A facile bacterial assisted electrochemical self-assembly of polypyrrole micro-pillars: towards underwater low adhesive superoleophobicity†
Zhe
Cheng‡
a,
Chunmei
Ding‡
a,
Huan
Liu
*a,
Ying
Zhu
a and
Lei
Jiang
ab
aKey Laboratory of Bio-Inspired Smart Interfacial Science and Technology of Ministry of Education, School of Chemistry and Environment, Beihang University, Beijing, 100191, P. R. China. E-mail: liuh@buaa.edu.cn
bBeijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China
First published on 14th October 2013
Abstract
By taking advantage of bacterial extracellular electron transfer behavior, a facile method was developed to fabricate oriented polypyrrole micro-pillars (PPy-MP) with nanoscale surface roughness. Microbes acted as a living conductive template on which PPy was in situ polymerized. The as-prepared PPy-MP exhibit the distinctive underwater low adhesive superoleophobicity which is attributable to the unique hierarchical micro/nano-structures and the high surface energy by doping with inorganic small anions.
Underwater superoleophobic surfaces, especially those with low adhesive force, are of paramount importance for practical applications in areas of self-cleaning of marine equipment,1 oil/water separation,2 anti-bioadhesion,3 lab-on-chip devices,4 and small droplet manipulations,5etc. Thus it has attracted extensive research attention recently.6 Many biological surfaces as fish skin,7 nacre's shell8 and the pallium-covered region of a short clam's shell9 have been recently revealed to bear the robust oil repellency in aqueous medium that were aroused from the typical surface structures and the high-energy nature of material itself like inorganic composites of CaCO3, Ca3(PO4)2, hydrophilic proteins and polymers.1,7–10 Drawing inspiration from these natural phenomena, incorporation of micro-/nano- surface structures and high energy materials is a valid approach for fabricating underwater superoleophobic surfaces, as has been well demonstrated recently.11 So far, many kinds of underwater superoleophobic surfaces have been developed based on this concept, for example, hydrogel,1a,7,11c,12 colloidal crystal,13 metal oxide,14 conducting polymer15 and so on. Among all the materials, conducting polymers with special wettability have attracted much research interest for their broad technological applications as biosensors,16 energy conversion devices17 and artificial muscles18 where interfacial interactions are greatly involved. Recent advances have demonstrated that tunable adhesion to oil droplets could be modulated in aqueous medium by applying potential on a polyaniline film.5a Besides, in situ electrochemical switching between being superoleophobic and superoleophilic underwater was realized on a polypyrrole (PPy) film made by a template method.19 Very recently, we fabricated a free-standing janus PPy film which exhibited underwater anisotropic superoleophobicity.20 However, preparing a robust, low adhesive superoleophobic surface from conducting polymers directly remains a challenge so far.
Extracellular electron transfer (EET), especially featured by members of the Gram-negative bacteria genus, Shewanella, is an important process involved in various natural biochemical and geological events as natural bioremediation and biogeochemical cycling of minerals.21Shewanella can respire on diverse solid state electron acceptors directly via outer membrane c-type cytochromes.22 Recently, bacterial EET has shown promising applications in fabricating various nano-materials like selenium,23 silver,24 palladium,25meta-schoepite26 and arsenic-sulfide27 where a reduction process is involved. However, preparing polymers with certain micro-/nano-structures in terms of bacteria EET is rarely reported; that might be because of the involvement of an oxidation process during polymerization.
Herein, by taking advantage of bacterial EET behaviour, we developed a facile approach to prepare oriented polypyrrole micro-pillars (PPy-MP) with nanoscale surface roughness. As is well known, microbes prefer to move to a biased anode where the metabolic electrons can be released and thus the bioactivity was maintained.28 By this property, we combined the bacterial EET and a traditional electrochemical polymerization method to fabricate a structure-controlled PPy film by properly modulating the polymerization condition. As a result, a PPy-MP film was successfully prepared, and what's more, it embodied a distinctive oil resistant property in water with its superoleophobicity and low adhesion.
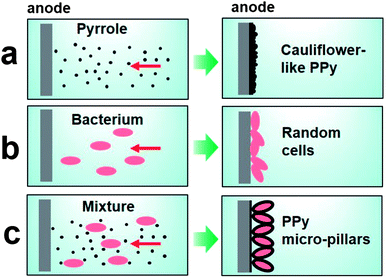
As schematically illustrated in Fig. 1, a random structured PPy film was expected when using pyrrole as the only reactant in a constant potential procedure (Fig. 1a), as we previously suggested.19 On the other hand, bacteria of Shewanella were prone to lie on the anode randomly where usually a biofilm was formed to release metabolic electrons29 when solely added in electrolyte (Fig. 1b). Therefore, anodes involved in these two processes play a similar role by uptaking electrons from pyrrole to oxidize it, as well as from microbes to maintain its metabolic activities. Therefore, we proposed a bacterial EET assisted self-assembly approach where the mixture of pyrrole and microbes with a well tuned ratio were added into the electrolyte (Fig. 1c). With a poised anode, pyrrole and microbes moved to the anode simultaneously in the process of electro-polymerization. As the speed of PPy polymerization was very fast,19 a thin layer of PPy was formed first on the anode. Besides, the bacteria can be regarded as tiny electrodes once they contacted the surface of the anode because of the conductivity of bacteria and PPy.30 Thus, the PPy was polymerized on the surface of bacteria that were fixed on the electrode and oriented mostly in the long axis direction (Fig. 1c). As a result, oriented PPy-MP were prepared.
Here, PPy-MP was synthesized by means of a galvanostatic current procedure (0.05 mA cm2, 5 h) in an electrolyte solution containing pyrrole, a certain amount of microbes and L-alanine in defined medium using L-alanine as the carbon source. An Au plate was used as a working electrode while a platinum plate and saturated calomel electrode were used as the counter and reference electrode, respectively. There was a visible change on the surface of the anode after reaction, indicating the formation of a PPy film. FT-IR spectroscopy was performed to further confirm the chemical nature of the PPy film (Fig. S1†).
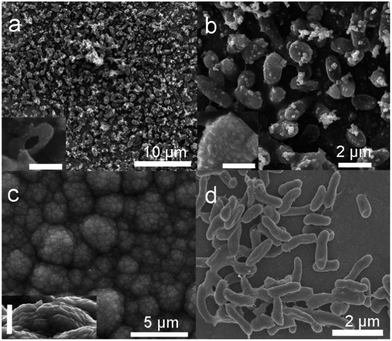
Fig. 2a shows a representative SEM image of the as-prepared PPy-MP film in large scale, where ordered and patterned structures were observed. The high-magnified SEM image (Fig. 2b, S2†) clearly suggested that the patterned structure is arisen from the arranged microbes clothed by PPy homogeneously (inset of Fig. 2b). That is to say, PPy was shaped into micro-pillar arrays by using the living microbes as templates during the polymerization process. It can be directly evidenced by the hollow PPy micro-pillar where the inner microbe was dehydrated and shrunk by the vacuum process during the SEM sample preparation (inset of Fig. 2a). To further confirm the role of the microbe, the same experiment was performed without microbes, and the as-prepared PPy film was shown in Fig. 2c. The film showed distinct different topological structures where the cauliflower-shaped PPy covered the entire surface. The cauliflower-like PPy exhibited clearly smaller surface roughness compared with that of PPy-MP. The SEM image of the biofilm when only microbes were added into the electrolyte (Fig. 2d) revealed the random distribution of microbes, as well the matched dimensions with that of PPy-MP, further confirming the role of the microbe as a living template.
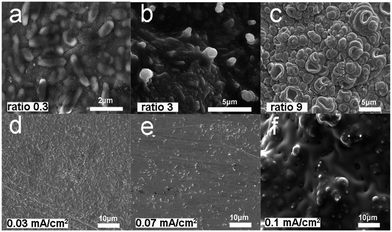
To explore how the concentration ratio of pyrrole to bacteria influences the morphology of PPy films, a variety of ratios (pyrrole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bacteria) were used from 0.3, 1.5, 3 to 9 (see detail in Experimental section) to prepare a PPy film with a constant current density of 0.05 mA cm−2 in the presence of microbes. As shown in Fig 3a–c, with increasing the ratio, the distinguishable contour profile of microbes is gradually diminished. Many visible contour profiles of microbes were observed (52 ± 6 per 100 μm2) when the ratio was 0.3 (Fig. 3a), while nearly no visible contour profile of microbes can be clearly viewed when the ratio was as high as 9 (Fig. 3c). Moreover, we also investigate the current density effect on the morphology of PPy films. When the concentration ratio of pyrrole to bacteria was fixed as 1.5, increasing current density from 0.03, 0.05, 0.07 to 0.1 mA cm−2 can also lead to a decrease of the visible contour profile of microbes (Fig. 3d–f). The number of visible bacteria was 54 ± 5 per 100 μm2 when the current density was set to 0.03 mA cm−2 (Fig. 3d). However, there were no visible bacteria on the electrode when the current density became 0.1 mA cm−2 (Fig. 3f). According to the results shown above, the most suitable condition to synthesize PPy-MP can be set as a pyrrole
bacteria) were used from 0.3, 1.5, 3 to 9 (see detail in Experimental section) to prepare a PPy film with a constant current density of 0.05 mA cm−2 in the presence of microbes. As shown in Fig 3a–c, with increasing the ratio, the distinguishable contour profile of microbes is gradually diminished. Many visible contour profiles of microbes were observed (52 ± 6 per 100 μm2) when the ratio was 0.3 (Fig. 3a), while nearly no visible contour profile of microbes can be clearly viewed when the ratio was as high as 9 (Fig. 3c). Moreover, we also investigate the current density effect on the morphology of PPy films. When the concentration ratio of pyrrole to bacteria was fixed as 1.5, increasing current density from 0.03, 0.05, 0.07 to 0.1 mA cm−2 can also lead to a decrease of the visible contour profile of microbes (Fig. 3d–f). The number of visible bacteria was 54 ± 5 per 100 μm2 when the current density was set to 0.03 mA cm−2 (Fig. 3d). However, there were no visible bacteria on the electrode when the current density became 0.1 mA cm−2 (Fig. 3f). According to the results shown above, the most suitable condition to synthesize PPy-MP can be set as a pyrrole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bacteria ratio of 1.5, and current density of 0.05 mA cm−2.
bacteria ratio of 1.5, and current density of 0.05 mA cm−2.
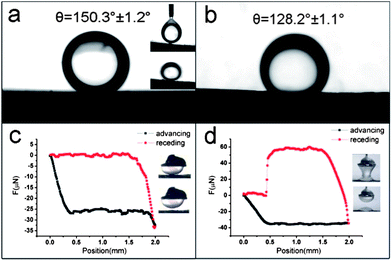
The surface wettability of PPy-MP and cauliflower-like PPy films were characterized by contact angle (CA) measurements in air and underwater. Both of them were superhydrophilic in air, (Fig. S3†) indicating the high energy of as-obtained PPy surfaces.31 In the underwater experiment, 1,2-dichloroethane was used as a probe oil for the measurement. The results showed that the PPy-MP films were superoleophobic with a CA of 150.3 ± 1.2° (Fig. 4a) while the cauliflower-like PPy films presented an oleophobic property with a CA of 128.2 ± 1.1°(Fig. 4b). To be noted, the oil droplets could not adhere to the PPy-MP films and rolled off the surface easily (inset in Fig. 4a). In contrast, the oil droplets adhered tightly on the cauliflower-like films. The adhesive force between oil and substrates was measured by using a high sensitivity micro-electromechanical balance system (Fig. 4c and d). As is shown in Fig. 4c, the adhesive force of oil on the PPy-MP film was too low to be detected (≈0 μN). Nearly no deformation of the oil droplet occurred when detaching from the film (inset of Fig 4c). However, the oil droplet was out of shape when leaving the cauliflower-like PPy film, giving an oil adhesive force of about 60.3 ± 1.5 μN (Fig 4d).
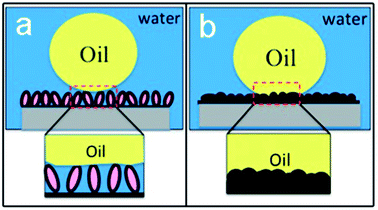
The wettability of a solid surface was commonly governed by the surface energy, which depends on the surface chemical composition and surface topographic roughness. Interestingly, surface roughness can enhance the wettability (hydro-/oleo- phobicity and philicity) and tune the dynamic wetting behavior as well.1a Here, by doping inorganic small anion Cl−, PPy exhibited enhanced water-favoring property due to the high energy,31 compared with that doped with organic large anions of dodecyl benzenesulfonic acid.19,32 The X-ray photoelectron spectroscopy (XPS) spectrum of PPy-MP proves that the inorganic anion Cl− is doped in as-prepared film (Fig. S4†). When an oil droplet was placed on PPy-MP underwater (Fig. 5a), the high content of water can be trapped within the highly textured hierarchical micro-/nano- structures of PPy-MPA films, which results in a minimized contact area between the oil and the solid surface, and hence a discrete three-phase contact line (TCL). The oil droplet maintained a sphere shape on PPy-MP, forming a typical Cassie–Baxter contact. Therefore, the PPy-MP film enables underwater superoleophobicity with undetectable oil adhesion. For a cauliflower-like PPy film, the surface roughness is too small to trap water efficiently in between the oil–solid interface (Fig. 5b), giving a typical Wenzel-contact with a continuous TCL. This gives rise to the high affinity with oil droplet.
In summary, a facile method for fabricating oxidation state PPy-MP was demonstrated by using the microbe as a living template. Through fine tuning the polymerization condition, the micro-scaled dimension of microbes was well maintained, while the nanoscale surface roughness in the normal polymerization process was also kept, thus forming a typical highly textured surface roughness for the as-prepared PPy-MP. The PPy-MPs enable a robust underwater superoleophobicity with low adhesive force. Here, the concept of using bacteria as a template reveals a new approach to fabricate micro/nano-scale structured materials for those that can be prepared by an electrochemical method. The as-resulted typical hierarchical micro-/nano-structures are desirable for various special wetting behaviours.
Experimental section
Microbe preparation
Shewanella loihica PV-4 was cultured aerobically in 20 mL of marine broth (MB) (20 g L−1) at 30 °C for 24 h. Cells were collected by centrifugation, and then placed into fresh defined medium at 30 °C for another 24 h using sodium lactate (10 mM L−1) as the sole carbon source (DM: NaHCO3 (2.5 g), CaCl2·2H2O (0.08 g), NH4Cl (1.0 g), MgCl2·6H2O (0.2 g), NaCl (10 g), and HEPES (7.2 g) per liter, pH 7.8). Microbes were washed three times with defined medium before electro-polymerization.PPy-MP electrode preparation
Pyrrole (Acros Co.) was distilled under vacuum prior to use. L-alanine and other chemicals were commercially available analytical grade reagents (Beijing Chemical, Co.) and were used without further purification. Ultrapure water (18.2 MΩ cm) was acquired using a Milli-Q water purification system (Millipore). PPy-MP was synthesized by a procedure involving electrochemical oxidative polymerization using galvanostatic current. A Au plate was used as a working electrode to prepare PPy-MP films using a Galvan static current procedure (0.05 mA cm2, 5 h) in an electrolyte solution containing 0.1 M L-alanine, pyrrole, and bacteria in defined medium where concentration ratios of pyrrole to bacteria were finely controlled. Herein, ratio was defined for concentration of pyrrole (mol L−1) to the OD600 of bacteria. A platinum plate and saturated calomel electrode were used as the counter and reference electrode, respectively. The temperature during the experiment ranged from 22 °C to 25 °C. After electrochemical polymerization, the working electrode was removed from the electrolyte solution, washed with ultrapure water and then dried under air for further use.Characterization
The morphology of the as-prepared film was investigated by a field-emission scanning electronic microscope (SEM) (JEOL 7500F, Japan). Contact angle (CA) measurements were conducted using a data-physics OCA20 contact angle system (Data-Physics, Germany). Drops of 1.5 μL 1,2-dichloroethane were used as the probe liquids for the underwater oil CA measurements, and the values reported were the average of at least five drops per sample at different locations. The adhesive force that prevented the oil droplet being drawn away from the surface was measured by using a high-sensitivity micro-electromechanical balance system (Data-Physics DCAT 11, Germany).Acknowledgements
This work was supported by the 973 Program (2013CB933000, 2010CB934701), Fok Ying Tong Education Foundation (132008), National Natural Science Foundation of China (20974006), Fundamental Research Funds for BeiHang University (300704) and the Innovation Foundation of BUAA for PhD Graduates (296181).Notes and references
- (a) M. Liu, S. Wang, Z. Wei, Y. Song and L. Jiang, Adv. Mater., 2009, 21, 665 CrossRef CAS; (b) S. Nishimoto and B. Bhushan, RSC Adv., 2013, 3, 671 RSC; (c) J. A. Callow and M. E. Callow, Nat. Commun., 2011, 2, 244 CrossRef PubMed.
- L. Zhang, Z. Zhang and P. Wang, NPG Asia Mater., 2012, 4, e8 CrossRef.
- L. Chen, M. Liu, H. Bai, P. Chen, F. Xia, D. Han and L. Jiang, J. Am. Chem. Soc., 2009, 131, 10467 CrossRef CAS PubMed.
- K. Tsougeni, D. Papageorgiou, A. Tserepi and E. Gogolides, Lab Chip, 2010, 10, 462 RSC.
- (a) C. Ding, Y. Zhu, M. Liu, L. Feng, M. Wan and L. Jiang, Soft Matter, 2012, 8, 9064 RSC; (b) B. Su, S. Wang, Y. Song and L. Jiang, Soft Matter, 2011, 7, 5144 RSC.
- (a) Y. C. Jung and B. Bhushan, Langmuir, 2009, 25, 14165 CrossRef CAS PubMed; (b) B. Bhushan and G. D. Bixler, Nanoscale, 2013, 5, 7685 RSC.
- L. Lin, M. Liu, L. Chen, P. Chen, J. Ma, D. Han and L. Jiang, Adv. Mater., 2010, 22, 4826 CrossRef CAS PubMed.
- L. Xu, J. Peng, Y. Liu, Y. Wen, X. Zhang, L. Jiang and S. Wang, ACS Nano, 2013, 7, 5077 CrossRef CAS PubMed.
- X. Liu, J. Zhou, Z. Xue, J. Gao, J. Meng, S. Wang and L. Jiang, Adv. Mater., 2012, 24, 3401 CrossRef CAS PubMed.
- K. Y. Tan, T. L. Hughes, M. Nagl and W. T. S. Huck, ACS Appl. Mater. Interfaces, 2012, 4, 6403 CAS.
- (a) G. Zhang, X. Zhang, Y. Huang and Z. Su, ACS Appl. Mater. Interfaces, 2013, 5, 6400 CrossRef CAS PubMed; (b) Y. Zhu, F. Zhang, D. Wang, X. Pei, W. Zhang and J. Jin, J. Mater. Chem. A, 2013, 1, 5758 RSC; (c) L. Chen, M. Liu, L. Lin, T. Zhang, J. Ma, Y. Song and L. Jiang, Soft Matter, 2010, 6, 2708 RSC.
- Z. Xue, S. Wang, L. Lin, L. Chen, M. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270 CrossRef CAS.
- Y. Huang, M. Liu, J. Wang, J. Zhou, L. Wang, Y. Song and L. Jiang, Adv. Funct. Mater., 2011, 21, 4436 CrossRef CAS.
- D. Tian, X. Zhang, Y. Tian, Y. Wu, X. Wang, J. Zhai and L. Jiang, J. Mater. Chem., 2012, 22, 19652 RSC.
- J. A. Halldorsson, S. J. Little, D. Diamond, G. Spinks and G. Wallace, Langmuir, 2009, 25, 11137 CrossRef CAS PubMed.
- B. C. Thompson, J. Chen, S. E. Moulton and G. G. Wallace, Nanoscale, 2010, 2, 499 RSC.
- J. Huang, K. Wang and Z. Wei, J. Mater. Chem., 2010, 20, 1117 RSC.
- (a) J. Liu, Z. Wang, Y. Zhao, H. Cheng, C. Hu, L. Jiang and L. Qu, Nanoscale, 2012, 4, 7563 RSC; (b) E. Smela, Adv. Mater., 2003, 15, 481 CrossRef CAS.
- M. Liu, F. Nie, Z. Wei, Y. Song and L. Jiang, Langmuir, 2010, 26, 3993 CrossRef CAS PubMed.
- J. Song, H. Liu, M. Wan, Y. Zhu and L. Jiang, J. Mater. Chem. A, 2013, 1, 1740 CAS.
- (a) K. H. Nealson and D. Saffarini, Annu. Rev. Microbiol., 1994, 48, 311 CrossRef CAS PubMed; (b) B. E. Logan, Nat. Rev. Microbiol., 2009, 7, 375 CrossRef CAS PubMed; (c) D. R. Lovley, Geobiology, 2008, 6, 225 CrossRef CAS PubMed.
- (a) J. K. Fredrickson, M. F. Romine, A. S. Beliaev, J. M. Auchtung, M. E. Driscoll, T. S. Gardner, K. H. Nealson, A. L. Osterman, G. Pinchuk, J. L. Reed, D. A. Rodionov, J. L. M. Rodrigues, D. A. Saffarini, M. H. Serres, A. M. Spormann, I. B. Zhulin and J. M. Tiedje, Nat. Rev. Microbiol., 2008, 6, 592 CrossRef CAS PubMed; (b) R. Nakamura, K. Ishii and K. Hashimoto, Angew. Chem., Int. Ed., 2009, 48, 1606 CrossRef CAS PubMed.
- C. T. Ho, J. W. Kim, W. B. Kim, K. Song, R. A. Kanaly, M. J. Sadowsky and H. G. Hur, J. Mater. Chem., 2010, 20, 5899 RSC.
- A. K. Suresh, D. A. Pelletier, W. Wang, J. W. Moon, B. Gu, N. P. Mortensen, D. P. Allison, D. C. Joy, T. J. Phelps and M. J. Doktycz, Environ. Sci. Technol., 2010, 44, 5210 CrossRef CAS PubMed.
- X. Wu, F. Zhao, N. Rahunen, J. R. Varcoe, C. Avignone-Rossa, A. E. Thumser and R. C. T. Slade, Angew. Chem., Int. Ed., 2011, 50, 427 CrossRef CAS PubMed.
- S. Jiang, M. G. Kim, S. J. Kim, H. S. Jung, S. W. Lee, D. Y. Noh, M. J. Sadowsky and H. G. Hur, Chem. Commun., 2011, 47, 8076 RSC.
- J. H. Lee, M. G. Kim, B. Yoo, N. V. Myung, J. Maeng, T. Lee, A. C. Dohnalkova, J. K. Fredrickson, M. J. Sadowsky and H. G. Hur, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20410 CrossRef CAS PubMed.
- U. Schröder, ChemSusChem, 2012, 5, 959 CrossRef PubMed.
- A. P. Borole, G. Reguera, B. Ringeisen, Z. W. Wang, Y. Feng and B. H. Kim, Energy Environ. Sci., 2011, 4, 4813 CAS.
- C. Ding, H. Liu, Y. Zhu, M. Wan and L. Jiang, Energy Environ. Sci., 2012, 5, 8517 CAS.
- B. P. Binks and J. H. Clint, Langmuir, 2002, 18, 1270 CrossRef CAS.
- M. Liu, X. Liu, C. Ding, Z. Wei, Y. Zhu and L. Jiang, Soft Matter, 2011, 7, 4163 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: The shape of a water drop on PPy-MPA and cauliflower-like PPy film in air. See DOI: 10.1039/c3nr03788f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |