Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Colouring crystals with inorganic nanoparticles†
Alexander N.
Kulak
a,
Pengcheng
Yang
b,
Yi-Yeoun
Kim
a,
Steven P.
Armes
b and
Fiona C.
Meldrum
*a
aSchool of Chemistry, University of Leeds, Leeds, LS2 9JT, UK. E-mail: F.Meldrum@leeds.ac.uk
bDainton Building, Department of Chemistry, University of Sheffield, Brook Hill, Sheffield, Yorkshire S3 7HF, UK. E-mail: S.P.Armes@sheffield.ac.uk
First published on 6th November 2013
Abstract
A simple, one-pot method is presented whereby gold nanoparticles coated with a zwitterionic diblock copolymer are incorporated within single crystals of calcite. This may provide a versatile alternative to dyeing crystal with organic molecules and could be extended to create a series of new nanocomposite crystals with novel properties.
That single crystals can be coloured through the occlusion of dye molecules within their lattice has been recognised for centuries.1 Often giving rise to symmetric patterns of colour, the number of dye and crystal combinations which lead to occlusion within an ionic crystal are typically rather limited and often difficult to predict. Indeed, from 962 experiments in which 26 dyes were crossed with 37 ionic crystals, a determined Retgers identified only four mixed crystals which showed significant levels of incorporation.2 The complex process of dye incorporation within crystals is therefore really quite intriguing; in addition to endowing the host crystal with potentially interesting physical and optical properties, it can also provide useful insights into the underlying mechanisms of important phenomena such as crystal growth, habit modification by additives and epitaxy.1
The ability to entrap impurities within single crystals extends well beyond dye molecules. Particulate occlusions are often observed within rocks or ice, where these usually form during a freezing transition.3–6 Biominerals are also invariably composite materials, whereby organic macromolecules can be embedded within single crystals.7–9 In this way, nature engineers materials with poor physical properties such as calcium carbonate to build complex skeletal structures with substantially enhanced fracture toughness.7,10 This strategy can also be adopted by the synthetic materials chemist to occlude small molecules11 and gel fibres12,13 within single crystals of calcite and to encapsulate latex particles within zinc oxide crystals.14,15 We have also recently described how remarkably high volume fractions of latex particles16 and copolymer micelles17 can be occluded within calcite single crystals. Here, successful occlusion critically depends on the particle surface chemistry, leading in the latter case to crystals with mechanical properties that rival those of calcite biominerals.
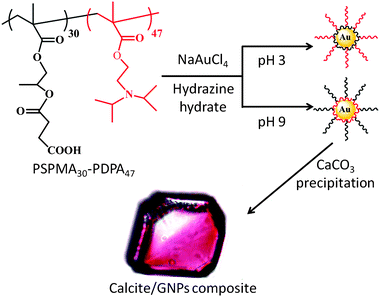
In this paper, we build on these results to develop a novel and potentially quite general strategy for colouring single crystals – based on the incorporation of inorganic nanoparticles, rather than organic molecules (Fig. 1). The occlusion of gold nanoparticles (GNPs) within calcite was identified as a suitable model system, since the gold particles are strongly coloured and they can be readily distinguished from the calcite matrix using electron microscopy. Pure calcite, in turn, is colourless and is known to occlude a wide range of impurities. By tailoring the GNP surface chemistry using an appropriate diblock copolymer,18,19 they can be occluded within the host crystal without perturbing its single crystal structure, thus changing its intrinsic properties and driving the formation of coloured calcite crystals. Design of a suitable diblock copolymer is therefore fundamental to this approach; one block should adsorb onto the gold nanoparticles, while the other block both promotes binding of the nanoparticle to the crystal and ensures colloidal stability of the nanoparticles in the crystal growth solution via electrosteric stabilisation.
A series of pH-responsive, zwitterionic diblock copolymers, PSPMAn–PDPA47 (where n = 16, 30, 50 and 70), were investigated as coatings for the GNPs. This copolymer structure was selected as the tertiary amine methacrylate-based PDPA block was expected to bind strongly to the GNPs at pH 7–9, which is the pH window used for CaCO3 formation, while the carboxylate-functionalised PSPMA block was anticipated to provide both effective electrosteric stabilisation of the nanoparticles in the crystal growth solution and promote their incorporation within the calcite crystal.17 GNPs were synthesised at 20 °C from a solution of PSPMAn–PDPA47 and NaAuCl4, through addition of hydrazine hydrate. During the reduction from Au(III) to Au(0), the transparent, yellow solution transformed into a wine-red suspension of copolymer-stabilised GNPs, while an immediate black precipitate of gold was observed in the absence of any copolymer stabiliser. After reduction, the reaction mixture was stirred overnight, before being dialysed against copious quantities of deionised water.
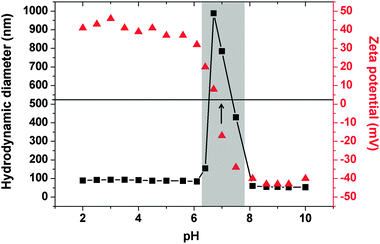
The colloidal stability of the GNPs was strongly dependent on the precise copolymer structure. Thus those sols prepared using PSPMAn–PDPA47 (n = 50, 70) were not stable during dialysis, and sedimented as a grey-purple powder. In contrast, GNPs prepared using PSPMAn–PDPA47 (n = 16 or 30) exhibited remarkable stability, not only during dialysis but also at pH values as low as 1.5 and as high as 12 (pH adjusted using HCl and NaOH, respectively). No precipitation was observed for at least two weeks over this wide pH range. Characterisation of these particles using transmission electron microscopy (TEM) indicated that the gold particles themselves had diameters of ∼10–70 nm, with a mean value of 10–20 nm (Fig. S1, ESI†). Measurement of the sizes of the PSPMA30–PDPA47-stabilised nanoparticles in solution using dynamic light scattering (DLS) indicated somewhat larger diameters due to the steric stabiliser thickness layer (see Fig. 2). Zeta potential measurements indicated that they possessed cationic surface character at low pH (+46 mV at pH 3.0) and weakly anionic surface character at high pH (−43 mV at pH 9.0). Thus the GNPs prepared using these PSPMA–PDPA diblock copolymers exhibit similar properties to the ‘schizophrenic’ copolymer micelles alone (Fig. S2, ESI†).
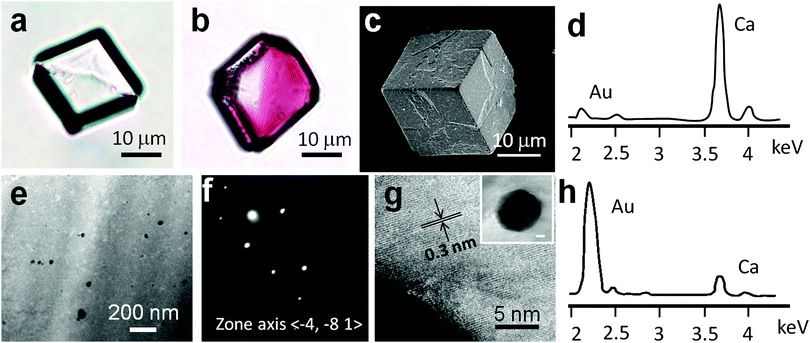
The use of these copolymer-stabilised GNPs to colour calcite crystals was then investigated. GNPs stabilised with either PSPMA16–PDPA47 or PSPMA30–PDPA47 gave very similar results, and thus only data using PSPMA30–PDPA47 copolymer is presented here for simplicity. CaCO3 was precipitated from aqueous CaCl2 solution ([Ca2+] = 3.0 mM) in the presence of 0.30 g dm−3 PSPMA30–PDPA47-stabilised GNPs using the ammonium carbonate diffusion method.20 Crystals were isolated from solution after one day and were rinsed with dilute aqueous HCl to remove any surface-adsorbed GNPs. In contrast to the colourless calcite crystals precipitated in control experiments (Fig. 3a and Fig. S3a, ESI†), the GNP/CaCO3 crystals were visibly pink in colour (Fig. 3b and Fig. S3b, ESI†). Further examination with scanning electron microscopy (SEM) showed that these coloured crystals were primarily rhombohedral calcite with roughened surfaces (Fig. 3c), where the polymorph was confirmed using Raman microscopy and powder XRD (Fig. S4, ESI†). Unfortunately, the gold content within the host crystal was too low for identification using powder XRD. In contrast, gold nanoparticles synthesised in the absence of any copolymer using the standard citrate reduction rapidly aggregated when added to CaCl2 solution.
The quantity and location of GNPs occluded within the calcite crystals was also investigated. Atomic absorption spectroscopy, which was performed after dissolving the hybrid crystals in aqua regia, showed that the CaCO3 crystals occluded ∼3 wt% of gold particles. The fracture surfaces of the crystals were also characterised using SEM after grinding crystals. Compared to pure calcite crystals, which fracture to give smooth surfaces, the cross-sections of the calcite–gold hybrid crystals were rough, providing strong evidence for particle incorporation (Fig. S5, ESI†). This was supported by Energy-Dispersive X-Ray (EDX) analysis, which revealed the presence of gold (Fig. 3d). Finally, the location of the GNPs within the calcite lattice was determined via TEM by examining thin sections, which were prepared using Focussed Ion Beam (FIB) (Fig. 3e). No evidence for aggregation of the GNPs within the crystal was obtained, as was confirmed by the calcite composite crystals being pink rather than blue in colour. These studies also confirmed that the GNPs did not disrupt the crystal lattice, as evidenced by selected area electron diffraction which yielded single crystal type patterns (Fig. 3f). High resolution TEM images also showed continuous lattice fringes (Fig. 3g and Fig. S6, ESI†) while EDX analysis of this sample area confirmed that the occluded particles were indeed gold (Fig. 3h).
The mechanisms by which impurities are incorporated within a crystal vary according to their physical size and chemical structure. The occlusion of (micron- to millimetre-sized) particles during a freezing transition has been widely studied both experimentally and theoretically and depends on many factors, including the thermal diffusivity of the particle and melt, wetting of the crystal face by the particles, the viscosity of the melt, the rate of crystallisation and the particle size.3–6 At much shorter length scales, small molecules are believed to bind stereospecifically to step edges or selectively to specific crystal faces, where they can be subsequently overgrown.21 This mechanism also appears to operate during the occlusion of proteins in biominerals, where synchrotron XRD experiments have revealed a remarkable correlation between defect patterns caused by protein occlusion and the gross morphologies of single crystals.22,23 The copolymer-stabilised gold nanoparticles used here are substantially larger than proteins and their occlusion is determined by the strength of adsorption to the active kink and step sites, where they compete with incoming crystal growth units.6,24 This therefore suggests the ability to tailor the degree of nanoparticle occlusion through appropriate design of the adsorbed copolymer; this hypothesis will be tested in future work.
In conclusion, nanoparticle occlusion can provide an effective route to colouring single crystals. While many crystals (including calcite) change colour when doped with specific impurity ions, a full spectrum of colours is seldom achievable using this approach. Similarly, while some crystals can occlude a wide range of organic dye molecules, others (including calcite) have proven extremely resistant to dyeing.1 Our new strategy, where crystals are precipitated in the presence of copolymer-stabilised nanoparticles as simple growth additives, is expected to be extremely versatile in that a single copolymer could be used to drive the occlusion of a range of nanoparticles – selected for their colours based on size and composition – in the desired host crystal. Finally, it is envisaged that the ability to incorporate inorganic nanoparticles within single crystals will facilitate identification of the fundamental design rules that dictate the incorporation of impurity species within crystals, and provide a novel route to the generation of composite crystals with novel properties.
We thank the EPSRC for financial support via grants EP/G00868X/1 and EP/K006304/1 (AK, FCM and SPA). This work was also supported by an EPSRC Leadership Fellowship (EP/H005374/1; FCM, YYK) and an ERC Advanced Investigator grant (PISA 320372; SPA).
Notes and references
- B. Kahr and R. W. Gurney, Chem. Rev., 2001, 101, 89 CrossRef PubMed.
- J. W. Retgers, Z. Phys. Chem., 1893, 12, 583 Search PubMed.
- R. Asthana and S. N. Tewari, J. Mater. Sci., 1993, 28, 5414 CrossRef CAS.
- A. W. Rempel and M. G. Worster, J. Cryst. Growth, 1999, 205, 427 CrossRef CAS.
- D. Coupard, F. Girot and J. M. Quenisset, J. Mater. Sci., 1996, 31, 5305 CrossRef CAS.
- A. A. Chernov, Springer Series in Solid State Sciences, Springer-Verlag, 1984 Search PubMed.
- H.-Y. Li, H. L. Xin, M. E. Kunitake, E. C. Keene, D. A. Muller and L. A. Estroff, Adv. Funct. Mater., 2011, 21, 2028 CrossRef.
- J. W. C. Dunlop and P. Fratzl, inAnn. Rev. Mater. Res., ed. D. R. Clarke, M. Ruhle and F. Zok, 2010, pp. 1–24 Search PubMed.
- F. C. Meldrum and H. Colfen, Chem. Rev., 2008, 108, 4332 CrossRef CAS PubMed.
- S. Weiner, L. Addadi and H. D. Wagner, Mater. Sci. Eng., C, 2000, 11, 1 CrossRef.
- S. Borukhin, L. Bloch, T. Radlauer, A. R. Hill, A. H. Fitch and B. Pokroy, Adv. Funct. Mater., 2012, 22, 2416 CrossRef.
- H.-Y. Li, H. L. Xin, D. A. Muller and L. A. Estroff, Science, 2009, 326, 1244 CrossRef CAS PubMed.
- H.-Y. Li and L. A. Estroff, Adv. Mater., 2009, 21, 470 CrossRef CAS.
- R. Munoz-Espi, Y. Qi, I. Lieberwirth, C. M. Gomez and G. Wegner, Chem.–Eur. J., 2006, 12, 118 CrossRef CAS PubMed.
- R. Munoz-Espi, A. Chandra and G. Wegner, Cryst. Growth Des., 2006, 1584 Search PubMed.
- Y. Y. Kim, L. Ribeiro, F. Maillot, O. Ward, S. J. Eichhorn and F. C. Meldrum, Adv. Mater., 2010, 22, 2082 CrossRef CAS PubMed.
- Y. Y. Kim, K. Ganesan, P. Yang, A. N. Kulak, S. Borukhin, S. Pechook, L. Ribeiro, R. Kröger, S. J. Eichhorn, S. P. Armes, B. Pokroy and F. C. Meldrum, Nat. Mater., 2011, 10, 890 CrossRef CAS PubMed.
- J. Shan and H. Tenhu, Chem. Commun., 2007, 4580 RSC.
- S. Y. Liu, J. V. M. Weaver, M. Save and S. P. Armes, Langmuir, 2002, 18, 8350 CrossRef CAS.
- J. Ihli, P. Bots, A. Kulak, L. G. Benning and F. C. Meldrum, Adv. Funct. Mater., 2013, 23, 1965 CrossRef CAS.
- C. A. Orme, A. Noy, A. Wierzbicki, M. T. McBride, M. Grantham, H. H. Teng, P. M. Dove and J. J. DeYoreo, Nature, 2001, 411, 775 CrossRef CAS PubMed.
- A. Berman, J. Hanson, L. Leiserowitz, T. F. Koetzle, S. Weiner and L. Addadi, Science, 1993, 259, 776 CAS.
- J. Aizenberg, J. Hanson, M. Ilan, L. Leiserowitz, T. F. Koetzle, L. Addadi and S. Weiner, FASEB J., 1995, 9, 262 CAS.
- L. Stappers and J. Fransaer, J. Electrochem. Soc., 2006, 153, C472 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, additional characterisation. See DOI: 10.1039/c3cc47904h |
| This journal is © The Royal Society of Chemistry 2014 |