Asymmetric copolymer vesicles to serve as a hemoglobin vector for ischemia therapy†
Bin
Li
ab,
Yanxin
Qi
a,
ShaSha
He
ab,
Yupeng
Wang
a,
Zhigang
Xie
a,
Xiabin
Jing
a and
Yubin
Huang
*a
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: ybhuang@ciac.ac.cn; Tel: +86-431-85262769
bUniversity of Chinese Academy of Sciences, Beijing 100039, P. R. China
First published on 17th June 2014
Abstract
Self-aggregated vesicles have been considered to be promising candidates for hemoglobin-based oxygen carriers. Here, amphiphilic hetero-triblock copolymers are designed and synthesized with the capacity to self-assemble into polymer vesicles (polymersomes). Conceivably, vesicles are formed with asymmetric membranes, which achieve enhanced encapsulation efficiency of hemoglobin (Hb) beyond the diblock counterpart. Furthermore, hemoglobin-loaded vesicles (HbV) are fabricated with high Hb content and submicron particle sizes. The gas-binding capability, oxygen affinity and methemoglobin (metHb) level of the HbV dispersions are all comparable to the natural erythrocytes. In vitro HbV stability studies further reveal that the encapsulation of Hb within vesicles can greatly avoid the existence of free Hb and shows no interference with cells, especially for blood components. To evaluate the efficacy on ischemia reperfusion, HbV suspended in a plasma expander is transfused as a resuscitation fluid into an acute anemia rat model. Results demonstrate that the combined infusion of the plasma expander with HbV effectively ameliorates the lethal shock symptom and reduces short-term mortality. Concurrently, rats transfused with HbV are void of the acute tubular necrosis caused by the filtration of dissociated Hb dimers from glomeruli. We envision that the oxygen carriers derived from polymer self-assembly technology will become an alternative strategy for future development of blood substitutes.
Introduction
Oxygen therapeutics (often termed “blood substitutes”) refers to alternatives to donated blood for transfusion. Since the HIV epidemic in the 1980s, chemically modified Hb was developed due to the simple synthesis and scalable production, and boomed in the 1990s; over ten products entered different stages of clinical trials, some of which have been approved for veterinary use in North America and Europe, and routine clinical uses are licensed in South Africa and Russia.1–3 However, further clinical studies reveal controversial adverse effects when hemoglobin-based oxygen carriers (HBOCs) are transfused into subjects.4,5 In 2009, a multicenter prehospital resuscitation trial revealed that initial resuscitation of HBOCs could significantly prolong the survival time of patients before access to stored RBCs compared with regular treatment with saline, yet accompanied by an increased risk of nonfatal myocardial infarction. Therefore, presently, HBOCs could not be used interchangeably with RBCs but would be used when the likelihood of dying without oxygen-carrying replacement is so great that the potential life-sustaining benefit would exceed any potential risks.6 Biochemical and physiological investigations into the mechanisms underlying the toxicity of HBOCs are ongoing, and will help to correlate the observed adverse effects to specific HBOC structural and functional properties.2,7 However, whether chemical modification may offer solutions to all the associated side effects is still doubtful. These considerations might lead to significant changes in the field, with a re-evaluation of the strategy for future clinical trials and the development of novel approaches and products.8–11Therefore, encapsulated Hb, both in the sections of conventional and innovative academic investigation, has been burgeoning in recent years, and numerous micro–nano systems have been explored for their potential application as oxygen carriers, such as liposomes,12,13 nanocapsules,14 microspheres,15 hydrogel nanoparticles,16–18 and porous particles,19 as well as the coating of the nanoparticle surface with Hb.20,21 Notably, distinctive from chemically modified Hb, Hb in particle-form does not possess a colloid osmotic pressure (COP), and must be suspended in a plasma expander for transfusion.
In recent years, the development of polymer synthesis and self-assembly technology has provided opportunities for the design of remarkably precise and versatile systems at the micro and nano levels for biomedical application.22,23 Due to their superior performance to liposomes, such as adjustable membranes, enhanced mechanical strength, reduced chemical permeability and easily realizable stimuli-responsiveness, polymersomes have emerged as excellent alternatives in areas ranging from enzymatic nanoreactors to drug delivery and in vivo imaging.9 As the analogue of natural erythrocytes, self-aggregated polymer vesicles with an inner cavity show great potential to incorporate Hb. Appropriate molecular design of the polymer architecture may enable the delivery systems to fulfill the performance requirements for HBOCs including the biocompatible surface properties, membrane stability, permeability, biodegradability, etc. With these thoughts in mind, we designed and synthesized a hetero-triblock copolymer with hydrophilic polyethylene glycol (PEG) chains and hydrophobic biodegradable chains (Scheme 1), and investigated the necessary copolymer composition that was required for polymersome formation. The excipient-aided film hydration strategy was established both for the study of the self-assembly behaviors and Hb loading, in consideration of the denaturation of Hb in contact with organic solvents. The hydrophilic segments with different molecular weights segregated to form an asymmetric membrane in the assembled vesicles. A pilot study has shown that the asymmetric polymersomes have entrapped adequate Hb without bioactivity damage, and can perform the O2 transporting capacity on an exchange transfusion into an acute anemia rat model.
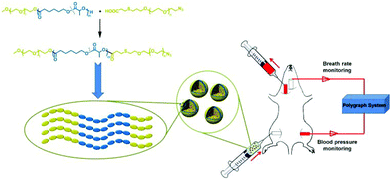 | ||
| Scheme 1 The schematic illustration of material structure, proposed packing in the vesicle membrane and exchange transfusion of hemoglobin-loaded vesicles (HbV) into an acute anemia rat model. | ||
Although polymersomes have been exploited to encapsulate Hb,9,24–26 the realm advanced slowly due to the scarce knowledge of the integration of polymer design, assembly technologies and protein entrapment. This work provides a systematic investigation of the feasibility of Hb-loaded polymeric vesicle as oxygen carriers. By combination with plasma substitutes, a resuscitation fluid is obtained, which could reduce short-term mortality, without the risk of overburdening the metabolic organs when a compatible blood transfusion is undertaken.
Materials and methods
Materials
mPEG-PCL-PEG or mPEG-P(CL-co-LA)-PEG was synthesized by esterification conjugation between mPEG-PCL or mPEG-P(CL-co-LA) with α-carboxy-ω-azide PEG (Scheme 1, experimental details in ESI†). Bovine Hb was purchased from Shanghai Kayon Biological Technology Co. Ltd and stabilized under a CO atmosphere to afford COHb. CO gas (99.95%) was obtained from Dalian Date Gas Co. Ltd. Hydroxyethyl starch (6% HES 130/0.4) was supplied by Medisan Pharmaceutical Co. Ltd (Harbin, China). Other solvents were of analytical grade and were used without further purification.Preparation of polymer vesicles (PV) and hemoglobin-loaded vesicles (HbV)
The polymersomes were prepared via a modified film hydration method. Typically, 10 mg of the copolymer and 5 mg of mPEG550 were co-dissolved in 5 mL of CHCl3. Afterwards, the solution was slowly evaporated on a rotary evaporator for 2 h at 60 °C to form a thin film. Then, 20 mL of ultrapure water was added to the dry film, followed by agitation at 60 °C for 10 h.Hb was loaded into polymersomes during the film hydration process. Typically, 1 g of the copolymer and 20 mg of mPEG550 were co-dissolved in 50 mL of CHCl3. Afterwards, the solution was slowly evaporated on a rotary evaporator for 2 h at 60 °C to form a thin film. Then, 200 mL of COHb solution (20 mM phosphate, pH 7.4) was added to the dry film, followed by agitation at 60 °C for 2 h and 30 °C for another 24 h. The obtained HbV suspension was subjected to centrifugation (1000 rpm × 10 min) to remove the macroscopic aggregates, and then collected by ultracentrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm × 10 min) with further washing with PB to remove the un-encapsulated Hb. The resulting HbV was re-dispersed in plasma substitute solutions (6% HES 130/0.4), deoxidized with ascorbic acid, and purged with CO for 1 h. Finally the vial was sealed and stored at 4 °C for future use. Hb encapsulation efficiency and Hb loading content were measured as described in our previous work.27
000 rpm × 10 min) with further washing with PB to remove the un-encapsulated Hb. The resulting HbV was re-dispersed in plasma substitute solutions (6% HES 130/0.4), deoxidized with ascorbic acid, and purged with CO for 1 h. Finally the vial was sealed and stored at 4 °C for future use. Hb encapsulation efficiency and Hb loading content were measured as described in our previous work.27
Characterization of PV and HbV
Transmission electron microscopy (TEM) measurements were performed on a JEOL JEM-1011 electron microscope operating at an acceleration voltage of 100 kV. Confocal Laser Scanning Microscopy (CLSM) images of RhB-conjugated polymersomes were collected with a Leica TCS SP2 CLSM system (Leica Microsystems Heidelberg GmbH, Germany) equipped with a 100× oil immersion objective. The samples were excited using a 543 nm He/Ne laser. A droplet of the polymersome suspension was dripped on a glass slide, covered with a coverslip and visualized directly. Inductively coupled plasma mass spectrometry (ICP-MS, Xseries II, Thermo Scientific, USA) was used for the quantitative determination of iron in heme to calculate the Hb content. The samples were subjected to dialysis against deionized water to remove the salt before measurement.1H NMR measurement was conducted for the qualitative determination of surface composition of polymersomes. The copolymer solid was directly dissolved in CDCl3 to obtain the full spectrum of protons assigned to all the block segments. For the measurement of the bulk copolymer in D2O, the sample was re-prepared as follows to avoid the formation of assemblies during the dialysis process: the synthesized copolymer solid after lyophilization was redissolved in CHCl3, precipitated into an excess of cold diethyl ether, collected and dried under vacuum. On the other hand, lyophilized polymersome solids were directly re-dispersed in D2O and subjected to 1H NMR measurement for the characterization of composition change of the copolymers after aqueous assembly.
Gas-binding capacity, oxygen affinity and metHb level
The gas-binding capacity of Hb at different atmospheres could be monitored by UV-vis spectrophotometry. Hb or HbV solution (∼0.3 g L−1) in an airproof cuvette was subjected to different gases (CO, N2 or air). COHb was converted to oxyHb by exposing the solution to visible light under an O2 atmosphere, while the deoxyHb state was obtained by exposing the solution to visible light under an N2 atmosphere with the addition of a trace amount of Na2S2O4.The O2 binding and release ability of Hb at different oxygen partial pressures was evaluated via oxygen half-saturation pressure (P50) and cooperativity (Hill coefficient). They could be obtained using an oxygen dissociation curve, which reflected the relationship between O2 partial pressure (P) and O2 saturation (Y) of oxygen carriers. To determine the value of P and Y, typically, the Hb solution was first converted to the complete deoxyHb state, and then different volumes of air were introduced into the airproof cuvette through a syringe. The O2 saturation (Y) was calculated using the following equations by monitoring the UV absorption of the solution, and P was determined from the introduced oxygen in air. The plot of Y vs. P was further fitted as a sigmoidal curve by a Boltzmann function, and P50 was determined as the oxygen partial pressures when the O2 saturation reached 0.5 in the sigmoidal curve. Furthermore, linear fitting of a Hill plot (Log (Y/(1 − Y)) versus Log P) was performed, and the Hill coefficient was obtained as the slope of the line. The sample solution was diluted with physiological saline solution (0.2 M phosphate, 0.9% NaCl, pH 7.4), and the test conditions were maintained the same for all the samples.
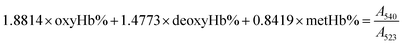 | (1) |
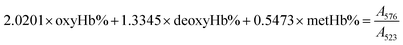 | (2) |
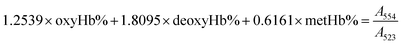 | (3) |
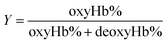 | (4) |
Within the equations, oxyHb%, deoxyHb% and metHb% represent the calculated percentage of oxyHb, deoxyHb and metHb respectively, and A523, A540, A554 and A576 stand for the absorbance of the solution at 523 nm, 540 nm, 554 nm and 576 nm respectively. As for the HbV solution, the real absorbance value was obtained by deducting the absorbance of blank polymersomes due to the baseline drift caused by the scattering effects of the vesicles.
The percentage of metHb was assayed via UV-vis spectroscopy using the cyanomethemoglobin method described elsewhere.28
In vitro hemoglobin leakage study of HbV
The HbV particles re-suspended in 20 mL of hydroxyethyl starch solutions (6% HES 130/0.4, 0.9% NaCl) were incubated at 37 °C under constant shaking at 100 rpm. At predetermined time intervals, the suspension was centrifuged (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm × 5 min). 2 mL of the supernatant was withdrawn, and replaced by isometric fresh media. The amount of released Hb in the supernatant was assayed via absorbance at 405 nm in the UV-vis spectrum using the established calibration curve. The total amount of encapsulated Hb was determined by quantification of ferrum in the HbV lyophilized powder via ICP-MS. And the percentage of cumulative Hb released with respect to the amount of Hb encapsulated was investigated as a function of incubation time.
000 rpm × 5 min). 2 mL of the supernatant was withdrawn, and replaced by isometric fresh media. The amount of released Hb in the supernatant was assayed via absorbance at 405 nm in the UV-vis spectrum using the established calibration curve. The total amount of encapsulated Hb was determined by quantification of ferrum in the HbV lyophilized powder via ICP-MS. And the percentage of cumulative Hb released with respect to the amount of Hb encapsulated was investigated as a function of incubation time.
Hemocompatibility of HbV in vitro
Fresh whole blood obtained from Wistar rats was stored in heparinized Eppendorf tubes, and mixed with an equal volume of HbV suspended in HES solutions. The mixture was incubated immediately at 37 °C in a water bath incubator with constant shaking. After 3 hours, one drop of the incubated sample was observed microscopically using a Nikon Eclipse Ti inverted microscope, and the blood cell counting was performed using an ABX Micros 60 counter (ABX Diagnostics, Montpellier, France). Following the same experimental protocol, HES solution and ultrapure water were also tested as the positive- or negative-control groups. All of the experiments were carried out in triplicate, and the counted cell number was shown as mean ± SD of independent groups.The resuscitation effect on an exchange transfusion of HbV into an acute anemia rat model
The animals were sacrificed after the experiments by acute bleeding from the common carotid artery. All of the study protocol was performed according to the Guidelines of the Committee on Animal Use and Care of Chinese Academy of Sciences.
Results and discussion
The polymeric formulation
Presumably, vesicles that had distinct interior environments separated from the outside by an asymmetric membrane were our first step towards the superfine mimicry of erythrocytes. Therefore, an ABA-type hetero-triblock copolymer structure was constituted by linking a hydrophobic PCL segment with two PEG segments of different molecular weights. It was assumed that when this type of copolymers formed vesicles, longer PEG chains segregated to the outer surface while shorter PEG chains were more liable to be located on the inner surface of the vesicles.22,23,30 A series of mPEG-PCL-PEG copolymers were synthesized by combination of anionic polymerization, ring-opening polymerization and esterification coupling (Scheme S1 and Fig. S1†). By varying the molecular weights of two coupled polymers in feed, triblock copolymers with the hydrophilic block fraction (wt%) spanning from 20% to 50% were obtained for further study (Table S1†).Complicated by the relationship of the self-assembled morphology with the hydrophilic–hydrophobic ratio of polymers, self-assembly behaviors were investigated ahead of Hb loading. In the film-rehydration process, both heating and mPEG were essential ingredients to form dispersible nano-aggregates, especially for vesicle-forming copolymers with a longer hydrophobic block. Vesicles could form with the hydrophilic fraction (wt%) in the range of 35% to 39% (Fig. 1A and B and Fig. S2†). The vesicle “region” was also applicable when LA units were inserted into the hydrophobic segment by random copolymerization of ε-CL and L-LA, and the addition of mPEGX with different molecular weights did not alter the vesicle morphology (Fig. 1C and Fig. S3†), indicating that the hydrosoluble excipient did not participate in membrane packing. The hybrid composition of the hydrophobic block showed superiority to the homo counterpart for the uniform distribution of vesicle diameters (Fig. 1). And the amorphous freezing state of P(CL-co-LA) could form a fluid and flexible membrane more favorable for flowing through the vasculature and post-processing of vesicles such as the reduction of diameters (determined using DSC curves in Fig. S1D†). Conclusively, the triblock copolymer PEG5K-P(CL-co-LA)11K-PEG2K was chosen for further characterization and Hb encapsulation.
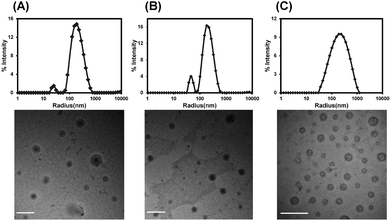 | ||
| Fig. 1 DLS results and representative TEM images of polymersomes formed from mPEG5K-PCL11K-PEG1K (A), mPEG5K-PCL11K-PEG2K (B) and mPEG5K-P(CL-co-LA)11K-PEG2K (C). Scale bars = 500 nm. | ||
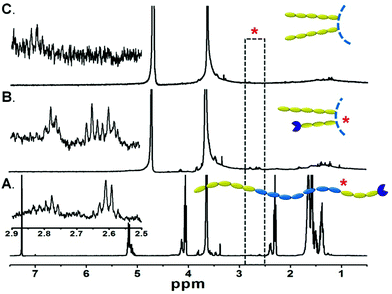
The orientation of the triblock copolymers within the artificial membrane systems was investigated by 1H NMR. First, the copolymer was solvated into single chains in CDCl3 and displayed the signals assigned to protons of all the hydrophilic and hydrophobic segments (Fig. 2A). When the bulk copolymer was dispersed in D2O (Fig. 2B), the persistence of signals at ∼3.65 ppm attributed to the backbone ether protons of PEG and the disappearance of characteristic peaks of the P(CL-co-LA) verified the amphiphilic nature of the copolymer. Furthermore, the resonances at ∼2.60 ppm attributed to methylene adjacent to sulfur and ∼2.80 ppm of the methylene adjacent to the carbonyl group implied that the relatively short PEG chains with functional groups also stretched towards the aqueous media. However, these characteristic signals completely disappeared when the freeze-dried polymersome was re-dispersed in D2O (Fig. 2C), in coincidence with our hypothesis that the short PEG segment was isolated in the membrane and formed the inner hydrophilic layer of the vesicles. It is noteworthy that the reactive terminal groups of the copolymers were reserved for further functionalization of the vesicles. For example, rhodamine B (RhB) could be conjugated with the azido-terminated copolymers via click chemistry, which made the vesicles detectable by CLSM observation (Fig. S4†).
The segregation of hydrophilic segments in the membranes avoided the stretching of long-chain PEG towards the internal cavity, leading to increased volume for Hb loading, as corroborated by the encapsulation efficiency improvement compared with the diblock precursor (Fig. 3A) in the same feeding ratio. Further optimization of the polymer/Hb weight ratio in feed could yield a formulation with Hb that comprised above 60% weight of the dehydrated HbV product (Fig. 3B). Although lower than the human erythrocytes (∼90%), it was equal to the liposome-based HbV (∼65.5%) reported by Sakai et al.10,12
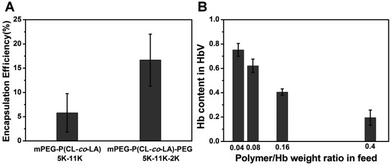 | ||
| Fig. 3 Effect of copolymer structures on Hb encapsulation efficiency (A). Effect of the polymer/Hb weight ratio in feed on Hb loading efficiency in mPEG5K-P(CL-co-LA)11K-PEG2K vesicles (B). | ||
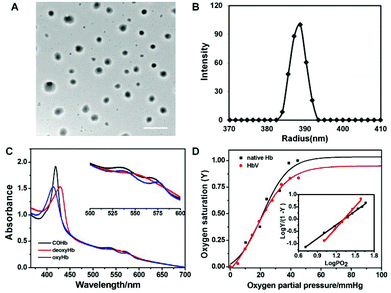
Characterization of HbV
HbV particles could be collected with very narrow size distributions by gradient centrifugation (809.3 ± 57.2 nm), and the spherical morphology was well preserved after encapsulation as observed by TEM (Fig. 4A). The stretching of long-chain hydrophilic segments eliminated the occurrence of multi-lamellar vesicles frequently encountered during liposome formation, and avoided the adverse effect toward Hb bioactivity during the destructive processing such as freeze–thawing cycles.Hb had a tetrameric structure made of two α and β globin chains with each unit containing a prosthetic moiety constituted of an iron atom conjugated to a porphyrinic ring (heme), capable of complexing different gas ligands under different atmospheres (Scheme S2†). The various Hb derivatives showed different absorption spectra when scanned in the range of the UV-vis region, and hence the gas-binding capacity of HbV could be monitored via UV-vis spectrophotometry to check whether the biological activity of Hb has been affected by the encapsulation process. As shown in Fig. 4C, the different states of encapsulated Hb could be converted freely along with the change of the maximum absorbance peaks. Furthermore, the evolution of the spectrum from deoxyHb to oxyHb was also acquired when the gas atmosphere of HbV dispersion changed from N2 to air in the airproof cuvette (Fig. S5†).
The quantitative characterization of the oxygen-carrying capacity of Hb was evaluated via an oxygen dissociation curve. P50 was defined as PO2 (oxygen partial pressure) when oxygen saturation (Y) reached 50% in the oxygen dissociation curve, and the Hill coefficient was the slope of the Hill plot, which represented the cooperative extent of the four subunits in Hb molecules. Y was calculated from eqn (1)–(4) by monitoring the UV absorption of the solution, and P was determined from the introduced oxygen in air. Fig. 4D shows the oxygen dissociation curves of native Hb and encapsulated Hb, and it was obvious that the plots were all fitted as sigmoidal curves. HbV displayed the oxygen-carrying capacity (P50 and Hill coefficient values were 21.6 mmHg and 2.89, respectively) close to that of native Hb (22 mmHg and 2.3). It was demonstrated that the cooperative binding of O2 to Hb was not compromised by encapsulation inside the aqueous cavity of the vesicles.
Without the protection of antioxidant enzymatic systems inside the RBCs, Hb was slowly oxidized to metHb. MetHb was unable to deliver oxygen to tissues because it could not form reversible complexes with molecular oxygen. Therefore the metHb level needed to be controlled when HBOCs were fabricated. As reported in the literature, the tolerable level of metHb was about 10–15% depending on different experimental animal models.31,32 Using the characteristic absorption peak at 630 nm, the metHb level of the HbV dispersion could be calculated and tracked during the preparation process. The metHb level of the stock COHb solution was 2.75%, and the value increased to 15.8% during the encapsulation procedure under the CO atmosphere. A drastic rise to 45.9% happened when the dispersion was subjected to post-processing although it was conducted at 4 °C. The reduction of metHb to Hb was realized through the post-addition of a chemical reductant. An obvious drop of metHb content (to 5.5%) was observed when L-ascorbic acid of a suitable molar ratio to Hb was added. Taken together, the metHb content of the HbV could be maintained at a low level by the combination of CO protection and reductant treatment.
Hb leakage and biocompatibility of HbV in vitro
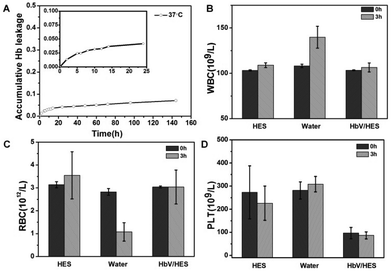
With Hb entrapped in the cavity of polymersomes, HbV circulated as a biomimetic erythrocyte when injected in vivo. Both the HbV stability and compatibility with cells, especially for blood cells, were prerequisites before transfusion. The Hb leakage studies in vitro revealed that 7% of the loaded Hb was leaked out during the time span of a week with release of 3.2% in the first 10 hours (Fig. 5A). Albeit with potential renal toxicity caused by free Hb, the trace level of Hb was tolerable for the existence of a natural protein in blood plasma with a high affinity to bind Hb, haptoglobin (Hp), to eliminate Hb escaped from erythrocytes.33 Therefore, aimed at serving as a synergistic agent for a plasma expander, the addition of HbV ingredients could endow an oxygen-delivery capability to the plasma substitute solutions, and circumvent the potential risk of overburdening the metabolic organs.Cytotoxicity of the fabricated HbV was evaluated against L929 cells in vitro by the MTT assay (Fig. S6†). The survival rates of L929 co-incubated with HbV at all tested concentrations up to 1 mg mL−1 all exceeded 90%, indicating low toxicity and good cytocompatibility of the HbV. Furthermore, the hemocompatibility of HbV was performed by incubation with isovolumetric heparinized blood in vitro and the succeeding blood cell counting of the mixture. Due to the altered environments in vitro for survival of blood cells, HES served as the positive control group for the generally proved biocompatibility as a plasma substitute. And as the negative control group, ultrapure water was mixed with blood to induce hypotonic hemolysis. As shown in Fig. 5, fluctuation of cell count happened in the HES group, and dramatic cell abnormalities of the water group indicated the hemolysis caused by the reduced osmotic pressure. Compared with HES, the contact of blood with HbV did not induce further cell damage. Microscopic observation of the morphology changes of the RBC (Fig. S7†) confirmed that no occurrences of deformation and aggregation of RBC were caused for the HES and HbV/HES groups, while cytopenia and cell fragments were induced after 3 hours of incubation of blood with ultrapure water. All these results revealed that vesicles formed from PEGylated copolymers provided the HbV with stealth behavior, and showed no interactions with blood components when infused into blood.
The resuscitation effect of HbV on an exchange transfusion into an acute anemia rat model
HES was widely used as a resuscitation fluid for volume expansion during severe blood loss caused by trauma, surgery or other emergency situations. However, without the function performed by blood cells, they showed limited effectiveness on patients suffering from severe ischemia or acute fatal hemorrhagic shock.34–36 As a biomimetic erythrocyte, HbV retained the O2-delivering function of Hb, and meanwhile avoided the side effects caused by stroma free Hb. Exploratively, the efficacy of low-dose HbV (10 g L−1) as the synergistic ingredients of HES was investigated to alleviate the organ hypoxia and prolong the survival time during hypovolemia in an acute anemia model.Acute hemorrhagic shock was induced by first normovolaemic hemodilution with HES and subsequent blood withdrawal. The blood loss in the severe hemodilution state was more sensitive to fluid resuscitation, and the acute toxicity of different fluids on ischemia reperfusion could be evaluated by analysis of the survival time and organ injury. As shown in Fig. 6, the physiological response of different groups showed a similar trend during the hemodilution and bleeding stages. Hematocrit (Hct) dramatically reduced to 1/3 of the initial value due to the dilution of blood with HES, and was maintained constant before the sample injection. The mean arterial pressure (MAP) showed a steady decline by hemodilution, and was further lowered to 39–42% of the basal value with blood loss induced hypovolemia. The breath rate (BR) was slightly increased as a response to compensate for the erythropenia, and decreased following the bleeding operation, indicative of the gradual respiratory failure in the acute fatal hemorrhagic shock state.
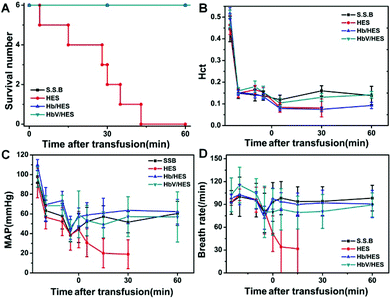 | ||
| Fig. 6 Changes in survived numbers (A), hematocrit (Hct), mean arterial pressure (MAP) and breath rate (BR) (B–D) during extreme hemodilution and exchange transfusion with infusion of HbV/HES with 10 g Hb L−1, Hb/HES with 10 g Hb L−1, shed self-blood (S.S.B.) and HES. The time span along the X-axis was labelled after the sample infusion (0 min), and the points before 0 min indicated the data before the 67% hemodilution, immediately after the hemodilution, 10 min after the hemodilution and after the 30% bleeding, respectively. The lines for S.S.B., Hb/HES and HbV/HES groups in Fig. 6A were overlapped for no morbidity in the monitoring period. | ||
Following the bleeding step, fluid resuscitation was performed to relieve the shock symptom. When heparinized autologous blood was transfused back, both the MAP and HR were recovered to the levels before bleeding, maintained constant during the monitoring period of 1 h (Fig. 6), and the rats even revived from anesthesia by long-term observation (data not shown). The rat groups infused with RBC-free solutions showed a further decline of Hct, and the resuscitation effects were varied depending on the addition of oxygen-supplying ingredients. The rats receiving intravenous infusion of HES showed no recovery from danger, and the physiological signals were further weakened until the disappearance (Fig. 6C and D). Although the administration of HES restored the blood volume, the hypoxia state of organs could not be alleviated without compensation for oxygenation. All the rats died in 43 min with an average survival time of 25.8 ± 14.1 min (Fig. 6A). The injection of Hb- or HbV-dispersed HES significantly controlled the worsening of severe shock, and achieved the therapeutic effect comparable to autologous blood in short-term observation (Fig. 6C and D). All the rats in the Hb/HES and HbV/HES groups survived for over 60 min, suggesting that the systemic oxygen delivery was vital to partly restore the functions of organs and prolong the survival time.
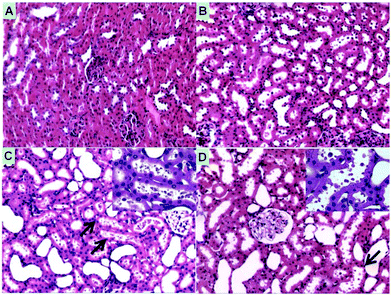
No acute inflammation was induced by the infusion of HbV/HES or Hb/HES groups as indicated by the insignificant leukocytosis relative to the control groups (Fig. S8†). On the other hand, macroscopic levels of hemoglobinuria were observed for rats transfused with Hb/HES solutions (Fig. S9A†), indicative of hemoglobin-induced nephrotoxicity when the plasma concentration exceeded the threshold of Hp-binding capacity and other removal mechanisms.37 The histology analysis of renal injury by light microscopy further implied that severe renal morphological changes occurred in rats injected with Hb/HES solutions (Fig. 7). As shown in Fig. 7C, the filtered hemoglobin from glomeruli caused the necrosis of epithelial cells, exfoliation from the basement membrane and further intraluminal aggregation of necrotic cell debris. As for groups transfused with HbV/HES solutions (Fig. 7D), only a slight swelling of the renal tubule and detachment of epithelial cells occurred, similar to the rats which survived from normovolaemic hemodilution (Fig. 7B), which was probably caused by the acute renal dysfunction during the ischemia induction stage. Collectively, the encapsulation of Hb within the polymeric vesicles maintained the bioactivity of Hb, and meanwhile effectively eliminated the disassociation of stroma free Hb tetramers into dimers and avoided the acute tubular necrosis caused by overburden of the nephrons.
Conclusions
An amphiphilic hetero-triblock copolymer could self-aggregate into dispersible vesicles with hydrophilic segment separation in the asymmetric bilayer membrane. The formed polymer vesicles could successfully entrap Hb within their internal cavity through optimization of the copolymer compositions and polymer/Hb weight ratio in feed. Undamaged gas-binding capability and oxygen affinity, plus high stability and biocompatibility of the particles, guaranteed a superior performance of HbV as biomimetic erythrocytes. In an acute anemia rat model, the combination of HbV with a plasma expander reduced short-term mortality, and meanwhile effectively circumvented hemoglobin-induced nephrotoxicity. Therefore, the fabricated artificial oxygen carriers could enhance the efficacy of plasma substitutes and serve as a “bridge” to preserve O2 transport until conventional RBC transfusion is undertaken.Acknowledgements
The authors would like to acknowledge the financial support from the Ministry of Science and Technology of China (863 Project, no. SS2012AA020603), National Natural Science Foundation of China (no. 51321062 and 21174143), “100 Talents Program” of the Chinese Academy of Sciences (no. KGCX2-YW-802), and Jilin Provincial Science and Technology Department (no. 20100588).Notes and references
- P. W. Buehler and A. I. Alayash, Biochim. Biophys. Acta, 2008, 1784, 1378–1381 CrossRef CAS PubMed.
- L. Ronda, S. Bruno, S. Abbruzzetti, C. Viappiani and S. Bettati, Biochim. Biophys. Acta, Proteins Proteomics, 2008, 1784, 1365–1377 CrossRef CAS PubMed.
- W. Robert M, Adv. Drug Delivery Rev., 2000, 40, 131–142 CrossRef.
- A. Mozzarelli, Biochim. Biophys. Acta, Proteins Proteomics, 2008, 1784, 1363–1364 CrossRef CAS PubMed.
- T. A. Silverman and R. B. Weiskopf, Anesthesiology, 2009, 111, 946–963 CrossRef PubMed.
- E. E. Moore, J. L. Johnson, F. A. Moore and H. B. Moore, Crit. Care Clin., 2009, 25, 325–356 CrossRef CAS PubMed.
- P. W. Buehler and A. I. Alayash, Transfusion, 2004, 44, 1516–1530 CrossRef CAS PubMed.
- J. L. Fridey, Oxygen carriers as alternatives to red cell transfusion, in UpToDate, ed. A. J. Silvergleid and J. S. Tirnauer, UpToDate, Waltham, MA (accessed November 2011) Search PubMed.
- T. M. S. Chang, Artificial Cells, World Scientific Publishing Co. Pte. Ltd., Singapore, 2007, pp. 93–129 Search PubMed.
- E. Tsuchida, K. Sou, A. Nakagawa, H. Sakai, T. Komatsu and K. Kobayashi, Bioconjugate Chem., 2009, 20, 1419–1440 CrossRef CAS PubMed.
- P. E. Graves, D. P. Henderson, M. J. Horstman, B. J. Solomon and J. S. Olson, Biochim. Biophys. Acta, Proteins Proteomics, 2008, 1784, 1471–1479 CrossRef CAS PubMed.
- H. Sakai, K. Hamada, S. Takeoka, H. Nishide and E. Tsuchida, Biotechnol. Prog., 1996, 12, 119–125 CrossRef CAS PubMed.
- H. Sakai, H. Horinouchi, E. Tsuchida and K. Kobayashi, in Chemistry and Biochemistry of Oxygen Therapeutics, John Wiley & Sons, Ltd, 2011, pp. 381–390 Search PubMed.
- T. Chang and W. Yu, US Pat., 5670173, 1997 Search PubMed; T. Chang, W. Yu and D. Powanda, US Pat., 7498045 B2, 2009 Search PubMed.
- F. T. Meng, G. H. Ma, Y. D. Liu, W. Qiu and Z. G. Su, Colloids Surf., B, 2004, 33, 177–183 CrossRef CAS PubMed.
- J. N. Patton and A. F. Palmer, Biomacromolecules, 2005, 6, 2204–2212 CrossRef CAS PubMed.
- J. N. Patton and A. F. Palmer, Biomacromolecules, 2005, 6, 414–424 CrossRef CAS PubMed.
- J. N. Patton and A. F. Palmer, Langmuir, 2006, 22, 2212–2221 CrossRef CAS PubMed.
- J. Zhao, C.-S. Liu, Y. Yuan, X.-Y. Tao, X.-Q. Shan, Y. Sheng and F. Wu, Biomaterials, 2007, 28, 1414–1422 CrossRef CAS PubMed.
- Q. Shi, Y. Huang, X. Chen, M. Wu, J. Sun and X. Jing, Biomaterials, 2009, 30, 5077–5085 CrossRef CAS PubMed.
- C. Chauvierre, M. C. Marden, C. Vauthier, D. Labarre, P. Couvreur and L. Leclerc, Biomaterials, 2004, 25, 3081–3086 CrossRef CAS PubMed.
- X. Q. Yang, J. J. Grailer, I. J. Rowland, A. Javadi, S. A. Hurley, V. Z. Matson, D. A. Steeber and S. Q. Gong, ACS Nano, 2010, 4, 6805–6817 CrossRef CAS PubMed.
- A. Blanazs, M. Massignani, G. Battaglia, S. P. Armes and A. J. Ryan, Adv. Funct. Mater., 2009, 19, 2906–2914 CrossRef CAS.
- S. Rameez, H. Alosta and A. F. Palmer, Bioconjugate Chem., 2008, 19, 1025–1032 CrossRef CAS PubMed.
- D. R. Arifin and A. F. Palmer, Biomacromolecules, 2005, 6, 2172–2181 CrossRef CAS PubMed.
- S. Rameez, U. Banerjee, J. Fontes, A. Roth and A. F. Palmer, Macromolecules, 2012, 45, 2385–2389 CrossRef CAS PubMed.
- B. Li, G. Chen, F. B. Meng, T. H. Li, J. Yue, X. B. Jing and Y. B. Huang, Polym. Chem., 2012, 3, 2421–2429 RSC.
- E. J. van Kampen and W. G. Zijlstra, in Advances in Clinical Chemistry, ed. A. L. Latner and K. S. Morton, Elsevier, 1983, vol. 23, pp. 199–257 Search PubMed.
- Y. Huang, T. Komatsu, H. Yamamoto, H. Horinouchi, K. Kobayashi and E. Tsuchida, Biomaterials, 2006, 27, 4477–4483 CrossRef CAS PubMed.
- O. Terreau, L. Luo and A. Eisenberg, Langmuir, 2003, 19, 5601–5607 CrossRef CAS.
- X. Y. He, B. Guan, X. W. Zhang, K. Xu, Y. H. Yu and Q. Liu, Artif. Cells Blood Substit. Immobil. Biotechnol., 2007, 35, 490–506 CrossRef CAS PubMed.
- R. Linberg, C. D. Conover, K. L. Shum and R. G. L. Shorr, Artif. Cells Blood Substit. Immobil. Biotechnol., 1998, 26, 133–148 CrossRef CAS.
- A. I. Alayash, Clin. Chim. Acta, 2011, 412, 493–498 CrossRef CAS PubMed.
- R. J. Bosman, J. Minten, H. R. Lu, H. V. Aken and W. Flameng, Anesth. Analg., 1992, 75, 811–817 CAS.
- E.-P. Horn, T. Standl, S. Wilhelm, E. E. Jacobs, U. Freitag, M. Freitag and J. S. a. Esch, Surgery, 1997, 121, 411–418 CrossRef CAS.
- M. Takaori and A. Fukui, Artif. Cells Blood Substit. Immobil. Biotechnol., 1996, 24, 643–653 CrossRef CAS.
- M. Feola, J. Simoni, R. Tran and P. C. Canizaro, Biomater. Artif. Cells Artif. Organs, 1990, 18, 233–249 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4bm00123k |
| This journal is © The Royal Society of Chemistry 2014 |