Stereoselective geminal difunctionalization of vinyl arenes mediated by the bromonium ion†
Pandur Venkatesan Balaji and
Srinivasan Chandrasekaran*
Department of Organic Chemistry, Indian Institute of Science, Bangalore-560012, India. E-mail: scn@orgchem.iisc.ernet.in; Fax: +91 80 2360 2423; Tel: +91 80 2293 2404
First published on 24th October 2013
Abstract
An anti-Markovnikov geminal oxyamination of styrenyl alkenes in an intermolecular fashion using the umpolung strategy mediated by the bromonium ion is reported. Isotope labeling studies confirm the migration of the phenyl group in the semipinacol rearrangement.
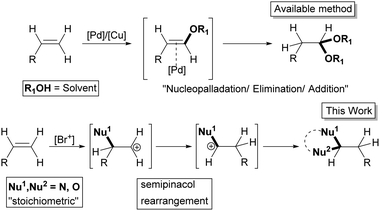
Difunctionalization of alkenes is a preeminent strategy used in organic synthesis which has been extensively studied and widely utilized as a transformative tool and has profound utiltity in various branches of chemistry.1 The much studied 1,2-difunctionalization (vicinal) of alkenes involves a simple addition across the double bond.2–7 Whereas the geminal difunctionalization of alkenes is an intricate process, where the single sp2 carbon of the C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond gets difunctionalized. The addition of two nucleophiles to the electron rich alkene moiety, especially in a geminal fashion, still remains challenging. Most of the reported methods utilize the modified Wacker process8 and are limited to simple oxidation9,10 and use of tethered nucleophiles to form heterocycles in an intramolecular fashion.11 No promising reagent system other than the Wacker type is available yet.
C bond gets difunctionalized. The addition of two nucleophiles to the electron rich alkene moiety, especially in a geminal fashion, still remains challenging. Most of the reported methods utilize the modified Wacker process8 and are limited to simple oxidation9,10 and use of tethered nucleophiles to form heterocycles in an intramolecular fashion.11 No promising reagent system other than the Wacker type is available yet.
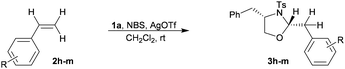
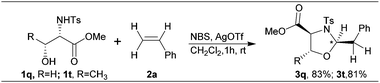
The methods that are available for the geminal difunctionalization of alkenes in an intermolecular process using stoichiometric nucleophiles are very scarce. Especially the intermolecular geminal oxyamination of alkenes still remains obscure and to the best of our knowledge it has not yet been reported under non-Wacker conditions.12 In this context, we report the first non-Wacker method for the intermolecular geminal oxyamination of alkenes using the umpolung strategy mediated by the bromonium ion through a semipinacol rearrangement (Scheme 1).
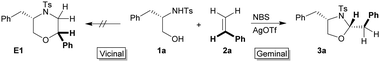
We originally intended to add 1,2-dinucleophiles (such as 1,2-aminoalcohol or 1,2-diol) across the double bond of alkenes to get 1,4-heterocycles by an annulation process. To examine the feasibility of this addition, we first attempted to add N-Ts-phenylalaninol 1a to styrene 2a using NBS as the electrophilic bromine source and there was no observable product formed.
Then we sought to increase the electrophilicity of the bromonium ion to facilitate this addition. After some optimization, we found that in the presence of NBS, which is activated by AgOTf, N-Ts-phenylalaninol 1a and styrene 2a effectively reacted to provide a single product within 1 h in a very good yield (88%).13 We were thrilled to find that the product formed was a 5-membered oxazolidine 3a (Scheme 2) with excellent diastereoselectivity, formed by the addition of the nitrogen and oxygen atoms to the same carbon rather than the expected morpholine derivative E1 which comes from vicinal addition.
This method allows the straightforward stereoselective umpolung installation of two new heteroatoms into a single carbon of an alkene in a domino process. Attracted by the novelty and the potential of this addition process, further studies were taken up to understand the scope and limitation of this protocol.
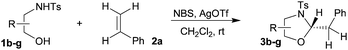
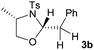
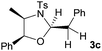
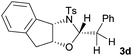
First the effect of substituents in the aminoalcohols on the stereoselectivity of addition was studied and the results are summarized in Table 1. The N-Ts-aminoalcohol 1b, the norephedrine derived 1c and the bicyclic 2-N-Ts-indanol 1d provided the oxazolidines 3b, 3c and a tricyclic, linearly fused oxazolidine 3d, respectively, as the sole product with excellent diastereoselectivity at the newly formed endocyclic stereogenic center (entries 1–3, Table 1). The X-ray diffraction analysis of the product 3b provided a robust proof for the oxazolidine structure assigned.14
| Entry | Amino alcohol | Product | Yieldc (%) |
|---|---|---|---|
a Conditions: 1 equiv. (0.2 mmol) of amino alcohol, 1.2 equiv. of 2a, 1.2 equiv. of NBS, 1.4 equiv. of AgOTf, CH2Cl2, rt, 60 min.b 1f and 1g: rt, 30 min.c Isolated yields.d Ratio of 3ea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3eb is 2 3eb is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.e d.r = 1 1.e d.r = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7.f d.r = 1 1.7.f d.r = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. 1.5. |
|||
| 1 | 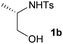 |
 |
75 |
| 2 | 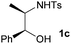 |
 |
72 |
| 3 | 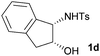 |
 |
79 |
| 4 | 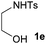 |
 |
65d |
| 5 | 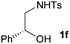 |
 |
74b,e |
| 6 | 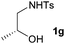 |
 |
65b,f |
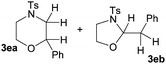
Interestingly, when the simple unsubstituted N-Ts-aminoalcohol 1e was treated with styrene (NBS, AgOTf, CH2Cl2, rt) [entry 4, Table 1] it yielded a mixture of two different heterocycles, 6-membered morpholine 3ea and 5-membered oxazolidine 3eb in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, which are formed by the vicinal and geminal addition respectively.
1 ratio, which are formed by the vicinal and geminal addition respectively.
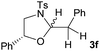
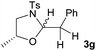
However, when the 1-substituted N-Ts-aminoalcohols 1f and 1g were reacted with styrene under the reaction conditions the corresponding oxazolidines were obtained in good yields with moderate diastereoselectivity (d.r. = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 for 3f; d.r. = 1
1.7 for 3f; d.r. = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 for 3g). This decrease in the diastereoselectivity can be attributed to the lack of geminal substituents at the annulating nucleophile.
1.5 for 3g). This decrease in the diastereoselectivity can be attributed to the lack of geminal substituents at the annulating nucleophile.
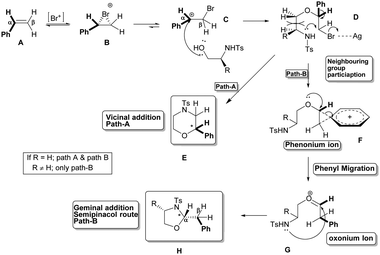
In light of the above observations, we propose a mechanism as depicted in Scheme 3 for this stereoselective intermolecular geminal difunctionalization of styrenes. Initially the NBS activated by AgOTf delivers an electrophilic bromonium ion, which gets added to the electron rich π-bond of vinyl benzene A (styrene) to form a bridged bromonium ion B. This gets equilibrated to the more stable open benzylic carbocation C. The hydroxyl group of the nucleophile adds intermolecularly to this carbocation C in a rate determining step to give a bromobenzylether intermediate D. The bromogroup of ether D gets activated by Ag; at this stage the intramolecular reaction can take place via two kinetically controlled pathways. (i) The tethered nitrogen can undergo a 6-exo-tet cyclization (Path-A) to give morpholine E (vicinal addition) and (ii) the phenyl group can render neighbouring group assistance to facilitate bromide ion departure to form a transient phenonium ion F.15 The species F then readily undergoes a semipinacol rearrangement involving the migration of the phenyl group, which is favoured by the formation of the planar oxonium ion G, which finally gets cyclized in a 5-endo-trig fashion to give oxazolidine H. In the reaction of unsubstituted N-Ts-aminoalcohol (e.g.1e) (when R = H) both the pathways become possible, whereas in the case of substituted N-Ts-aminoalcohols like 1a–d (when R ≠ H) path-B dominates due to steric interactions (D, Scheme 3) and forms only the oxazolidine as the product. The mechanism shown above also explains the greater control over the stereoselectivity offered by the geminal substituent of nitrogen than that of oxygen, since the former heteroatom gets added at the cyclization step.
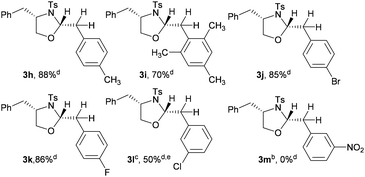
Since we envisaged that this addition process must involve a benzylic carbocationic intermediate (C, Scheme 3) and also a semipinacol rearrangement (F, Scheme 3) by a Whitmore 1,2 shift of the phenyl group, the effect of the substituent in the migrating phenyl group of styrene on the course of the reaction was studied and the results are shown in Table 2.
The p-methyl substituted styrene 2h and 2,4,6-trimethyl substituted styrene 2i gave the corresponding oxazolidines 3h and 3i, respectively, in good yields and high diastereoselectivity as observed for styrene 2a. Interestingly, the substrates with electron withdrawing groups p-bromo 2j and the p-fluoro 2k also gave the products 3j and 3k, respectively, in high yields. In the case of m-Cl-styrene 2l the oxazolidine 3l was obtained along with the uncyclized bromoether (intermediate D, Scheme 3). The highly electron deficient m-NO2 styrene 2m failed to undergo the addition reaction. This relative decrease/absence of reactivity of electron deficient styrenes to undergo oxidative addition-cum-annulation provides additional support for the intermediacy of the benzylic carbocation and the migration of the nucleophile.
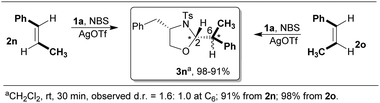
Next, the influence of stereochemistry of olefin on the diastereoselectivity of product formation was examined and the results are depicted in Scheme 4.
Intriguingly, when the diastereomeric alkenes of β-methylstyrene 2n and 2o were subjected to this addition (NBS, AgOTf, CH2Cl2, rt) they were found to furnish oxazolidine 3n as the sole product with the same stereochemistry at both the newly formed endocyclic and exocyclic stereogenic centers. The observed stereoidentity of the products formed strongly implies the convergence of the diastereomeric intermediates formed from the diastereomeric olefins. It is pertinent to speculate that the rotation of the Cα–Cβ bond (intermediate C, Scheme 3) of the open or weakly bound carbocation causes the loss of stereogenicity of the olefin, which eventually causes the loss of stereospecificity through stereoconvergence.
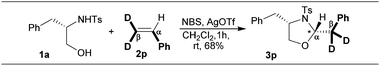
To follow the trajectory of the migration process, the β,β-di-deuterated-styrene 2p was chosen as the olefin for investigation.
This styrene containing two deuteriums and a phenyl group at the vicinal position, under these oxidative addition conditions, got transformed into an oxazolidine 3p containing the exocyclic methylene group constituting two deuteriums and a phenyl group on the same carbon (Scheme 5). This result unequivocally confirms the migration of the phenyl group from the hydrogen attached carbon (Cα, migration origin) to the di-deuterium attached carbon (Cβ, migration terminus) through a semipinacol rearrangement (intermediates D–G, Scheme 3).
The potency and the expediency of this geminal oxidative addition were exemplified in the straightforward synthesis of pseudoprolines 3q and 3t from styrene 2a in a single pot with a very high stereoselectivity (Scheme 6).
In conclusion, the first report on the non-Wacker intermolecular geminal oxyamination of vinyl arenes (styrenes) through a domino process is described. This highly stereoselective oxidative geminal addition is found to involve a semipinacol rearrangement. The diastereomeric alkenes were found to show stereoconvergence in the product formation. The migration of the phenyl group in the semipinacol rearrangement was confirmed by deuterium labeling studies. The efficacy of this method was exemplified in the synthesis of pseudoprolines from styrene.
We thank Mr. Amol G. Dikundwar and Prof. T. N. Guru Row for their help in X-ray crystal structure determination of compounds 3b and 3n. P.V.B. thanks IISc for an Int.PhD Fellowship and CSIR, India, for a CSIR-SRF fellowship. S.C. thanks DST, India, for a JC Bose Fellowship.
Notes and references
- E. Block and A. L. Schwan, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon, Oxford, 1st edn, 1991, vol. 4, p. 329 Search PubMed.
- (a) A. Wang, H. Jiang and H. Chen, J. Am. Chem. Soc., 2009, 131, 3846 CrossRef CAS PubMed; (b) L. Emmanuvel, T. M. A. Shaikh and A. Sudalai, Org. Lett., 2005, 7, 5071 CrossRef CAS PubMed; (c) R. B. Woodward and F. V. Brutcher Jr., J. Am. Chem. Soc., 1958, 80, 209 CrossRef CAS.
- T. J. Donohoe, C. K. A. Callens, A. Flores, A. R. Lacy and A. H. Rathi, Chem.–Eur. J., 2011, 17, 58 CrossRef CAS PubMed.
- (a) K. Muñiz and C. Martínez, J. Org. Chem., 2013, 78, 2168 CrossRef PubMed; (b) F. Cardona and A. Goti, Nat. Chem., 2009, 1, 269 CrossRef CAS PubMed.
- S. D. R. Christie and A. D. Warrington, Synthesis, 2008, 1325 CrossRef CAS PubMed.
- (a) U. Farid and T. Wirth, Angew. Chem., Int. Ed., 2012, 51, 3462 CrossRef CAS PubMed; (b) V. A. Schmidt and E. J. Alexanian, J. Am. Chem. Soc., 2011, 133, 11402 CrossRef CAS PubMed; (c) H. M. Lovick and F. E. Michael, J. Am. Chem. Soc., 2010, 132, 1249 CrossRef CAS PubMed.
- (a) S. E. Denmark, W. E. Kuester and M. T. Burk, Angew. Chem., Int. Ed., 2012, 51, 10938 CrossRef CAS PubMed; (b) L. Zhou, D. W. Tay, J. Chen, G. Y. C. Leung and Y.-Y. Yeung, Chem. Commun., 2013, 49, 4412 RSC.
- J. Tsuji, Synthesis, 1984, 369 CrossRef CAS.
- (a) P. Teo, Z. K. Wickens, G. Dong and R. H. Grubbs, Org. Lett., 2012, 14, 3237 CrossRef CAS PubMed; (b) C. N. Cornell and M. S. Sigman, Org. Lett., 2006, 8, 4117 CrossRef CAS PubMed; (c) J. Chen and C.-M. Che, Angew. Chem., Int. Ed., 2004, 43, 4950 CrossRef CAS PubMed; (d) H. Kikuchi, K. Kogure and M. Toyoda, Chem. Lett., 1984, 341 CrossRef CAS.
- (a) U. Farid, F. Malmedy, R. Claveau, L. Albers and T. Wirth, Angew. Chem., Int. Ed., 2013, 52, 7018 CrossRef CAS PubMed; (b) M. A. Kumar, P. Swamy, M. Naresh, M. M. Reddy, C. N. Rohitha, S. Prabhakar, A. V. S. Sarma, J. R. P. Kumar and N. Narender, Chem. Commun., 2013, 49, 1711 RSC; (c) A. D. Chowdhury and G. K. Lahiri, Chem. Commun., 2012, 48, 3448 RSC; (d) A. M. Balija, K. J. Stowers, J. Schultz and M. S. Sigman, Org. Lett., 2006, 8, 1121 CrossRef CAS PubMed; (e) T. Hosokawa, T. Ohta, S. Kanayama and S.-I. Murahasi, J. Org. Chem., 1987, 52, 1758 CrossRef CAS.
- R. M. McDonald, G. Liu and S. S. Stahl, Chem. Rev., 2011, 111, 2981 CrossRef CAS PubMed.
- (a) A. F. Ward and J. P. Wolfe, Org. Lett., 2011, 13, 4728 CrossRef CAS PubMed; (b) L. D. Elliott, J. W. Wrigglesworth, B. Cox, G. C. Lloyd-Jones and K. I. Booker-Milburn, Org. Lett., 2011, 13, 728 CrossRef CAS PubMed.
- When the reaction was performed with the lesser equivalents (catalytic) of NBS (0.2 equiv.) or AgOTf (0.2 equiv.), the yield of the product was found to decrease proportionally. For optimization data refer to ESI†.
- CCDC 942034 and 942035 contain the supplementary crystallographic data for the compounds 3b and 3n.
- D. J. Cram, J. Am. Chem. Soc., 1952, 84, 2129 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectral data of compounds 1a–1t and 3a–3t. CCDC 942034 and 942035. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc46003g |
| This journal is © The Royal Society of Chemistry 2014 |