Biocompatible macro-initiators controlling radical retention in microfluidic on-chip photo-polymerization of water-in-oil emulsions†
Yujie
Ma‡
,
Julian
Thiele‡
,
Loai
Abdelmohsen
,
Jinge
Xu
and
Wilhelm T. S.
Huck
*
Institute for Molecules and Materials, Heyendaaleweg 135, 6525 AJ Nijmegen, The Netherlands. E-mail: w.huck@science.ru.nl; Fax: +31 2436 52929; Tel: +31 2436 52138
First published on 12th November 2013
Abstract
A series of water-soluble macro-initiators is synthesized to avoid radical loss in microfluidic on-chip photo cross-linking of hyaluronic acid methacrylate-containing water-in-oil emulsions. Their superior performance over known photo-initiators through the generation of water-soluble radicals and excellent biocompatibility are demonstrated.
In recent years droplet-based microfluidics has emerged as a powerful tool in chemical and biological research as it provides new opportunities for the miniaturization of chemical and biochemical reactions, novel platforms for single cell analysis, and innovative ways for microstructure fabrication.1,2 In materials science, droplet-based microfluidics is particularly useful for preparing uniform, monodisperse polymer particles with precise control over their size, composition and morphology. Heat, redox reactions and light are most commonly used to initiate polymerization reactions in droplets. A UV-initiated polymerization reaction is generally preferred due to the rapid kinetics that preserve the internal microstructures and the precise spatial and temporal control over the reaction.3 Various reports have shown UV-initiated polymerization in combination with droplet microfluidics for the fabrication of porous microspheres,4 microcapsules5 and microgels.6 In most of these examples UV irradiation takes place offline in a bulk emulsion. This is not ideal, as homogeneous UV irradiation on each droplet cannot be ensured, which may lead to heterogeneity in the polymer particles produced. On-chip photo-polymerization could not only overcome this problem, but also offer the possibility of locking the internal droplet microstructure.5 Recent advances in microfluidics have made it possible to precisely control on-chip incubation time by incorporating delay lines.7 For the reliable utilization of on-chip photo-polymerization in emulsion droplets, droplets need to be stable and retain their contents, which is normally realized through the use of surfactants.8 Most commonly used and commercially available photo-initiators are type I initiators that dissociate into two radicals following photon absorption in the visible wavelength range. One common feature of this class of photo-initiators is that they generally possess very limited water solubility, especially those intended for the formation of biomaterials where biocompatibility also has to be taken into account.9 In the case of water soluble monomers/macromers, the solubility difference of the monomers and initiators could result in a preferential polymerization reaction at the water–oil interface.5 Moreover, it is known that the surfactant layer stabilizing microdroplets is in principle permeable to small molecules.10,11 UV polymerization of water-soluble monomers/macromers in water-in-oil droplets with commercially available UV initiators in the aqueous phase thus presents potential challenges as the partitioning of the water-insoluble initiators and radicals into the oil phase may take place, lowering the effective concentration of radicals. In fact, similar molecular transport phenomena have been studied in both controlled and conventional radical polymerization reactions in dispersed systems.12–14 However, on-chip polymerization in microfluidic emulsion droplets differs from both homogeneous as well as heterogeneous polymerization reactions as the reaction not only happens inside a secondary phase, but also in a continuous flow. So far, the distribution of photo-initiators in microfluidic two-phase systems and its influence on the outcome of the on-chip photo-polymerization have not been studied.
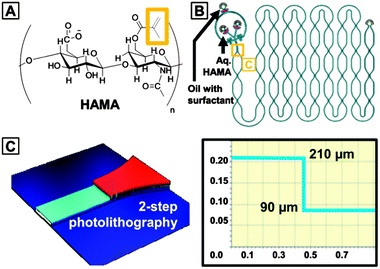
Here, we present studies on photo-initiator partitioning in on-chip photo-polymerization in droplet microfluidic water-in-oil two phase systems. A model reaction, UV-initiated cross-linking of hyaluronic acid methacrylate (HAMA) (Fig. 1), was selected as it potentially presents a novel hydrogel microbead platform for the study of cell–matrix interactions15 at the single cell level.16
As shown in Fig. 1, a microfluidic flow-focusing device was combined with an on-chip incubation module containing delay lines for the production and subsequent UV cross-linking of HAMA-containing droplets. The continuous oil phase consists of a fluorinated oil (HFE7500, 3 M) containing 1% (w/w) of a biocompatible surfactant (Krytox–Jeffamine–Krytox A–B–A triblock copolymer). The dispersed, aqueous phase consists of an aqueous solution of HAMA (0.5% w/w) and a photo-initiator. Immediately after formation in the flow-focusing nozzle which is 90 μm in height, droplets flow through wide, deep channels 210 μm in height, containing constrictions every 5.5 mm. The 3D design of the incubation chamber provides spherical, non-squeezed droplets and increases their incubation time. The constrictions provide sufficient mixing of the droplets, ensuring equal residence time for all droplets.7
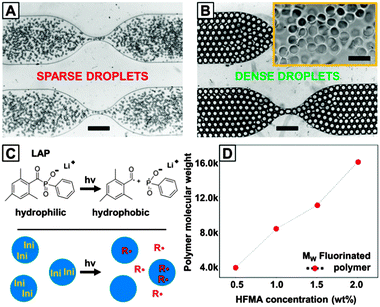
2-Hydroxy-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone (Irgacure 2959), a UV initiator that is generally considered cytocompatible,17 mainly resulted in cross-linking of HAMA in droplets at the oil–water interface (data not shown). Therefore we used a water soluble and biocompatible UV initiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) (Fig. 2C, ∼3 mM)9 to study the on-chip photo cross-linking. The cross-linking of HAMA was tested by breaking the collected emulsion using perfluorooctanol (PFO) in the presence of PBS and checking for microparticles in the aqueous phase. Upon varying oil/aqueous flow ratio and maintaining a fixed total flow rate of 1200 μL h−1 (250 to 450 nm, 4 mW cm−2, approx. 5 min), we found that HAMA hydrogel microbeads were only obtained when droplets were densely packed in the incubation chamber (Fig. 2B). No hydrogel microbeads were obtained in the case of loosely packed droplets, where the droplets were largely surrounded by the oil phase (Fig. 2A).
A close examination of the chemical structure of LAP and its UV degradation products revealed that even though LAP itself is water-soluble, the trimethylbenzoyl radical that is actively participating in the initiation process is not. The fact that UV-initiated HAMA cross-linking only took place when droplets were in close contact with each other strongly suggests radical partitioning into the oil phase. Upon UV irradiation, an aqueous solution of LAP immediately became turbid, indicating the formation of water-insoluble compounds. In the case of loosely packed droplets (oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water volume ratio ≥1) flowing through the UV-incubation chambers, the water-insoluble radicals generated by UV will most likely re-distribute between the oil and the aqueous phase, ending predominantly in the oil phase. For densely packed droplets, the balance of partitioning might be towards the dispersed phase as the oil
water volume ratio ≥1) flowing through the UV-incubation chambers, the water-insoluble radicals generated by UV will most likely re-distribute between the oil and the aqueous phase, ending predominantly in the oil phase. For densely packed droplets, the balance of partitioning might be towards the dispersed phase as the oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio is much smaller (0.5). In order to verify this hypothesis, sparse droplets were generated again with the oil phase replaced by a fluorinated monomer (2,2,3,3,4,4,4-heptafluorobutyl methacrylate, HFMA) solution in HFE7500. Upon evaporating HFE7500 from the eluent collected at the outlet of the microfluidic device, a white solid compound was obtained and tested by 1H NMR and GPC. The tests confirm that the solid compound is indeed polymerized HFMA, whose molecular weight increases with increasing HFMA concentration between 0.5% and 2% (w/w) in the aqueous phase (Fig. 2D).
water ratio is much smaller (0.5). In order to verify this hypothesis, sparse droplets were generated again with the oil phase replaced by a fluorinated monomer (2,2,3,3,4,4,4-heptafluorobutyl methacrylate, HFMA) solution in HFE7500. Upon evaporating HFE7500 from the eluent collected at the outlet of the microfluidic device, a white solid compound was obtained and tested by 1H NMR and GPC. The tests confirm that the solid compound is indeed polymerized HFMA, whose molecular weight increases with increasing HFMA concentration between 0.5% and 2% (w/w) in the aqueous phase (Fig. 2D).
As the partitioning of water-insoluble radicals from LAP obviously makes on-chip hydrogel microbead fabrication rather uncontrolled, we set out to look for UV macro-initiators that circumvent the partitioning problem associated with small molecule radicals and generate water-soluble radicals. In recent years some examples of water-soluble macro-initiators have been reported. However, the biocompatibility of these macro-initiators has not been fully addressed.18–20 To this end, we designed and synthesized a series of water-soluble LAP- and I2959-based initiators containing poly(ethylene glycol) (PEG) tails of varying molar masses (Fig. 3). Briefly, PEG-LAP was synthesized in a 3-step reaction starting from 2,4,6-trimethylbenzoyl chloride. LAP-CH2Cl was first obtained by a Michaelis–Arbuzov reaction and subsequently reacted with PEG methyl ether (MW 550 and 2000) in the presence of pyridine to give PEG-LAP. To circumvent the very low yield of this reaction (1 to 2% w/w), PEG methyl ether tosylate (MW 550 and 2000) was reacted with the primary alcohol present on I2959 in the presence of pyridine to yield PEG-I2959. All four compounds readily dissolve in water and show degradation under UV irradiation (Fig. S2, ESI†). Bulk cross-linking experiments in HAMA solutions (0.5% w/w) containing equal concentration of PEG-LAP and PEG-I2959 (∼3 mM) resulted in the formation of bulk hydrogels under the same UV irradiation conditions, demonstrating their good initiation efficiencies.
Next, microfluidic on-chip photo cross-linking of droplets containing HAMA hydrogel precursors (0.5% w/w) was performed under the above experimental conditions, but using the newly synthesized photo-initiators (∼3 mM) instead. Interestingly, homogeneous and spherical hydrogel microbeads (Fig. S4 and S5, ESI†) were obtained in the case of loosely packed droplets for all four macro-initiators. Moreover, when HFMA was present in the oil phase, no fluorinated polymer was obtained. The hydrophilic PEG tails ensure that the more active benzoyl radicals that are attached to them stay in the aqueous phase in the oil–water two phase system. In other words, the retention of the radicals in the water phase was effectively controlled by using the new class of macro-initiators.
For biological applications21–24 the cytocompatibility of these new macro-initiators was tested. A standard MTT assay shows that at the useful concentration range (≤3 mM),17 PEG-I2959 is most cytocompatible among the five different UV initiators tested in this study (Fig. S6, ESI†). It is also shown that a longer PEG tail appears to induce higher toxicity. As radicals are most often the cytotoxic species, a more relevant experiment was carried out by embedding NIH/3T3 fibroblast cells in HAMA hydrogels prepared under the same UV irradiation conditions with the same HAMA (0.5% w/w) and initiator (∼3 mM) concentrations. The viability of the cells was tested by a membrane integrity assay using the Live/Dead cytotoxicity kit (Invitrogen) following 24 h of culture of the 3D hydrogel/cell constructs under standard cell culture conditions.9 Both PEG containing initiators support over 90% of cell survival in the hydrogel matrix (Fig. S7, ESI†), demonstrating their excellent biocompatibility.
In summary, we have shown the redistribution of small molecule radicals in microfluidic water–oil two phase systems due to the differences in water solubility of the UV-generated radicals. Radical partitioning results in uncontrolled photo-polymerization of water-soluble monomers and macromers on-chip when susceptible photo-initiators such as LAP are used. A series of water-soluble macro-initiators containing PEG tails were synthesized and their superior ability in controlling radical retention in the original water phase by generating water soluble radicals was demonstrated. PEG-LAP and PEG-I2959 are both particularly useful for controlled microfluidic on-chip photo-polymerization. The biocompatibility of PEG-I2959 makes it an interesting candidate for droplet microfluidics-based, biologically oriented applications, for example the fabrication of hydrogel microbeads for live cell studies at the single cell level.
J. T. is a Feodor-Lynen fellow of the Alexander von Humboldt Foundation. Work in the Huck group was supported by a European Research Council (ERC) Advanced Grant (246812 Intercom), a VICI grant from the Netherlands Organization for Scientific Research (NWO), and by funding from the Ministry of Education, Culture and Science (Gravity program 024.001.035).
Notes and references
- A. B. Theberge, F. Courtois, Y. Schaerli, M. Fischlechner, C. Abell, F. Hollfelder and W. T. S. Huck, Angew. Chem., Int. Ed., 2010, 49, 5846–5868 CrossRef CAS PubMed.
- H. N. Joensson and H. A. Svahn, Angew. Chem., Int. Ed., 2012, 51, 12176–12192 CrossRef CAS PubMed.
- D. K. Hwang, D. Dendukuri and P. S. Doyle, Lab Chip, 2008, 8, 1640–1647 RSC.
- X. Q. Gong, W. J. Wen and P. Sheng, Langmuir, 2009, 25, 7072–7077 CrossRef CAS PubMed.
- C. H. Choi, J. H. Jung, D. W. Kim, Y. M. Chung and C. S. Lee, Lab Chip, 2008, 8, 1544–1551 RSC.
- S. Seiffert and D. A. Weitz, Polymer, 2010, 51, 5883–5889 CrossRef CAS PubMed.
- L. Frenz, K. Blank, E. Brouzes and A. D. Griffiths, Lab Chip, 2009, 9, 1344–1348 RSC.
- J.-C. Baret, Lab Chip, 2012, 12, 422–433 RSC.
- B. D. Fairbanks, M. P. Schwartz, C. N. Bowman and K. S. Anseth, Biomaterials, 2009, 30, 6702–6707 CrossRef CAS PubMed.
- F. Courtois, L. F. Olguin, G. Whyte, A. B. Theberge, W. T. S. Huck, F. Hollfelder and C. Abell, Anal. Chem., 2009, 81, 3008–3016 CrossRef CAS PubMed.
- Y. Skhiri, P. Gruner, B. Semin, Q. Brosseau, D. Pekin, L. Mazutis, V. Goust, F. Kleinschmidt, A. El Harrak, J. B. Hutchison, E. Mayot, J. F. Bartolo, A. D. Griffiths, V. Taly and J. C. Baret, Soft Matter, 2012, 8, 10618–10627 RSC.
- J. Qiu, B. Charleux and K. Matyjaszewski, Prog. Polym. Sci., 2001, 26, 2083–2134 CrossRef CAS.
- A. Sogabe, J. D. Flores and C. L. McCormick, Macromolecules, 2010, 43, 6599–6607 CrossRef CAS.
- I. Capek, Adv. Colloid Interface Sci., 2010, 156, 35–61 CrossRef CAS PubMed.
- M. Güvendiren and J. A. Burdick, Nat. Commun., 2012, 3, 792 CrossRef PubMed.
- D. Velasco, E. Tumarkin and E. Kumacheva, Small, 2012, 8, 1633–1642 CrossRef CAS PubMed.
- C. G. Williams, A. N. Malik, T. K. Kim, P. N. Manson and J. H. Elisseeff, Biomaterials, 2005, 26, 1211–1218 CrossRef CAS PubMed.
- E. Groison, S. Brusseau, F. D'Agosto, S. Magnet, R. Inoubli, L. Couvreur and B. Charleux, ACS Macro Lett., 2012, 1, 47–51 CrossRef CAS.
- G. Y. Liu, Q. Qiu, W. Q. Shen and Z. S. An, Macromolecules, 2011, 44, 5237–5245 CrossRef CAS.
- S. R. Li, J. P. Deng and W. T. Yang, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 936–942 CrossRef CAS.
- S. Ma, M. Natoli, X. Liu, M. P. Neubauer, F. Watt, A. Fery and W. T. S. Huck, J. Mater. Chem. B, 2013, 1, 5128–5136 RSC.
- S. Khetan and J. A. Burdick, Soft Matter, 2011, 7, 830–838 RSC.
- S. Khetan, M. Guvendiren, W. R. Legant, D. M. Cohen, C. S. Chen and J. A. Burdick, Nat. Mater., 2013, 12, 458–465 CrossRef CAS PubMed.
- T. Rossow, J. A. Heyman, A. J. Ehrlicher, A. Langhoff, D. A. Weitz, R. Haag and S. Seiffert, J. Am. Chem. Soc., 2012, 134, 4983–4989 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis details and Fig. S1–S7. See DOI: 10.1039/c3cc46733c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |