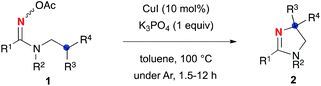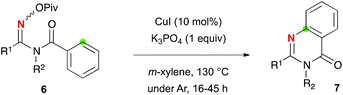Copper-catalyzed redox-neutral C–H amination with amidoximes†
Hui
Chen
and
Shunsuke
Chiba
*
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore. E-mail: shunsuke@ntu.edu.sg; Fax: +65-67911961
First published on 1st November 2013
Abstract
CuI-catalyzed reactions of N-alkylamidoximes afforded dihydroimidazoles via sp3 C–H amination. On the other hand, the reactions of N-benzoylamidoximes resulted in sp2 C–H amination to form quinazolinones. The reaction mechanisms could be characterized as a redox-neutral radical pathway including a Cu(I)–Cu(II) redox catalytic cycle.
Abundance of nitrogen-containing heterocycles (azaheterocycles) in biologically active alkaloids and pharmaceutical drugs has stimulated synthetic chemists to develop a variety of reactions enabling efficient construction of them.1,2 Among these methodologies, intramolecular C–H amination offers atom- and step-economical routes to access the azaheterocyclic frameworks, and various sets of the strategies have recently been exploited, especially with transition-metal catalysts.3
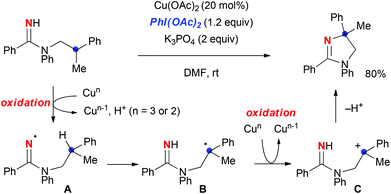
Our group has worked on the development of C–H oxidation based on radical-mediated (non-nitrene-based) tactics,4 where we have recently made use of amidine derivatives for Cu-catalyzed aliphatic C–H oxidation reactions towards the synthesis of azaheterocycles.5,6 For example, we disclosed Cu-catalyzed PhI(OAc)2-mediated C–H amination of N-alkyl amidines for the synthesis of dihydroimidazoles (Scheme 1).5a This strategy is initiated by the 1,5-H-radical shift of amidinyl radical A oxidatively generated from the amidine, which results in the formation of C-radical B. Further oxidation of C-radical B leads to carbocation C that is subsequently aminated to give the dihydroimidazole. However, the drawback of this reaction is that it requires a stoichiometric use of PhI(OAc)2 to maintain the catalytic turnover, obviously because of the redox nature of this strategy, needing two-electron oxidation (for the generation of amidinyl radical A from the amidine and the oxidation of transient C-radical B to carbocation C) to realize the aliphatic C–H amination.
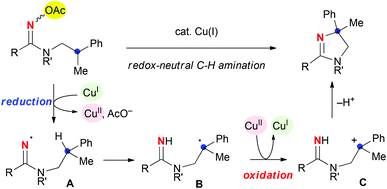
To develop an entirely catalytic system for the C–H amination, employment of amidoximes as a precursor of the amidinyl radical A was envisioned.7 As shown in Scheme 2, the reaction is initiated by reduction of the oxime N–O bond with Cu(I) species, generating amidinyl radicals A along with Cu(II) species.8 After the 1,5-H shift, the resulting C-radicals B are oxidized to the carbocation C by the Cu(II)9 to result in the formation of dihydroimidazole products and re-generation of Cu(I) species. This reductive initiation–oxidative termination sequence with amidoximes potentially enables redox-neutral C–H amination.10–13
With the hypothesis outlined in Scheme 2, our investigation commenced with the reaction of amidoxime 1a for the construction of dihydroimidazole 2a (Table 1). Amidoxime 1a was synthesized by treatment of benzaldoxime with NCS followed by the secondary amine (see the ESI† for more details) and was obtained as a 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of E/Z isomers (the stereochemistry was not determined). First of all, the major isomer of oxime 1a was utilized for the optimization of the reaction conditions. Treatment of amidoxime 1a with a series of Cu(I) salts as a catalyst (20 mol%) in the presence of K3PO4 in DMF resulted in the formation of 2a in moderate to good yields (entries 1–6) and the best yield of 2a was provided by CuI (72% yield, entry 3). Screening of the solvents (entries 7–9) revealed that toluene is optimal for this transformation (entry 9). The catalyst loading of CuI could be reduced to 10 mol% in toluene, giving 2a in 76% yield (entry 10). These optimized reaction conditions (10 mol% of CuI in toluene at 100 °C) were applicable to the minor isomer of amidoxime 1a as well as the 1.3
1 mixture of E/Z isomers (the stereochemistry was not determined). First of all, the major isomer of oxime 1a was utilized for the optimization of the reaction conditions. Treatment of amidoxime 1a with a series of Cu(I) salts as a catalyst (20 mol%) in the presence of K3PO4 in DMF resulted in the formation of 2a in moderate to good yields (entries 1–6) and the best yield of 2a was provided by CuI (72% yield, entry 3). Screening of the solvents (entries 7–9) revealed that toluene is optimal for this transformation (entry 9). The catalyst loading of CuI could be reduced to 10 mol% in toluene, giving 2a in 76% yield (entry 10). These optimized reaction conditions (10 mol% of CuI in toluene at 100 °C) were applicable to the minor isomer of amidoxime 1a as well as the 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of major/minor isomers of 1a, affording comparable yields of dihydroimidazole 2a (77% and 74% yields, respectively).
1 mixture of major/minor isomers of 1a, affording comparable yields of dihydroimidazole 2a (77% and 74% yields, respectively).
| Entry | Cu salts (mol%) | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|
a The reactions were carried out using 0.3 mmol of 1a in solvents (0.1 M). Unless otherwise noted, the major isomer of amidoxime 1a was utilized for this optimization.
b Isolated yields.
c The yield of 2a in the reaction of the minor isomer of 1a.
d The yield of 2a in the reaction of the 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of major/minor isomers of oxime 1a. TC = 2-thiophene carboxylate. Bn = benzyl. 1 mixture of major/minor isomers of oxime 1a. TC = 2-thiophene carboxylate. Bn = benzyl.
|
||||
| 1 | CuCl (20) | DMF | 4 | 51 |
| 2 | CuBr (20) | DMF | 4 | 68 |
| 3 | CuI (20) | DMF | 2 | 72 |
| 4 | (CuOTf)2·C6H6 (10) | DMF | 4 | 52 |
| 5 | CuOAc (20) | DMF | 3.5 | 66 |
| 6 | CuTC (20) | DMF | 2 | 61 |
| 7 | CuI (20) | DMA | 3 | 66 |
| 8 | CuI (20) | DMSO | 3 | 61 |
| 9 | CuI (20) | Toluene | 2 | 75 |
| 10 | CuI (10) | Toluene | 4 | 76 (77,c74d) |
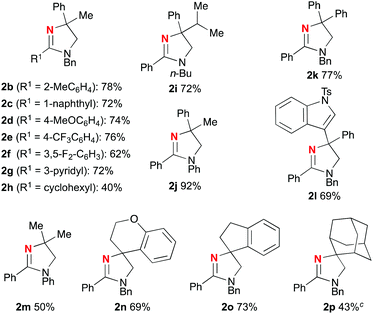
This CuI-catalyzed redox-neutral aliphatic C–H amination was extended to various amidoximes 1 as compiled in Table 2. The scope was first explored by varying the substituents R1, in which sterically hindered aromatic rings (2b, 2c) and both electron-rich (2d) and -deficient (2e, 2f) ones as well as 3-pyridyl motif (for 2g) could be installed. A cyclohexyl group could be used as the R1, while the yield of dihydroimidazole 2h was moderate (40% yield). The method allowed installing not only a benzyl (Bn) but also n-butyl and phenyl groups (2i and 2j) as the substituent R2. The tertiary carbon aminated (marked in blue) could possess a bulky isopropyl group (2i), a diphenyl moiety (2k), and an indolyl part (2l) to afford the corresponding dihydroimidazoles 2 in good yields, while the yield of C–H amination on non-benzylic dimethyl methine carbon was moderate (2m in 50% yield). Spirocyclic structures could be nicely constructed by the present method (2n–p), while amination of a non-benzylic adamantyl C–H bond (2p) resulted in moderate yield.
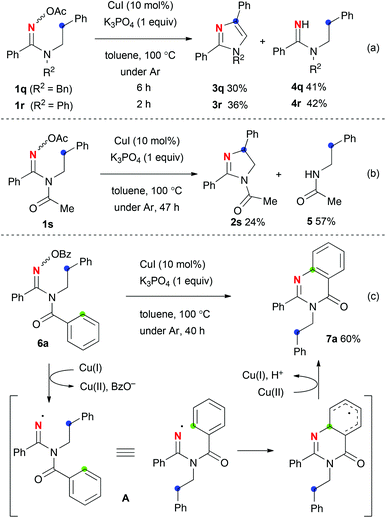
The reaction of oximes 1q and 1r with a secondary methylene C–H bond delivered aromatic imidazoles 3q (30% yield) and 3r (36% yield) as well as uncyclized amidine 4q (41% yields) and 4r (42% yield), respectively (Scheme 3-a). To prevent the formation of imidazoles 3 presumably via oxidative aromatization of putative dihydroimidazole intermediates, the reaction of N-acetylamidoxime 1s was examined (Scheme 3-b). While dihydroimidazole 2s was isolated as expected, the yield of 2s was low (24% yield) and amide 5 was obtained in 57% yield via C–N bond fission. In sharp contrast, the reaction of N-benzoyl amidoxime 6a produced quinazolinone 7a in 60% yield via addition of the amidinyl radical A to the benzoyl part (marked in green) (Scheme 3-c). This redox neutral sp2 C–H amination inspired us further to examine its scope and limitation for the synthesis of substituted quinazolinones.
Optimization of the reaction conditions14 revealed that the reactions of O-pivaloyl oximes 6b–h with 10 mol% of CuI in m-xylene at 130 °C were optimal for this quinazolinone formation (Table 3). By varying substituents R1, benzene rings bearing electron-donating and -withdrawing groups as well as a cyclohexyl motif could be installed (7b–7e). As R2, not only benzyl and p-methoxybenzyl (for 7f) but also n-butyl and cyclopropyl units (7g, 7h) were introduced.
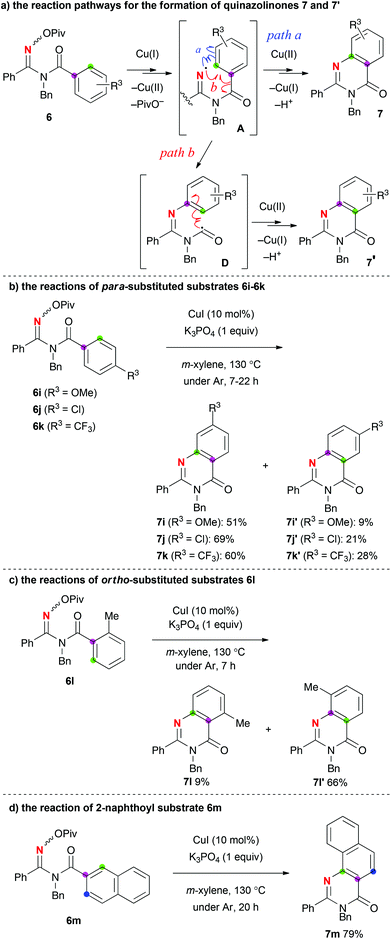
Previous precedents of the quinazolinone formation by cyclization of the amidinyl radical to the benzoyl benzene ring15,16 demonstrated that the addition of amidinyl radical A happens to not only the ortho-carbon to the carbonyl (marked in green, path a) but also the ipso-carbon (marked in purple, path b via carbamoyl radical D), which resulted in the formation of regioisomeric mixtures of quinazolinones 7 and 7′ (Scheme 4-a). We also observed the same cases when additional substituents R3 were put on the benzoyl benzene rings. By installing para-substituents R3, the reactions provided ortho-addition products 7i–7k as major products along with minor ipso-addition products 7i′–7k′ regardless of the electronic nature of the substituents R3 (Scheme 4-b).17 On the other hand, putting a methyl group at the ortho position (for the reaction of 6l) rendered the ipso addition product 7l′ major presumably because the helical sense of the C–C bond due to the ortho methyl group made interaction between the amidinyl radical and the ipso carbon smoother (path b) (Scheme 4-c). In the case of N-2-naphthoylamidoxime 6m, the amination reaction exclusively occurred with the ortho α-carbon (marked in green) to give 7m in 79% yield (Scheme 4-d).
Conclusions
In summary, we have developed CuI-catalyzed redox neutral sp3 and sp2 C–H amination reactions of readily available N-alkyl- and N-benzoylamidoximes, affording dihydroimidazoles and quinazolinones, respectively. Application of these catalytic redox-neutral radical strategies to intermolecular C–H amination is now under investigation in our laboratory.This work was supported by funding from Nanyang Technological University and the Singapore Ministry of Education (Academic Research Fund Tier 2: MOE2012-T2-1-014).
Notes and references
- For general reviews, see: (a) L. D. Quin and J. Tyrell, Fundamentals of Heterocyclic Chemistry: Importance in Nature and in the Synthesis of Pharmaceuticals, John Wiley & Sons Inc., New York, 2010 Search PubMed; (b) Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Pergamon, Oxford, 2008 Search PubMed; (c) Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, E. F. V. Scriven and C. W. Rees, Pergamon, Oxford, 1996 Search PubMed; (d) Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon, Oxford, 1984 Search PubMed; (e) T. Eicher and S. Hauptmann, The Chemistry of Heterocycles, Wiley-VCH, Weinheim, 2003 CrossRef.
- For selected reviews, see: (a) G. L. Thomas and C. W. Johannes, Curr. Opin. Chem. Biol., 2011, 15, 516 CrossRef CAS PubMed; (b) R. Tohme, N. Darwiche and H. Gali-Muhtasib, Molecules, 2011, 16, 9665 CrossRef CAS PubMed; (c) S. Dandapani and L. A. Marcaurelle, Curr. Opin. Chem. Biol., 2010, 14, 362 CrossRef CAS PubMed; (d) M. E. Welsch, S. A. Snyder and B. R. Stockwell, Curr. Opin. Chem. Biol., 2010, 14, 347 CrossRef CAS PubMed; (e) J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337 RSC.
- For recent reviews on C–H amination, see: (a) J. L. Jeffrey and R. Sarpong, Chem. Sci., 2013, 4, 4092 RSC; (b) R. T. Gephart III and T. H. Warren, Organometallics, 2012, 31, 7728 CrossRef; (c) J. L. Roizen, M. E. Harvey and J. Du Bois, Acc. Chem. Res., 2012, 45, 911 CrossRef CAS PubMed; (d) J. Du Bois, Org. Process Res. Dev., 2011, 15, 758 CrossRef CAS PubMed; (e) F. Collet, C. Lescot and P. Dauban, Chem. Soc. Rev., 2011, 40, 1926 RSC; (f) D. N. Zalatan and J. Du Bois, Top. Curr. Chem., 2010, 292, 347 CrossRef CAS; (g) F. Collet, R. H. Dodd and P. Dauban, Chem. Commun., 2009, 5061 RSC; (h) M. M. Díaz-Requejo and P. J. Pérez, Chem. Rev., 2008, 108, 3379 CrossRef PubMed.
- For recent examples of radical-mediated (non-nitrene-based) aliphatic C–H amination reactions, see: (a) Y. Amaoka, S. Kamijo, T. Hoshikawa and M. Inoue, J. Org. Chem., 2012, 77, 9959 CrossRef CAS PubMed; (b) Q. Michaudel, D. Thevenet and P. S. Baran, J. Am. Chem. Soc., 2012, 134, 2547 CrossRef CAS PubMed; (c) Z. Ni, Q. Zhang, T. Xiong, Y. Zheng, Y. Li, H. Zhang, J. Zhang and Q. Liu, Angew. Chem., Int. Ed., 2012, 51, 1244 CrossRef CAS PubMed; (d) R. T. Gephart III, D. L. Huang, M. J. B. Aguila, G. Schmidt, A. Shahu and T. H. Warren, Angew. Chem., Int. Ed., 2012, 51, 6488 CrossRef PubMed; (e) S. Sakagushi, T. Hirabayashi and Y. Ishii, Chem. Commun., 2002, 516 RSC; (f) H. Togo, Y. Harada and M. Yokoyama, J. Org. Chem., 2000, 65, 926 CrossRef CAS.
- (a) H. Chen, S. Sanjaya, Y.-F. Wang and S. Chiba, Org. Lett., 2013, 15, 212 CrossRef CAS PubMed; (b) Y.-F. Wang, H. Chen, X. Zhu and S. Chiba, J. Am. Chem. Soc., 2012, 134, 11980 CrossRef CAS PubMed.
- For Cu-catalyzed oxidative transformation of N-alkenylamidines for the synthesis of azaheterocycles, see: (a) S. Sanjaya and S. Chiba, Org. Lett., 2012, 14, 5342 CrossRef CAS PubMed; (b) Y.-F. Wang, X. Zhu and S. Chiba, J. Am. Chem. Soc., 2012, 134, 3679 CrossRef CAS PubMed.
- Zard reported the generation of amidinyl radicals from O-benzoyl amidoximes under the stannane-diazo initiator system or the Ni-AcOH conditions, see: D. Gennet, S. Z. Zard and H. Zhang, Chem. Commun., 2003, 1870 RSC.
- For reports on single-electron-reduction of the oxime N–O bonds with Cu(I) species, see: (a) M.-N. Zhao, H. Liang, Z.-H. Ren and Z.-H. Guan, Synthesis, 2012, 1501 CAS; (b) K. Tanaka, M. Kitamura and K. Narasaka, Bull. Chem. Soc. Jpn., 2005, 78, 1659 CrossRef CAS; (c) Y. Koganemaru, M. Kitamura and K. Narasaka, Chem. Lett., 2002, 784 CrossRef CAS; (d) J. Boivin, A.-M. Schiano, S. Z. Zard and H. Zhang, Tetrahedron Lett., 1999, 40, 4531 CrossRef CAS; (e) J. Boivin, A.-M. Schiano and S. Z. Zard, Tetrahedron Lett., 1992, 33, 7849 CrossRef CAS.
- J. K. Kochi, A. Bemis and C. L. Jenkins, J. Am. Chem. Soc., 1968, 90, 4616 CrossRef CAS.
- For reviews on redox-neutral C–H functionalization, see: (a) B. Peng and N. Maulide, Chem.–Eur. J., 2013, 19, 13274 CrossRef CAS PubMed; (b) F. W. Patureau and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 1977 CrossRef CAS PubMed.
- For reports on other types of redox-neutral transition-metal-catalyzed C–H functionalizations with oximes, see: (a) N. Guimond, S. I. Gorelsky and K. Fagnou, J. Am. Chem. Soc., 2011, 133, 6449 CrossRef CAS PubMed; (b) P. C. Too, S. Z. Chua, S. H. Wong and S. Chiba, J. Org. Chem., 2011, 76, 6159 CrossRef CAS PubMed; (c) P. C. Too, T. Noji, Y. J. Lim, X. Li and S. Chiba, Synlett, 2011, 2789 CAS; (d) T. K. Hyster and T. Rovis, Chem. Commun., 2011, 47, 11846 RSC; (e) X. Zhang, D. Chen, M. Zhao, J. Zhao, A. Jia and X. Li, Adv. Synth. Catal., 2011, 353, 719 CrossRef CAS; (f) Y. Tan and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 3676 CrossRef CAS PubMed; (g) P. C. Too, Y.-F. Wang and S. Chiba, Org. Lett., 2010, 12, 5688 CrossRef CAS PubMed.
- For reports on Cu-catalyzed redox-neutral transformation with hydroxylamine derivatives, see: (a) W.-M. Liu, Z.-H. Liu, W.-W. Cheong, L.-Y. Teo Priscilla, Y. Li and K. Narasaka, Bull. Korean Chem. Soc., 2010, 31, 563 CrossRef CAS; (b) M. Noack and R. Göttlich, Chem. Commun., 2002, 536 RSC.
- For a report on metal-free intramolecular sp3-C–H amination with ketoximes by thermal treatment of ketoximes with acetic anhydride, see: C. G. Savarin, C. Grisé, J. A. Murry, R. A. Reamer and D. L. Hughes, Org. Lett., 2007, 9, 981 CrossRef CAS PubMed.
- See the ESI† for the optimization of the reaction conditions.
- (a) M.-H. Larraufie, C. Courillon, C. Ollivier, E. Lacôte, M. Malacria and L. Fensterbank, J. Am. Chem. Soc., 2010, 132, 4381 CrossRef CAS PubMed; (b) M.-H. Larraufie, C. Ollivier, L. Fensterbank and M. Malacria, Angew. Chem., Int. Ed., 2010, 49, 2178 CrossRef CAS PubMed; (c) A. Beaume, C. Courillon, E. Derat and M. Malacria, Chem.–Eur. J., 2008, 14, 1238 CrossRef CAS PubMed; (d) A. Servais, M. Azzouz, D. Lopes, C. Courillon and M. Malacria, Angew. Chem., Int. Ed., 2007, 46, 576 CrossRef CAS PubMed.
- Y.-F. Wang, F.-L. Zhang and S. Chiba, Org. Lett., 2013, 15, 2842 CrossRef CAS PubMed.
- The reactions of oximes 6a–h bearing a non-substituted benzoyl group might involve not only ortho-addition but also ipso-addition pathways for the formation of quinazolinones 7a–h.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data of products, and copies of 1H and 13C NMR spectra. See DOI: 10.1039/c3ob41871e |
| This journal is © The Royal Society of Chemistry 2014 |