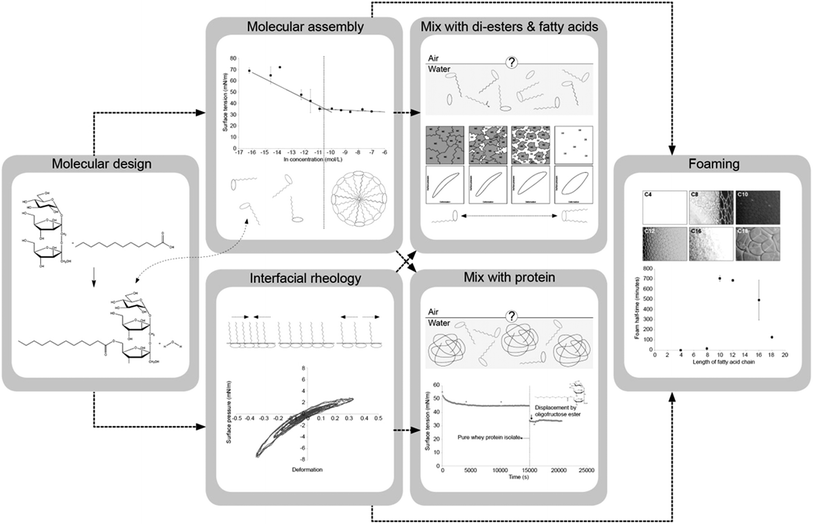
Molecular assembly, interfacial rheology and foaming properties of oligofructose fatty acid esters
Silvia E. H. J.
van Kempen
ab,
Henk A.
Schols
c,
Erik
van der Linden
a and
Leonard M. C.
Sagis
*a
aLaboratory of Physics and Physical Chemistry of Foods, Wageningen University, Bornse Weilanden 9, 6708 WG Wageningen, The Netherlands. E-mail: leonard.sagis@wur.nl; Fax: +31 317 483669; Tel: +31 317 485023
bDutch Polymer Institute DPI, P.O. Box 902, 5600 AX Eindhoven, The Netherlands
cLaboratory of Food Chemistry, Wageningen University, Bornse Weilanden 9, 6708 WG Wageningen, The Netherlands
First published on 11th November 2013
Abstract
Two major types of food-grade surfactants used to stabilize foams are proteins and low molecular weight (LMW) surfactants. Proteins lower the surface tension of interfaces and tend to unfold and stabilize the interface by the formation of a visco-elastic network, which leads to high surface moduli. In contrast, LMW surfactants lower the surface tension more than proteins, but do not form interfaces with a high modulus. Instead, they stabilize the interface through the Gibbs–Marangoni mechanism that relies on rapid diffusion of surfactants, when surface tension gradients develop as a result of deformations of the interface. A molecule than can lower the surface tension considerably, like a LMW surfactant, but also provide the interface with a high modulus, like a protein, would be an excellent foam stabilizer. In this article we will discuss molecules with those properties: oligofructose fatty acid esters, both in pure and mixed systems. First, we will address the synthesis and structural characterization of the esters. Next, we will address self-assembly and rheological properties of air/water interfaces stabilized by the esters. Subsequently, this paper will deal with mixed systems of mono-esters with either di-esters and lauric acid, or proteins. Then, the foaming functionality of the esters is discussed.
1. Introduction
The food industry produces food products with a wide range of textures. These textures are obtained using a wide variety of functional ingredients and structural elements, for example, three-dimensional networks formed by gluten proteins in bread,1 partially coalesced fat structures formed by milk fats in ice creams,2 and air bubbles stabilized by surfactants in mousses.3 In this paper, we have focused on the latter type of product, aerated food products.The production of aerated products with sufficient stability throughout their shelf life is complicated. Air bubbles can be introduced into a food product by for instance whipping, shaking, or gas injection.3 After the introduction of air bubbles, they need to be stabilized, so that at least during the shelf life of the product no significant changes in the product texture occur. The stability of the bubble depends on different factors. First, it is important that during the formation of an air bubble, surfactants adsorb quickly at its interface.4 Next, the functional properties of the continuous phase are important. A continuous phase with a high viscosity leads to slower drainage, which is one of the instability mechanisms in foams.5–8 Furthermore, the functional properties of the interfaces between air and water influence disproportionation and coalescence, two other instability mechanisms in foams.5,9–14 The interfacial properties are highly dependent on the type of surfactant that is used to stabilize the foam films.15
Two types of surfactants that are mostly used in the food industry are proteins and low molecular weight (LMW) surfactants.13 Proteins stabilize the interface by lowering the surface tension and by the formation of a viscoelastic layer upon adsorption to the interface.4,8,13 LMW surfactants generally lower the surface tension more than proteins and stabilize the interface through the Gibbs–Marangoni mechanism,4,13,16–18 which relies on rapid surface diffusion of surfactants, that will reduce surface concentration gradients that can develop after deformation of the interface.17,19
A surfactant that can lower the surface tension significantly, like a LMW surfactant, and at the same time provide the interface with a high dilatational modulus, like a protein, would be an excellent foam stabilizer. In this paper we describe the properties of a series of molecules that obey these criteria: oligofructose fatty acid esters. The molecular structure of the esters is expected to have a major impact on the functional properties.13,20 Therefore, the following variations in the molecular structure were investigated: the degree of esterification (mono-esters versus di-esters), the length of the fatty acid chain, the degree of saturation of the fatty acid chain, and the size of the hydrophilic head group. After synthesis, purification and structural characterization,21 the basic functional properties, such as micelle formation and the area per molecule, were studied,22 as well as rheological properties of air/water interfaces stabilized by the esters.23 Next, the functional properties of the mono-esters were determined when mixed with components present in the crude reaction product24 or proteins.25 Finally, the potential of the esters as foaming ingredients was studied.26 The outline of this research is graphically presented in Fig. 1. The results represent different length scales present in foam: the molecular scale (chemical structure of the esters), the interfacial scale (organization of the esters at the interface), and the macroscopic scale (stability of a macroscopic volume of foam), and all these scales are interdependent. In this paper we will elaborate on details of the results to appreciate the interdependencies between these different length scales and focus on new insights that were gained by combining all these results.
2. Synthesis and structural characterization
In this section the synthesis of oligofructose esters with different fatty acid chain lengths is discussed,21 which was based on previous studies by Ter Haar et al.27 and Sagis et al.28 Both chemical and enzymatic reaction procedures may be considered to synthesize oligofructose fatty acid esters. Due to the disadvantages of the chemical procedure (the use of alkaline catalysts at high temperatures29,30 and a low selectivity, which leads to a mixture of esters with different degrees of esterification31–34) an enzymatic reaction procedure was used to obtain a well-defined reaction product.31,33,35,36 Lipase B from Candida antarctica was selected due to the fact that this is a commonly used enzyme in similar reaction procedures,32,37–41 and due to the fact that it has already been successfully used to synthesize oligofructose esters.27,28 At a later stage in the research, a range of enzymes was screened for their transesterification activity in a model reaction between 1-octanol and vinyl laurate in hexane. There were both immobilized and free enzymes, and the enzymes were from different origins (yeasts, bacteria and plants). As expected,42 immobilized enzymes displayed significantly higher activities than free enzymes. Of all enzymes tested, the immobilized lipase B from Candida antarctica had the highest activity. This confirmed that the earlier choice to employ this enzyme for the synthesis reactions was the right one. One of the complications that one faces when producing these types of derivatives in liquid solvents is the difference in polarity between the oligofructose part of the molecule and the fatty acid. This limits the choice of organic solvents.31,43 Relatively polar solvents that lead to high solubility of the oligofructose part of the molecule decrease the solubility of the fatty acid and decrease the stability of the lipase.27 A combination of DMSO and ButOH (0.1/0.9) was used based on the fact that previous research27,28 had already shown that reasonable yields could be obtained when using this combination. Nott et al.44 have recently showed that during lipase-catalyzed esterification the yield of mannose myristic acid esters was the highest when a ratio DMSO–ButOH of 0.1/0.9 was used. It may of course be possible that with (a combination of) different solvents, for example acetone, diethyl ether, hexane or 2-methyl-2-butanol,37,45 a higher yield could be obtained.As a first step in the establishment of the synthesis reaction conditions, three different reaction procedures were considered: (1) esterification in the presence of molecular sieves, (2) transesterification in the absence of molecular sieves, and (3) transesterification in the presence of molecular sieves. No major differences in yield were found between the three different reaction procedures. These results are similar to recently reported results obtained during the esterification of mannose myristic acid esters in DMSO–ButOH 0.1/0.9,44 where no major differences in yield between esterification with molecular sieves and transesterification without molecular sieves were found. Because a similar yield was obtained for all reaction procedures, esterification reactions were selected for further syntheses of oligofructose esters.
Next, the inactivation of the enzyme was studied under the selected reaction conditions. The enzyme activity significantly decreased as a result of temperature, mechanical abrasion and stripping of the essential layer of water molecules. These results are similar to results recently reported by Nott et al.,44 who found a significant decrease in the yield of mannose myristic acid esters when the DMSO fraction was increased. They attributed this to the disruption of the hydration shell of the enzyme. In our study, after 24 hours, only 17% of the original activity was retained. Since the total incubation time was 68 hours, after 19 and 43 hours an additional batch of enzyme was added to compensate for enzyme inactivation. However, from an economic point of view, this is not an optimal solution to the problem. Many different approaches may be considered to reduce enzyme inactivation. Changes to the solvent will also affect the activity of the enzyme. It was proposed, for example, to replace the solvent with a hydrophobic solvent such as hexane or toluene.46 To reduce inactivation due to mechanical shear the speed of agitation may be reduced. Another approach, which was tested during preliminary experiments (results not shown), is the development of a continuous process for the enzymatic esterification of the oligofructose ester using a fixed-bed reactor with an immobilized enzyme and a rehydration step between the cycles. Using this set-up, it was possible to synthesize oligofructose esters using both an esterification reaction and a transesterification reaction.
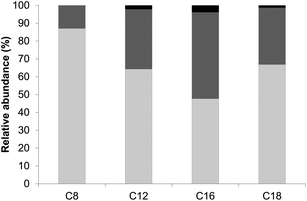
For all fatty acid chain lengths, as a result of the highly specific reaction procedure,47 mono-esters were the main reaction products (Fig. 2). The conversion of oligofructose into esters increased with the fatty acid chain length (Fig. 2), and is consistent with earlier reports of enzyme specificity towards more lipophilic substrates.33,48
The oligofructose was a mixture with different degrees of polymerization (DP). We found that for all fatty acid chain lengths, DP 3 and 4 were slightly better substrates for the enzyme than DP 5, 6, 7 and 8. For the shorter fatty acids DP5 was also a slightly better substrate than the longer oligomers. Furthermore, di-esters were only formed for the shortest fatty acids. These differences may be related to the lack of space in the catalytic cleft of the enzyme, which limits the access of oligomers with a higher DP and mono-esters.49–51
The reaction yielded a mixture of mono-esters, di-esters, unreacted oligofructose and unreacted fatty acids. Because of expected differences in functionality between the different fractions,24 it was desired to fractionate the crude reaction product. Reverse phase solid phase extraction was used to fractionate the mixture into different individual components. A gradient of methanol and water was used and the composition of the solvent that eluted the esters from the column could be correlated with the hydrophobicity of the esters.
The purified fractions were characterized using MALDI-TOF MS and (2D) NMR. Using MALDI-TOF MS, a purity of >90% was obtained for mono-esters and a purity of >80% for di-esters. Using (2D) NMR techniques, it was possible to show that the location of the esterification was restricted to the C-1 and C-6 positions of the monosaccharides. Unfortunately, the analysis did not conclusively show on which position on the oligofructose chain the esterification was located. However, due to geometric constraints in the catalytic cleft of the enzyme, it is likely that the esterification locations were at either ends of the oligofructose chain. The location of the esterification is likely to influence the functional properties of the esters, more specifically the arrangement of the molecule at the interface. The importance of the position of the esterification is demonstrated by Ferrer et al.52 for di-esters who found a much lower surface tension for the 6,6'-di-ester compared to the 6,1′-di-ester. Razafindralambo et al.53 have demonstrated how the location of the esterification can significantly change both the surface tension and surface rheological properties. Therefore, we recommend further investigation of ways to improve our understanding on the exact molecular structure of the esters. One approach that would facilitate the interpretation of the 2D NMR spectra would be the fractionation of the oligofructose on the basis of DP.
Most importantly, since major differences in functionality were observed between the oligofructose esters and sucrose esters,22,23,26 the size of the hydrophilic moiety apparently plays an important role in the functional properties of interfaces stabilized by those esters. Therefore, we recommend that further research should focus on ways to separate the oligomers with different degrees of polymerization from each other, either before or after synthesis. To obtain oligofructose with a more defined DP, one can focus on improving the selectivity of the partial enzymatic hydrolysis of inulin, using an endo-inulinase, or by making use of the oligofructose synthesized from sucrose using fructosyl-transferase.54,55
3. Effects of molecular structure on molecular assembly and interfacial properties
As already indicated, it is expected that differences in the molecular structure of the ester, such as the length of the fatty acid or the degree of esterification, have a major impact on the functionality of the esters. Therefore, the influence of molecular structure on some basic functional properties of these esters was studied. These include micelle formation, the amount of space that a molecule occupies at the air/water interface and rheological properties of interfaces stabilized by those esters.Micelle formation
Due to their amphiphilicity, surfactants that are present in the bulk will migrate to the interface where they lower the surface tension. With increasing bulk concentration of the surfactant, more molecules will migrate to the interface, where they further lower the surface tension. At a certain bulk concentration, the surfactants start to form aggregates. After this critical concentration is reached, the surface tension will not decrease much further. It is referred to as the critical micelle concentration (CMC) and is an important specification of a surfactant.56 The CMC is expected to depend on the molecular structure of the ester. Therefore, we have used a profile analysis tensiometer (PAT 1M, Sinterface, Berlin, Germany) to determine how the surface tension of air/water interfaces stabilized by oligofructose esters with different molecular structures depended on the bulk concentration.22 For all esters, a typical CMC curve was obtained. The CMC decreased with increasing fatty acid chain lengths (both for mono-esters and di-esters). Furthermore, di-esters had a lower CMC than the corresponding mono-esters (Table 1).| Surfactant | Type | CMC (mM) | Molecular area determined from fitting Gibbs equation (Å2) | Molecular area determined from ellipsometry (Å2) |
|---|---|---|---|---|
| OF–C8m | Oligofructose mono-ester | 28 ± 7.5% | 61 ± 15 | |
| OF–C8d | Oligofructose di-ester | 0.209 ± 7.5% | 55 ± 10 | 137 ± 5.7 |
| OF–C12m | Oligofructose mono-ester | 3.21 ± 20% | 91 ± 25 | 94 ± 2 |
| OF–C12d | Oligofructose di-ester | 0.0195 ± 5% | 46 ± 15 | 113 ± 8.4 |
| OF–C16m | Oligofructose mono-ester | 0.225 ± 20% | 85 ± 15 | 86 ± 3.3 |
| OF–C16:1m | Oligofructose mono-ester | 81 ± 2.6 | ||
| OF–C18m | Oligofructose mono-ester | 0.1438 ± 20% | 73 ± 20 | 76 ± 12.1 |
| S–C12m | Sucrose ester | 58 ± 0 | ||
| S–C16 | Sucrose ester | 58 ± 0.4 | ||
| S–C18 | Sucrose ester | 56 ± 1.2 |
Similar effects were found by other authors when studying sugar esters.20,52,57–62 Additionally, light scattering experiments were performed to establish the nature of the aggregates that were formed at concentrations higher than the CMC. For all esters except the mono-ester with a fatty acid chain length of 18 carbon atoms, aggregates in the size range of simple micelles were found. For the mono-esters with a fatty acid chain length of 18 carbon atoms, the size distribution was characterized by large and polydisperse aggregates. The nature of these aggregates is unclear. They could be caused by the presence of insoluble aggregates, as a result of the high hydrophobicity of the ester. Furthermore, it is possible that the long fatty acid chain affects the critical packing parameter of the esters and leads to micelles with a different morphology, such as worm-like micelles63 or rods.60 A possible method for studying the morphology of the aggregates could be cryo-TEM.
From the CMC curves, it is possible to determine the effectiveness and efficiency of the esters. These terms are often used to characterize surfactants.58 The efficiency of a surfactant gives information about the amount of material that is necessary to obtain a certain surface tension reduction, often 20 mN m−1. The efficiency has practical relevance because it determines the amount of material that is necessary in a product formulation. The efficiency of the esters increased with increasing hydrophobicity. Therefore, especially esters of longer fatty acid chains seem to be interesting as potential food-grade functional additives.52 The effectiveness is defined as the surface pressure that is obtained at the CMC and is not necessarily the same among a series of surfactants. In the case of oligofructose esters, a similar effectiveness was found for all esters, which may imply that the amount of space occupied by a molecule is determined by the oligofructose head group.
Surface area per molecule
The amount of space occupied by a single molecule after adsorption at the air/water interface gives information about the interactions between the molecules. Therefore, it is important for the rheological behavior of interfaces. Two different methods were used to determine the amount of space occupied by a single molecule at the interface.22 First, the CMC curves were fitted with the Gibbs equation for non-ionic surfactants, as often performed for sugar based esters.20,52,60,64,65 Furthermore, this parameter was determined using ellipsometry. As far as we are aware, this method was not applied before for sugar esters, possibly because the accuracy decreases, as interfacial layers get thinner.66 With both methods, a similar area per molecule was found for the oligofructose esters with different fatty acid chain lengths (Table 1). Indications were found that di-esters occupy a slightly larger area than mono-esters (Table 1), which means that two fatty acid tails could occupy a larger area than the oligofructose part of the molecule. This will have consequences on the rheological response and microstructure of interfaces stabilized by these esters.23,24 These consequences are discussed in subsequent sections. To study the influence of the size of the hydrophilic group, oligofructose esters were compared to sucrose esters. It was found that oligofructose esters occupy a larger molecular area than the corresponding sucrose esters with the same fatty acid chain length (Table 1). All (sucrose and oligofructose) esters occupied a significantly larger area than a single fatty acid chain. This shows that the oligofructose part determines the amount of space occupied by one molecule and that the fatty acid chains are relatively far apart. Therefore, it is likely that the rheological response of interfaces stabilized by oligofructose fatty acid esters is largely determined by interactions between the oligofructose part of the molecule, and is less affected by the fatty acid tail–tail interactions.Rheological properties
An important reason to study the rheological properties of air/water interfaces is their link to foaming properties.67–69 The shear and dilatational properties of interfaces have been linked to instability mechanisms of foams, such as disproportionation and coalescence.70 In this research, the focus was on dilatational deformation. The rheological properties were studied using a drop tensiometer (PAT 1M, Sinterface, Berlin, Germany), by applying sinusoidal area deformations with a frequency of 0.1 Hz and an amplitude of 5%.To further characterize the rheological response of the interfaces, frequency sweeps were performed after reaching equilibrium in the frequency range of 0.005 Hz to 1 Hz at a deformation amplitude of 5%. For all oligofructose esters (mono-esters with saturated and unsaturated fatty acid chains and di-esters) a low scaling exponent in the frequency dependency was found at surface pressures lower than πCMC (Table 2), as a result of slow transport from the bulk to the interface caused by the low concentration of molecules in the bulk. When the surface pressure was higher than πCMC the degree of variability in the scaling exponents increased. However, for the oligofructose mono-esters they remained relatively low. This could again be indicative of the presence of a soft interfacial glass phase formed by the oligofructose part of the molecule.74 For di-esters in some cases a scaling exponent in the frequency dependency close to 0.5 was obtained. This indicates that processes were diffusion-controlled and adequately described by the Lucassen–Van Den Tempel model,75 which is typical for LMW surfactants. For sucrose esters, scaling exponents that approached 0.5 were obtained which indicates typical LMW surfactant behavior without mesophase formation. The difference in the rheological response between oligofructose esters and sucrose esters and the fact that they have the same fatty acid chain make it unlikely that interactions between the fatty acids play a major role in the surface rheological response.
| Surfactant | Type | Scaling exponent | |
|---|---|---|---|
| π < πCMC | π > πCMC | ||
| OF–C8m | Oligofructose mono-ester | 0.07 (0–0.23) | 0.25 (0.2–0.3) |
| OF–C8d | Oligofructose di-ester | 0.12 (0–0.3) | 0.28 (0.15–0.48) |
| OF–C12m | Oligofructose mono-ester | 0.13 (0–0.33) | 0.1 (0.03–0.19) |
| OF–C12d | Oligofructose di-ester | 0.05 (0–0.12) | 0.25 (0.06–0.46) |
| OF–C16m | Oligofructose mono-ester | 0.03 (0–0.04) | 0.18 (0–0.32) |
| OF–C16:1m | Oligofructose mono-ester | 0.07 (0.03–0.18) | 0.28 (0.12–0.48) |
| OF–C18m | Oligofructose mono-ester | 0.05 (0–0.09) | 0.04 (−0.23–0.18) |
| S–C12m | Sucrose ester | 0.24 (0.18–0.28) | |
| S–C16 | Sucrose ester | 0.53 (0.51–0.55) | |
| S–C18 | Sucrose ester | 0.44 (0.43–0.45) | |
The first-harmonic Fourier moduli of air/water interfaces stabilized by oligofructose mono-esters with a fatty acid chain length of 18 carbon atoms strongly depended on the applied deformation amplitude.23 With increasing deformation amplitude, the surface dilatational modulus became lower. This could be interpreted as significant structural changes in the interfacial microstructure. In fact, no linear viscoelastic regime could be identified within the range of applied deformation amplitudes between 1.5% and 30%. This could point to the presence of a brittle layer, and could support the hypothesis of the formation of a soft interfacial glass phase by the oligofructose part of the molecule. The strong films formed by the low molecular weight surfactant sorbitan tristearate showed a similar brittle behavior.71 Strain dependent moduli were also observed by Humblet-Hua et al.,76 when studying oil/water interfaces stabilized by complexes of high methoxyl-pectin and proteins (ovalbumin and lysozyme) and by protein (ovalbumin and lysozyme) fibrils. The dependence of the modulus on the amplitude deformation is not specific for oligofructose esters and we stress the importance of including strain sweeps during rheological characterizations of interfaces.
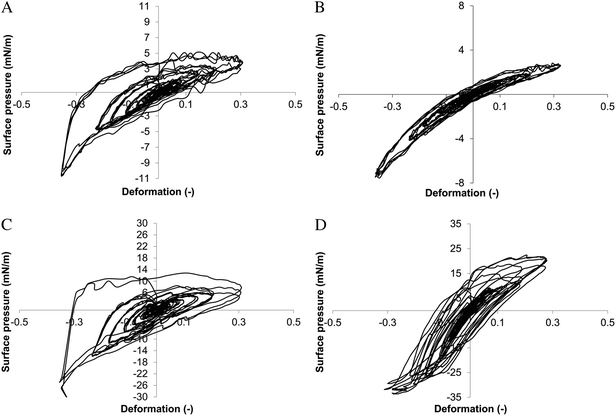
Using the first-harmonic Fourier moduli, non-linearities in the raw signal are disregarded, as already pointed out by Ewoldt et al.77 To acquire information about non-linearities, the results of the amplitude sweeps were presented in the form of Lissajous plots, where surface pressure was taken as a measure of stress. Recently, these Lissajous plots were used when studying the interfacial rheology of Acacia gum78 and β-lactoglobulin fibrils.79 We show that over the whole range of surface pressures, oligofructose mono-esters showed a predominantly elastic response. Furthermore, the Lissajous plots showed asymmetries where during compression the slope of the curve increased with increasing strain, and during expansion the slope of the curve decreased with increasing strain (Fig. 3). This points to strain hardening during compression and strain softening during extension. It is not likely that the shape of the curves is influenced by hydrodynamics: at the employed frequency of 0.1 Hz hydrodynamics only will play a significant role when bulk viscosities approach 1000 Pa s.80 Our results support the hypothesis that the oligofructose part of the molecule forms a soft interfacial glass phase.
For oligofructose di-esters at high surface pressures, the response became more viscous and non-linearities disappeared. Combined with the scaling exponent in the frequency dependence close to 0.5, we conclude that the presence of the two fatty acid chains interfered with the formation of the soft interfacial glass phase by the oligofructose part of the molecule. For sucrose esters a rather viscous response without asymmetries was found, typical for low molecular weight surfactants. These results demonstrate that Lissajous plots can be very useful in understanding the link between surface rheological properties and the interfacial microstructure.
The surface dilatational modulus increased with decreasing temperature. The fact that the surface dilatational modulus was dependent on temperature was indicative of a structural transition upon cooling of the interface. This is another argument that supports the hypothesis of a soft interfacial glass phase formed by the oligofructose part of the molecule.
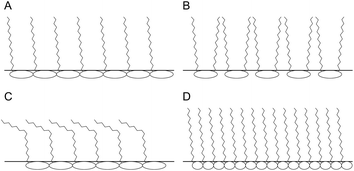
To summarize, interfaces stabilized by oligofructose mono-esters had relatively high dilatational moduli, low scaling exponents in the frequency dependency, and elastic and highly asymmetrical Lissajous plots with strain hardening during compression and strain softening during expansion. These properties were the same for esters with saturated or unsaturated fatty acid tails. Furthermore, the moduli were dependent on temperature. In contrast, sucrose esters had relatively low moduli, scaling exponents in the frequency dependency close to 0.5, and fairly viscous Lissajous plots without asymmetries. Based on these facts, we conclude with a fair amount of certainty that the oligofructose part of the ester forms a soft interfacial glass phase (Fig. 4). All hypotheses about the interfacial microstructure that were formulated based on rheological information23–26 need to be confirmed using appropriate optical techniques such as Brewster angle microscopy, particle tracking observed by microscopy,81 or grazing incidence X-ray diffraction.82
In this research we have shown that especially for interfaces with complex microstructures, it is essential to study the dependence of the modulus on the deformation amplitude. Therefore, we propose that the study of the rheological properties of interfaces during oscillatory dilatational deformations should always include strain sweeps. Furthermore, we have shown that Lissajous plots of surface pressure versus deformation can be powerful tools in understanding the link between surface rheological properties and the interfacial microstructure.
4. Mixing with other components
After having established the basic functional properties of purified mono-esters and di-esters, it is important to consider what will happen when the esters are mixed in the bulk with other components. First, one should realize that the crude reaction product that is obtained is a mixture of esters with different degrees of esterification, and of unreacted oligofructose and fatty acids.21 Since purification is a time-consuming and expensive process, one may wonder, from a practical point of view, what are the differences in functionality between the crude and purified products, and what is the contribution of each component that is present in the crude product to the functional properties. Second, when thinking about the application in food products, it is important to consider that many food products contain components that are surface active, mostly proteins. Hence, one must carefully consider what the interfacial structure of such a system will look like. Therefore, mixed systems are discussed.Mixtures of a mono-ester with a di-ester and fatty acid
Oligofructose fatty acid esters are produced by esterification of fatty acids with oligofructose.21 After synthesis, the reaction mixture is composed of unreacted oligofructose, unreacted fatty acids, mono-esters and di-esters, which are expected to have completely different functional properties.Due to the low solubility of fatty acids in the bulk at room temperature, properties of interfacial layers stabilized by fatty acids are often studied after spreading of the fatty acid on the interface.83 We have taken a different approach,24 because we wanted to know to what extent lauric acid could migrate from the bulk to the interface at room temperature, despite its low solubility. Pure lauric acid was present in the form of (visible) aggregates and hardly migrated to the interface, as evidenced by a high surface tension and a low dilatational modulus. The small amount of material that did migrate to the interface formed a purely elastic layer. The presence of mono-esters caused a small increase in the surface concentration of fatty acids, possibly by the formation of mixed micelles, as hypothesized by Golemanov et al.84 for their system containing both surfactants and fatty acids. The mixed interface of an oligofructose mono-ester and fatty acid was characterized by an intermediate frequency dependency (scaling exponent of 0.34) and a slightly asymmetric Lissajous plot. This could be caused by the presence of islands of glass phase formed by the mono-ester surrounded by lauric acid.
Oligofructose was not surface active and the mixing of oligofructose with a mono-ester only diluted the system, which did not lead to significant changes in surface properties because the concentration of mono-esters was, even after mixing, still close to the CMC.
So the impact of lauric acid and oligofructose on the properties of mixed interfaces was limited. The two main surface active components, mono-esters and di-esters, had a more pronounced influence on interfacial properties. We have established that both mono-esters and di-esters migrated to the interface.22 However, it is possible that, as a result of increased hydrophobicity, di-esters are present in the form of aggregates that will only migrate to the interface slowly. We have assessed the surface tension of air/water interfaces stabilized by both mono-esters and di-esters.24 Additionally, we have focused on differences in the speed of surfactant adsorption. Since major differences were found in the rheological response of mono-esters and di-esters,23 mixing of mono-esters and di-esters at different ratios may have significant consequences on the interfacial microstructure.
Pure OF mono-esters could lower the surface tension more quickly than pure OF di-esters. However, at equilibrium, di-esters lead to a lower surface tension. This means that the increase in hydrophobicity as a result of the second fatty acid chain does slow down the migration to the interface, but the reduction in solubility is insufficient to render the di-esters surface-inactive. This is in contrast to the behavior reported for smaller hydrophilic groups. Ferrer et al.52 describe how the incorporation of a second acyl chain into a sucrose ester led to a significant loss in solubility that made it impossible to collect reliable CMC data. Husband et al.61 describe how the surface tension obtained with the sucrose di-ester was much higher than the surface tension obtained with the sucrose mono-ester. Di-esters also had lower foamability. They attributed these differences to a less compact structure and to an increase in the amount of micelles that was present in the bulk leading to slower adsorption kinetics. In the case of oligofructose esters these effects are much less pronounced as a result of the increased hydrophilicity due to the larger hydrophilic group. Oligofructose mono-esters were studied at a concentration around the critical micelle concentration,22 which means that the surface concentration of the mono-esters was sufficiently high for the formation of the soft interfacial glass phase.23 The surface rheological characterization confirmed this. A low modulus was combined with a low exponent in the frequency dependency and a Lissajous plot that was mostly elastic with strain hardening during compression and strain softening during expansion. Di-esters led to a high modulus, combined with a high frequency dependency of the modulus and a fairly viscous and symmetric Lissajous plot.
When mono-esters and di-esters were mixed at a ratio of 0.8/0.2, the initial surface tension was lower than the surface tension obtained with either pure product. This shows that the presence of mono-esters increased the speed of migration of di-esters from the bulk to the interface, possibly through the formation of mixed micelles. The equilibrium surface tension was similar to that of the di-ester, indicating that there was a considerable amount of di-ester on the interface. We have found a low surface dilatational modulus, combined with intermediate frequency dependency and asymmetry in the Lissajous plots. The asymmetry was far less pronounced than in the case of the pure mono-ester but strain hardening during compression and strain softening during expansion were still observed. This might be caused by a structure composed of islands of mono-ester glass phase surrounded by a viscous phase formed by the di-ester. In the crude product, where the ratio between mono-esters and di-esters was higher compared to the previous case, there were a few differences. Here the surface tension was also similar to that of the di-ester but the modulus was high. The scaling exponent in the slope was low, and the Lissajous plot asymmetric, similar to those of the mono-ester. The hypothesis was formulated that here the interfacial structure is also composed of islands of glass phase present in a viscous phase formed by di-esters. However, in contrast to the previous case, here the surface fraction of di-esters was low, which means that the glassy patches formed by the mono-ester dominate the interface (Fig. 5).
The results show that there may be significant differences in the interfaces stabilized by crude products and purified products, which may have consequences on the application of the esters. Husband et al.61 have studied the interfacial and foaming properties of sucrose esters and found major differences between interfaces stabilized by pure mono-esters, pure di-esters and crude samples that resulted in important differences in foam stability. They found a higher foamability and foam stability of the crude sample compared to either component. Furthermore, they found that the addition of di-esters to mono-esters could improve the foaming properties to the level of the crude sample. Therefore, further research on the oligofructose esters should focus on the foaming properties.
Mixtures of a mono-ester with whey protein isolate
The functional properties of mixtures of surfactants and proteins at interfaces have been the subject of investigation for many years.70,85–87 One of the reasons for studying these mixtures is that protein formulations often contain traces of small lipid-like surfactants that are considered as contaminants.15 Since proteins generally lead to higher foam stability than small surfactants, proteins are the preferred substance for interface stabilization.6,10 However, small surfactants generally lower the surface tension more than proteins and will therefore always try to displace the proteins, leading to a dramatic decrease in stability when the interface is mixed.17 When virtually all protein is displaced the stability increases again.17,88,89Since the oligofructose fatty acid esters are intended to be food ingredients, it should be considered what happens when the ester is added to a protein-containing formulation. In contrast to other studies, in our study25 the ester is the preferred molecule at the interface instead of the protein. Because of the low surface tension combined with a high dilatational modulus,22,23 an oligofructose mono-ester with a chain length of 16 carbon atoms was used. Whey protein isolate was selected as a protein source, because it is a widely used protein in the food industry and because it is a globular protein, known to form a viscoelastic layer with a high modulus14 that might be difficult to displace. As expected, whey protein isolate formed an interfacial layer with an intermediate surface tension and a high dilatational modulus, which may be attributed to the formation of a viscoelastic network. The pure oligofructose fatty acid ester formed a layer with a lower surface tension than the one obtained with WPI at all three concentrations that were studied. The similarity in surface tension may be explained by the fact that all three concentrations were higher than the CMC.22 Furthermore, the interfaces had a high dilatational modulus, attributed to the presence of a soft interfacial glass phase formed by the oligofructose part of the molecule.23 The development of the modulus was highly irregular, with sometimes sudden decreases following a steady increase. These observations were attributed to the high brittleness of the interfacial layer, which means that the amplitude of deformation was too high, leading to structural rearrangements, and leading to a lower surface dilatational modulus after reaching equilibrium.23,90 Mixtures of protein and oligofructose fatty acid ester were studied at three different concentrations of the protein. Only at the highest bulk concentration of the protein did the surface tension obtained with the mixture deviate from the surface tension obtained with a pure oligofructose ester, indicating that there was a significant amount of protein at the interface. At all protein concentrations, the modulus of the mixed system was low. This shows that at the lowest protein bulk concentrations, the surface concentration of protein was low, but still high enough to prevent the formation of the soft interfacial glass phase by the oligofructose ester. We conclude that the stabilization mechanisms of proteins and oligofructose esters are mutually exclusive, which may have consequences on the foaming properties of their mixtures.
The foam stability of the systems was probed by aerating solutions containing the surface active substance from below in a glass foam tube (diameter 2 cm, glass grid at the bottom with pore size 40–100 μm, LGS BV, Ubbena, The Netherlands). After reaching a designated foam height, aeration was discontinued and the foam and liquid height as well as the foam structure were observed by the naked eye. The higher foam stability of the pure oligofructose ester compared to the protein was attributed to differences in the speed of surfactant adsorption and thus the surface tension reduction. Despite the low modulus that was obtained at the lowest protein concentrations, in these cases still a high foam stability was obtained. This may be attributed to the occurrence of the Gibbs–Marangoni mechanism. At the highest protein concentration, the surface concentration of protein was sufficiently high to interfere with the Gibbs–Marangoni mechanism, which resulted in a dramatic decrease in the foam stability.91,92
Because a fully developed WPI network may be more difficult to access by the esters,93,94 the next issue that was addressed was whether the oligofructose ester would be capable of displacing WPI. Immediately upon addition of the ester to the subphase of a maturated WPI stabilized interface, the surface tension and surface dilatational modulus dropped dramatically, indicating that the oligofructose ester could displace part of the WPI. Similar results were reported when studying mixed interfaces of milk proteins and sucrose esters.14 At the highest whey protein isolate concentration, a more connected network was formed that was more difficult to disrupt. In an attempt to gain understanding about the displacement mechanism, a similar experiment was performed while observing the interface using Brewster angle microscopy. The interface was immobile when stabilized by pure WPI. The mobility of the interface quickly increased after the oligofructose ester was injected below the surface, which supports the rheological results. Unfortunately, there were no other features visible that could increase the understanding of the displacement mechanism, possibly due to the small difference in relative refractivity or due to the small magnification of the camera.
Results showed that oligofructose esters have the potential to be used as a food additive, even in the presence of proteins. However, this statement needs to be further supported with experiments using different types of proteins, at different concentrations of surfactant and in actual food products.
5. Foaming properties
In this section the paper deals with the application of the oligofructose fatty acid esters as foam stabilizers.26 Depending on the surfactant structure, major differences in the functional properties were found. All esters were studied at the same bulk concentration. Oligofructose fatty acid esters containing short fatty acids of 4 and 8 carbon atoms have a relatively hydrophilic character and a relatively high affinity for the aqueous phase. They only start to form micelles in the bulk at concentrations much higher than the concentration that was used for the foaming experiments.22 Due to this, the interface could not be fully saturated when using these molecules. This led to incomplete surface coverage and high surface tensions, both at short time scales and after reaching equilibrium. In contrast, oligofructose mono-esters (both saturated and unsaturated chains) and di-esters containing fatty acids with chain lengths of 10 carbon atoms or longer all had a similar low surface tension at equilibrium, which means that they were able to completely saturate the interface. The fact that they could reach a low surface tension may be attributed to their higher affinity for the interface, which is a result of increased hydrophobicity.22 The similarity in surface tension may be attributed to the fact that the amount of area occupied by one molecule is determined by the size of the oligofructose part of the molecule.22 However, there were differences in the surface tension at short time scales that have not yet been addressed. For the esters with the highest hydrophobicity, which were the mono-esters with the longest fatty acid chains and the di-esters, the surface tensions at short time scales were higher (Table 3). These observations for the surface tension values had serious consequences on the foamability of the aqueous solutions of the esters. Only esters with intermediate hydrophobicity were able to generate foams with small bubbles and a monodisperse bubble size distribution, which resulted in high foam stability (Table 3). The conclusion that was drawn before,22 that esters of longer fatty acid chains are the most promising food-grade additives, is therefore not valid for this specific application as a foam stabilizer. For this application, it is more important that a surfactant is able to migrate to the interface quickly. It was shown that the surface tension at short time scales is the most accurate predictor of foam stability. However, at similar surface tensions at short time scales the foam stability of oligofructose esters was higher than the foam stability of sucrose esters.| Surfactant | Surface tension t = 0 (mN m−1) | Indication of bubble size (mm) | Foam half time (min) |
|---|---|---|---|
| OF–C4m | 70 ± 0.2 | 0 | 0 ± 0 |
| OF–C8m | 65 ± 1.2 | 0.1–2.6 | 17 ± 17 |
| OF–C10m | 41 ± 0.3 | 0.4 | 708 ± 29 |
| OF–C12m | 47 ± 2.0 | 0.4 | 690 ± 11 |
| OF–C16m | 40 ± 1.8 | 0.5 | 496 ± 197 |
| OF–C16:1m | 46 ± 1.7 | 0.5 | 597 ± 39 |
| OF–C18m | 53 ± 2.2 | 1.6 | 128 ± 15 |
| OF–C12d | 70 ± 4.1 | 0 | 0 ± 0 |
| S–C12m | 37 ± 1.0 | 0.4 | 716 ± 329 |
| S–C16 | 37 ± 0.1 | 1.3 | 245 ± 7 |
| S–C18 | 53 ± 0.5 | 3.5 | 61 ± 4 |
This is likely caused by the differences in the rheological response of the interfacial layers.23 Interfaces stabilized by sucrose esters had relatively low moduli, scaling exponents in the frequency dependency close to 0.5, and Lissajous plots that were fairly viscous and free from asymmetries. These results support the idea that interfaces were stabilized by the Gibbs–Marangoni mechanism. However, with increasing fatty acid chain length, the speed of surfactant adsorption also reduces, which will decrease the efficiency of this mechanism. The oligofructose esters, whose interfaces were characterized by relatively high moduli, low scaling exponents in the frequency dependency, and asymmetric Lissajous plots with strain hardening during compression and strain softening during expansion, will lead to interfaces with high mechanical stability and resistance to compression.
Results show that oligofructose esters may be used as high-performance foaming ingredients.
6. Concluding remarks
In this paper, we have shown that by using an oligosaccharide instead of a disaccharide as the hydrophilic head group in a sugar ester, dramatic changes in functionality are induced. When using an oligosaccharide, as a result of the larger hydrophilic group, interactions between the head groups are present on a much larger length scale than in the case of a disaccharide. This leads to the formation of an interfacial soft glass. This different interfacial microstructure has pronounced consequences on the rheological response of the interface, which eventually leads to differences in macroscopic properties. Oligofructose fatty acid esters are surface-active molecules that combine the best properties of both LMW surfactants and proteins, a low surface tension and a high dilatational modulus.Acknowledgements
This research forms part of the research program of the Dutch Polymer Institute DPI, project #687. We gratefully acknowledge Orafti (Tienen, Belgium) for supplying the oligofructose, Novozymes, the Netherlands B.V. (Amersfoort, the Netherlands) for supplying the lipase and Acatris (Bunschoten, the Netherlands) for supplying the RYOTO sucrose esters. The authors wish to thank Carmen Boeriu, Guus Frissen and Roy Delahaije for contributions to the preparation of the oligofructose esters. Furthermore, we wish to thank Karlijn Maas, Ricarda Enke and Ania Warczak for contributions to the functionality experiments. Finally, we wish to thank the Laboratory of Physical Chemistry and Colloid Science of Wageningen University for the use of the ellipsometer and Remco Fokkink for his help with the ellipsometry experiments.References
- N. Therdthai and W. Zhou, Food Sci. Technol. Res., 2003, 9, 219–226 CrossRef.
- H. D. Goff, Curr. Opin. Colloid Interface Sci., 2002, 7, 432–437 CrossRef CAS.
- G. M. Campbell and E. Mougeot, Trends Food Sci. Technol., 1999, 10, 283–296 CrossRef CAS.
- P. J. Wilde, Curr. Opin. Colloid Interface Sci., 2000, 5, 176–181 CrossRef CAS.
- S. A. Koehler, S. Hilgenfeldt and H. A. Stone, Langmuir, 2000, 16, 6327–6341 CrossRef CAS.
- D. Langevin, ChemPhysChem, 2008, 9, 510–522 CrossRef CAS PubMed.
- S. Hilgenfeldt, S. A. Koehler and H. A. Stone, Phys. Rev. Lett., 2001, 86, 4704–4707 CrossRef CAS.
- J. B. Bezelgues, S. Serieye, L. Crosset-Perrotin and M. E. Leser, Colloids Surf., A, 2008, 331, 56–62 CrossRef CAS PubMed.
- D. Monin, A. Espert and A. Colin, Langmuir, 2000, 16, 3873–3883 CrossRef CAS.
- B. S. Murray and R. Ettelaie, Curr. Opin. Colloid Interface Sci., 2004, 9, 314–320 CrossRef CAS PubMed.
- D. Langevin, Adv. Colloid Interface Sci., 2000, 88, 209–222 CrossRef CAS.
- B. S. Murray, Curr. Opin. Colloid Interface Sci., 2007, 12, 232–241 CrossRef CAS PubMed.
- M. A. Bos and T. Van Vliet, Adv. Colloid Interface Sci., 2001, 91, 437–471 CrossRef CAS.
- S. Rouimi, C. Schorsch, C. Valentini and S. Vaslin, Food Hydrocolloids, 2005, 19, 467–478 CrossRef CAS PubMed.
- A. Mackie and P. Wilde, Adv. Colloid Interface Sci., 2005, 117, 3–13 CrossRef CAS PubMed.
- C. Kotsmar, D. O. Grigoriev, F. Xu, E. V. Aksenenko, V. B. Fainerman, M. E. Leser and R. Miller, Langmuir, 2008, 24, 13977–13984 CrossRef CAS PubMed.
- P. Wilde, A. Mackie, F. Husband, P. Gunning and V. Morris, Adv. Colloid Interface Sci., 2004, 108–109, 63–71 CrossRef CAS PubMed.
- I. Kralova and J. Sjöblom, J. Dispersion Sci. Technol., 2009, 30, 1363–1383 CrossRef CAS.
- L. L. Schramm, E. N. Stasiuk and D. G. Marangoni, Annu. Rep. Prog. Chem., Sect. C: Phys. Chem., 2003, 99, 3–48 RSC.
- S. Soultani, S. Ognier, J. M. Engasser and M. Ghoul, Colloids Surf., A, 2003, 227, 35–44 CrossRef CAS.
- S. E. H. J. Van Kempen, C. G. Boeriu, H. A. Schols, P. De Waard, E. Van der Linden and L. M. C. Sagis, Food Chem., 2013, 138, 1884–1891 CrossRef CAS PubMed.
- S. E. H. J. Van Kempen, H. A. Schols, E. Van der Linden and L. M. C. Sagis, 2013, submitted.
- S. E. H. J. Van Kempen, H. A. Schols, E. Van der Linden and L. M. C. Sagis, Soft Matter, 2013, 9, 9579–9592 RSC.
- S. E. H. J. Van Kempen, H. A. Schols, E. Van der Linden and L. M. C. Sagis, J. Agric. Food Chem., 2013, 61, 7829–7837 CrossRef CAS PubMed.
- S. E. H. J. Van Kempen, K. Maas, H. A. Schols, E. Van der Linden and L. M. C. Sagis, Food Hydrocolloids, 2013, 32, 162–171 CrossRef CAS PubMed.
- S. E. H. J. Van Kempen, H. A. Schols, E. Van der Linden and L. M. C. Sagis, Food Biophysics , 2013, accepted Search PubMed.
- R. Ter Haar, H. A. Schols, L. A. M. Van den Broek, D. Sağlam, A. E. Frissen, C. G. Boeriu and H. Gruppen, J. Mol. Catal. B: Enzym., 2010, 62, 183–189 CrossRef CAS PubMed.
- L. M. C. Sagis, C. G. Boeriu, G. E. Frissen, H. A. Schols and P. A. Wierenga, Langmuir, 2008, 24, 359–361 CrossRef CAS PubMed.
- L. Cao, U. T. Bornscheuer and R. D. Schmid, J. Mol. Catal. B: Enzym., 1999, 6, 279–285 CrossRef CAS.
- D. B. Sarney and E. N. Vulfson, Trends Biotechnol., 1995, 13, 164–172 CrossRef CAS.
- D. Coulon, A. Ismail, M. Girardin and M. Ghoul, J. Mol. Catal. B: Enzym., 1998, 5, 45–48 CrossRef CAS.
- P. Degn, L. H. Pedersen, J. Ø. Duus and W. Zimmermann, Biotechnol. Lett., 1999, 21, 275–280 CrossRef CAS.
- S. Soultani, J.-M. Engasser and M. Ghoul, J. Mol. Catal. B: Enzym., 2001, 11, 725–731 CrossRef CAS.
- F. J. Plou, M. A. Cruces, M. Ferrer, G. Fuentes, E. Pastor, M. Bernabé, M. Christensen, F. Comelles, J. L. Parra and A. Ballesteros, J. Biotechnol., 2002, 96, 55–66 CrossRef CAS.
- S. Tarahomjoo and I. Alemzadeh, Enzyme Microb. Technol., 2003, 33, 33–37 CrossRef CAS.
- D. Coulon, A. Ismail, M. Girardin, B. Rovel and M. Ghoul, J. Biotechnol., 1996, 51, 115–121 CrossRef CAS.
- F. Cauglia and P. Canepa, Bioresour. Technol., 2008, 99, 4065–4072 CrossRef CAS PubMed.
- J. A. Arcos, M. Bernabé and C. Otero, Biotechnol. Bioeng., 1998, 57, 505–509 CrossRef CAS.
- P. Degn, K. L. Larsen, J. Ø. Duus, B. O. Petersen and W. Zimmermann, Carbohydr. Res., 2000, 329, 57–63 CrossRef CAS.
- M. Ferrer, J. Soliveri, F. J. Plou, N. López-Cortéz, D. Reyes-Duarte, M. Christensen, J. L. Copa-Patiño and A. Ballesteros, Enzyme Microb. Technol., 2005, 36, 391–398 CrossRef CAS PubMed.
- S. Šabeder, M. Habulin and Ž. Knez, J. Food Eng., 2006, 77, 880–886 CrossRef PubMed.
- K. E. Jaeger, B. W. Dijkstra and M. T. Reetz, Annu. Rev. Microbiol., 1999, 53, 315–351 CrossRef CAS PubMed.
- D. B. Sarney, M. J. Barnard, D. A. MacManus and E. N. Vulfson, J. Am. Oil Chem. Soc., 1996, 73, 1481–1487 CrossRef CAS.
- K. Nott, A. Brognaux, G. Richard, P. Laurent, A. Favrelle, C. Jérôme, C. Blecker, J. P. Wathelet, M. Paquot and M. Deleu, Prep. Biochem. Biotechnol., 2012, 42, 348–363 CrossRef CAS PubMed.
- T. Chaiyaso, A. H. -kittikun and W. Zimmermann, J. Ind. Microbiol. Biotechnol., 2006, 33, 338–342 CrossRef CAS PubMed.
- A. Widjaja, T. H. Yeh and Y. H. Ju, J. Chin. Inst. Chem. Eng., 2008, 39, 413–418 CrossRef CAS PubMed.
- M. Woudenberg-Van Oosterom, F. Van Rantwijk and R. A. Sheldon, Biotechnol. Bioeng., 1996, 49, 328–333 CrossRef CAS.
- L. Cao, A. Fischer, U. T. Bornscheuer and R. D. Schmid, Biocatal. Biotransform., 1997, 14, 269–283 CrossRef CAS.
- J. Pleiss, M. Fischer and R. D. Schmid, Chem. Phys. Lipids, 1998, 93, 67–80 CrossRef CAS.
- J. F. Cramer, M. S. Dueholm, S. B. Nielsen, D. S. Pedersen, R. Wimmer and L. H. Pedersen, Enzyme Microb. Technol., 2007, 41, 346–352 CrossRef CAS PubMed.
- N. R. Pedersen, R. Wimmer, J. Emmersen, P. Degn and L. H. Pedersen, Carbohydr. Res., 2002, 337, 1179–1184 CrossRef CAS.
- M. Ferrer, F. Comelles, F. J. Plou, M. A. Cruces, G. Fuentes, J. L. Parra and A. Ballesteros, Langmuir, 2002, 18, 667–673 CrossRef CAS.
- H. Razafindralambo, C. Blecker, S. Mezdour, C. Deroanne, J. M. Crowet, R. Brasseur, L. Lins and M. Paquot, J. Phys. Chem. B, 2009, 113, 8872–8877 CrossRef CAS PubMed.
- A. Franck, Br. J. Nutr., 2002, 87, S287–S291 CrossRef CAS PubMed.
- K. R. Niness, J. Nutr., 1999, 129, 1402S–1406S CAS.
- H. Wennerström and B. Lindman, Phys. Rep., 1979, 52, 1–86 CrossRef.
- A. Ducret, A. Giroux, M. Trani and R. Lortie, J. Am. Oil Chem. Soc., 1996, 73, 109–113 CrossRef CAS.
- T. Zhang and R. E. Marchant, J. Colloid Interface Sci., 1996, 177, 419–426 CrossRef CAS.
- C. J. Drummond and D. Wells, Colloids Surf., A, 1998, 141, 131–142 CrossRef CAS.
- G. Garofalakis, B. S. Murray and D. B. Sarney, J. Colloid Interface Sci., 2000, 229, 391–398 CrossRef CAS PubMed.
- F. A. Husband, D. B. Sarney, M. J. Barnard and P. J. Wilde, Food Hydrocolloids, 1998, 12, 237–244 CrossRef CAS.
- N. Becerra, C. Toro, A. L. Zanocco, E. Lemp and G. Günther, Colloids Surf., A, 2008, 327, 134–139 CrossRef CAS PubMed.
- L. Choplin, V. Sadtler, P. Marchal, D. Sfayhi, M. Ghoul and J. M. Engasser, J. Colloid Interface Sci., 2006, 294, 187–193 CrossRef CAS PubMed.
- L. Yanke, Z. Shufen, W. Qinghui and Y. Jinzong, Tenside, Surfactants, Deterg., 2004, 41, 26–30 Search PubMed.
- C. Blecker, S. Piccicuto, G. Lognay, C. Deroanne, M. Marlier and M. Paquot, J. Colloid Interface Sci., 2002, 247, 424–428 CrossRef CAS PubMed.
- C. Kotsmar, V. Pradines, V. S. Alahverdjieva, E. V. Aksenenko, V. B. Fainerman, V. I. Kovalchuk, J. Krägel, M. E. Leser, B. A. Noskov and R. Miller, Adv. Colloid Interface Sci., 2009, 150, 41–54 CrossRef CAS PubMed.
- D. O. Grigoriev, S. Derkatch, J. Krägel and R. Miller, Food Hydrocolloids, 2007, 21, 823–830 CrossRef CAS PubMed.
- L. Liggieri and R. Miller, Curr. Opin. Colloid Interface Sci., 2010, 15, 256–263 CrossRef CAS PubMed.
- R. Miller, J. K. Ferri, A. Javadi, J. Krägel, N. Mucic and R. Wüstneck, Colloid Polym. Sci., 2010, 288, 937–950 CAS.
- E. Dickinson, R. Ettelaie, B. S. Murray and Z. Du, J. Colloid Interface Sci., 2002, 252, 202–213 CrossRef CAS PubMed.
- P. Erni, P. Fischer and E. J. Windhab, Langmuir, 2005, 21, 10555–10563 CrossRef CAS PubMed.
- A. Torcello-Gómez, J. Maldonado-Valderrama, M. J. Gálvez-Ruiz, A. Martín-Rodríguez, M. A. Cabrerizo-Vílchez and J. De Vicente, Journal of Non-Newtonian Fluid Mechanics, 2011, 166, 713–722 Search PubMed.
- G. Garofalakis and B. S. Murray, Langmuir, 2002, 18, 4765–4774 CrossRef CAS.
- P. Cicuta, E. J. Stancik and G. G. Fuller, Phys. Rev. Lett., 2003, 90, 2361011–2361014 CrossRef.
- J. Lucassen and M. Van Den Tempel, Chem. Eng. Sci., 1972, 27, 1283–1291 CrossRef CAS.
- N. P. K. Humblet-Hua, E. Van Der Linden and L. M. C. Sagis, Soft Matter, 2013, 9, 2154–2165 RSC.
- R. H. Ewoldt, A. E. Hosoi and G. H. McKinley, J. Rheol., 2008, 52, 1427–1458 CrossRef CAS.
- P. Erni and A. Parker, Langmuir, 2012, 28, 7757–7767 CrossRef CAS PubMed.
- P. A. Rühs, C. Affolter, E. J. Windhab and P. Fischer, J. Rheol., 2013, 57, 1003–1022 CrossRef.
- L. M. C. Sagis, Eur. Phys. J.: Spec. Top., 2013, 222, 31–38 CrossRef.
- E. Van Der Linden, L. Sagis and P. Venema, Curr. Opin. Colloid Interface Sci., 2003, 8, 349–358 CrossRef CAS.
- V. M. Kaganer, H. Möhwald and P. Dutta, Rev. Mod. Phys., 1999, 71, 779–819 CrossRef CAS.
- L. V. N. Avila, S. M. Saraiva and J. F. Oliveira, Colloids Surf., A, 1999, 154, 209–217 CrossRef CAS.
- K. Golemanov, N. D. Denkov, S. Tcholakova, M. Vethamuthu and A. Lips, Langmuir, 2008, 24, 9956–9961 CrossRef CAS PubMed.
- E. Dickinson, Food Hydrocolloids, 2011, 25, 1966–1983 CrossRef CAS PubMed.
- G. Garofalakis and B. S. Murray, Colloids Surf., B, 2001, 21, 3–17 CrossRef CAS.
- J. Maldonado-Valderrama and J. M. Rodríguez Patino, Curr. Opin. Colloid Interface Sci., 2010, 15, 271–282 CrossRef CAS PubMed.
- J. Chen and E. Dickinson, Colloids Surf., A, 1995, 100, 267–277 CrossRef CAS.
- E. Dickinson and S. Tanai, J. Agric. Food Chem., 1992, 40, 179–183 CrossRef CAS.
- L. M. C. Sagis, Rev. Mod. Phys., 2011, 83, 1367–1403 CrossRef CAS.
- J. Krägel, S. R. Derkatch and R. Miller, Adv. Colloid Interface Sci., 2008, 144, 38–53 CrossRef PubMed.
- B. Rippner Blomqvist, M. J. Ridout, A. R. Mackie, T. Wärnheim, P. M. Claesson and P. Wilde, Langmuir, 2004, 20, 10150–10158 CrossRef CAS PubMed.
- L. A. Pugnaloni, E. Dickinson, R. Ettelaie, A. R. Mackie and P. J. Wilde, Adv. Colloid Interface Sci., 2004, 107, 27–49 CrossRef CAS PubMed.
- R. Ettelaie, E. Dickinson, Z. Du and B. S. Murray, J. Colloid Interface Sci., 2003, 263, 47–58 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |