and M. Andersson, Chem. Mater., 2011, 24, 892–902 CrossRef.
- B. Cengiz, Y. Gokce, N. Yildiz, Z. Aktas and A. Calimli, Colloids Surf., A, 2008, 322, 29–33 CrossRef CAS PubMed.
- S. A. Hutchens, R. S. Benson, B. R. Evans, H. M. O'Neill and C. J. Rawn, Biomaterials, 2006, 27, 4661–4670 CrossRef CAS PubMed.
- A. Tiselius, S. Hjertén and Ö. Levin, Arch. Biochem. Biophys., 1956, 65, 132–155 CrossRef.
- T. Li and Y. Q. Feng, Talanta, 2009, 80, 889–894 CrossRef CAS PubMed.
- C. de Perre, I. Corbin, M. Blas and B. R. McCord, J. Chromatogr., A, 2012, 1267, 259–265 CrossRef CAS PubMed.
- Z. Zhang, R. a. Wu, M. Wu and H. Zou, Electrophoresis, 2010, 31, 1457–1466 CrossRef CAS PubMed.
- J. Zhang, T. Bao and Z. Chen, Anal. Methods, 2012, 4, 4140–4145 RSC.
- Y. Zhu, C. Zhou, S. Qin, Z. Ren, L. Zhang, H. Fu and W. Zhang, Electrophoresis, 2012, 33, 340–347 CrossRef CAS PubMed.
- R. P. Liang, C. M. Liu, X. Y. Meng, J. W. Wang and J. D. Qiu, J. Chromatogr., A, 2012, 1266, 95–102 CrossRef CAS PubMed.
- Q. Qu, C. Gu and X. Hu, Anal. Chem., 2012, 84, 8880–8890 CrossRef CAS PubMed.
- I. Mikšík, K. Lacinová, Z. Zmatlíková, P. Sedláková, V. Král, D. Sýkora, P. Řezanka and V. Kašička, J. Sep. Sci., 2012, 35, 994–1002 CrossRef PubMed.
- J. Krenkova, N. A. Lacher and F. Svec, Anal. Chem., 2010, 82, 8335–8341 CrossRef CAS PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- S. Hong, Y. S. Na, S. Choi, I. T. Song, W. Y. Kim and H. Lee, Adv. Funct. Mater., 2012, 22, 4711–4717 CrossRef CAS.
- J. Ryu, S. H. Ku, H. Lee and C. B. Park, Adv. Funct. Mater., 2010, 20, 2132–2139 CrossRef CAS.
- W. O. Yah, H. Xu, H. Soejima, W. Ma, Y. Lvov and A. Takahara, J. Am. Chem. Soc., 2012, 134, 12134–12137 CrossRef CAS PubMed.
- S. M. Kang, I. You, W. K. Cho, H. K. Shon, T. G. Lee, I. S. Choi, J. M. Karp and H. Lee, Angew. Chem., Int. Ed., 2010, 49, 9401–9404 CrossRef CAS PubMed.
- S. Ryu, Y. Lee, J. W. Hwang, S. Hong, C. Kim, T. G. Park, H. Lee and S. H. Hong, Adv. Mater., 2011, 23, 1971–1975 CrossRef CAS PubMed.
- B. H. Kim, D. H. Lee, J. Y. Kim, D. O. Shin, H. Y. Jeong, S. Hong, J. M. Yun, C. M. Koo, H. Lee and S. O. Kim, Adv. Mater., 2011, 23, 5618–5622 CrossRef CAS PubMed.
- W. Zhang and Z. Chen, J. Chromatogr., A, 2013, 1278, 29–36 CrossRef CAS PubMed.
- W. Zhang, J. Zhang, T. Bao, W. Zhou, J. Meng and Z. Chen, Anal. Chem., 2013, 85, 6846–6854 CrossRef CAS PubMed.
- S. Müller, K. U. Totsche and I. Kögel-Knabner, Eur. J. Soil Sci., 2007, 58, 918–931 CrossRef.
- L. Dattolo, E. L. Keller and G. Carta, J. Chromatogr., A, 2010, 1217, 7573–7578 CrossRef CAS PubMed.
- Q. Qu, C. Gu, Z. Gu, Y. Shen, C. Wang and X. Hu, J. Chromatogr., A, 2013, 1282, 95–101 CrossRef CAS PubMed.
- X. Diao, F. Zhang, B. Yang, X. Liang, Y. Ke and X. Chu, J. Chromatogr., A, 2012, 1267, 127–130 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |