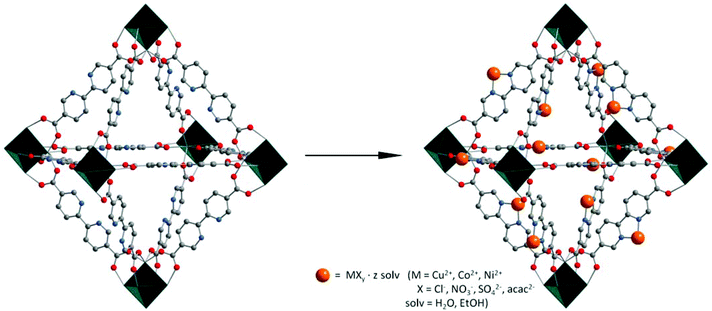
Integration of accessible secondary metal sites into MOFs for H2S removal†
Georg
Nickerl
a,
Matthias
Leistner
b,
Stella
Helten
a,
Volodymyr
Bon
a,
Irena
Senkovska
a and
Stefan
Kaskel
*ab
aDepartment of Inorganic Chemistry, Dresden University of Technology, Bergstraße 66, 01069 Dresden, Germany. E-mail: stefan.kaskel@chemie.tu-dresden.de; Fax: +49 351 46337287; Tel: +49 351 46334885
bFraunhofer Institute for Material and Beam Technology (IWS) Dresden, Winterbergstraße 28, 01277 Dresden, Germany
First published on 7th March 2014
Abstract
2,2′-Bipyridine-5,5′-dicarboxylic acid (H2bipy) was used for the synthesis of a zirconium based metal–organic framework with the aim to integrate an additional N-donor coordination functionality into the framework. The resulting MOF (UiO-67(bipy)) with a structure isoreticular to UiO-67 (UiO – University of Oslo) has a BET surface area up to 2500 m2 g−1. The chelating bipyridine moiety was used for postsynthetic functionalization of the MOF with different metal salts (MIIXy; M = Cu2+, Ni2+, Co2+; X = Cl−, NO3−, SO42−, acac2−) to give the corresponding metal_salt@UiO-67(bipy) materials. Due to the highly dispersed metal ions, metal_salt@UiO-67(bipy) shows good performance in toxic hydrogen sulfide capture. Especially, the copper loaded samples have high capacities of up to 7.8 wt%.
Introduction
Metal–organic frameworks (MOFs) exhibit large surface areas along with a modular design,1 which allows the individual selection of building blocks for the synthesis of materials with desired properties.2 MOFs are excellent materials for gas storage and separation,3 catalysis,4 and sensing,5 outperforming other porous materials, such as zeolites and activated carbons. Another emerging field of application is the removal of toxic gases such as hydrogen sulfide from natural gas or from air, e.g. in respiratory protection devices for firemen.6 In 2009 members of the MIL (MIL – Matériaux Institut Lavoisier) family were the first MOFs to be tested more in depth in the adsorption of hydrogen sulfide by De Weireld et al.7 They showed reversible adsorption of pure hydrogen sulfide for MIL-47(V) and MIL-53(Al, Cr) and a partially reversible adsorption for MIL-100(Cr) and MIL-101(Cr). Another promising MOF for the adsorption of hydrogen sulfide is CPO-27(Ni) (CPO – Coordination Polymer of Oslo),8 which shows a high affinity even at very low pressures due to open metal sites in the dehydrated compound.9 In addition, CPO-27(Ni) is unaltered by the presence of hydrogen sulfide. HKUST-1 (HKUST – Hong Kong University of Science and Technology) shows a hydrogen sulfide uptake of around 8 wt% that can be further increased by synthesizing a composite material of HKUST-1 and graphitic compounds.10Since transition metals like copper or cobalt show a high affinity towards representative toxic gases like ammonia and hydrogen sulfide, inclusion of these metals into the framework would be favorable. However, MOFs based on these metals, like HKUST-1, show a rather low chemical stability making applications under ambient conditions almost impossible.11,12 Thus, the aim of this study was to design a MOF which could offer chemical stability combined with a high sorption ability for H2S. Reversibility of H2S adsorption is not a requirement for respiratory filters and was thus not a target in this study.
A secondary building unit exhibiting a high chemical as well as thermal stability is the Zr6O4(OH)4(COO)12 unit which makes zirconium based MOFs an interesting class of MOFs for application under ambient or even harsh conditions. The UiO series were the first zirconium based MOFs reported.13 Since their discovery numerous other MOFs with the same or similar SBU were synthesized.14–16 Thus, to ensure framework stability the [Zr6O4(OH)4]12+ cluster was chosen as a building block. Unfortunately, zirconium based MOFs intrinsically do not posses remarkable adsorption capacity for hydrogen sulfide. The idea was to incorporate an accessible metal ion with high affinity to sulfide into the zirconium based MOF with the aim to enhance the sorption ability for hydrogen sulfide. In order to incorporate a second coordinatively bonded metal species into the framework, a functional linker which could interact strongly with transition metals is needed. For this purpose, 2,2′-bipyridine-5,5′-dicarboxylate (bipy) was chosen, since the bipyridine is a versatile ligand in molecular complex chemistry. Bipyridine is also known to interact strongly with metals in a chelating fashion. Bipyridine complexes with rhenium or manganese incorporated into manganese based frameworks were successfully used as photocatalysts by Champness et al. in 2010.17 The bipyridine moiety was further introduced into aluminum based MOFs and used for postsynthetic loading with noble metals such as ruthenium and palladium for gas separation and catalytic applications.18,19 The introduction of lanthanides into bipyridine based MOFs leads to materials with tunable luminescence.20 Thus, it is shown that integration of the free bipyridine moiety into a MOF is feasible, if the metal ions exclusively react with the carboxylic groups rather than with the bipyridine moiety during the MOF synthesis.
In 2011 Lin et al. synthesized a zirconium based MOF with UiO-67 topology using a mixture of biphenyl-4,4′-dicarboxylic acid (H2bpdc) and metal complexes of 2,2′-bipyridine-5,5′-dicarboxylic acid (H2bipy).21 The metal complex containing MOFs were used for photocatalytic reactions like the oxidation of thioanisole. Recently, Walton et al. synthesized a zirconium based framework using 2,2′-bipyridine-5,5′-dicarboxylate and investigated the stability of this framework against ambient conditions and moisture. The authors have shown that it lacks some stability against liquid water.22 Zhao et al. used the same framework for adsorption studies showing that the Lewis basic bipyridine sites increase the adsorption properties for different gases like hydrogen, methane, and carbon dioxide.23
In this work we report the use of a MOF based on zirconium and 2,2′-bipyridine-5,5′-dicarboxylate as a ligand for the postsynthetic functionalization with various transition metal salts leading to an increase of the adsorption capacity for hydrogen sulfide (Scheme 1).
Results and discussion
A white microcrystalline powder of UiO-67(bipy) was obtained by heating a solution of ZrCl4, H2bipy and acetic or benzoic acid as a modulator in DMF at 120 °C for 3 days. Under these conditions no single crystals suitable for crystal structure determination could be obtained. However, in order to confirm the isomorphism of UiO-67(bipy) to UiO-67(Zr6O4(OH)4(bpdc)6), a single crystal suitable for single crystal X-ray analysis of UiO-67 could be grown (for more details see ESI†). Utilization of a large amount of modulator in the synthesis allows to slow down the kinetics of the crystal growth to a level that yields high quality octahedral single crystals, suitable for X-ray diffraction experiment. It should be mentioned that no single crystal data for UiO-67 have been published up to now.As expected, UiO-67 crystallizes in the cubic space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m with cell parameter a = 26.896(3) Å and therefore is isoreticular to UiO-66, in which terephthalate was used as the organic ligand (see ESI†). The crystal structure is based on a 12-connected [Zr6O4(OH)4]12+ cluster. Each inorganic node is connected to 12 ones by bpdc linkers, building the uninodal 12-connected framework with fcu topology. The latter provides two types of pores: octahedral and tetrahedral (Fig. 1). Increasing the distance between the carboxylates from 5.64 Å in bdc2− in UiO-66 to 10.10 Å in bpdc2− in UiO-67 leads to an enlargement of the pores and pore windows in UiO-67. Thus, the diameter of the triangular pore window increases from 4.16 Å in UiO-66 to 6.52 Å in UiO-67 allowing for a wider range of adsorption applications. Comparison of powder X-ray diffraction data of UiO-67 and UiO-67(bipy) confirms the expected assumption of the structural analogy (Fig. S1†).
m with cell parameter a = 26.896(3) Å and therefore is isoreticular to UiO-66, in which terephthalate was used as the organic ligand (see ESI†). The crystal structure is based on a 12-connected [Zr6O4(OH)4]12+ cluster. Each inorganic node is connected to 12 ones by bpdc linkers, building the uninodal 12-connected framework with fcu topology. The latter provides two types of pores: octahedral and tetrahedral (Fig. 1). Increasing the distance between the carboxylates from 5.64 Å in bdc2− in UiO-66 to 10.10 Å in bpdc2− in UiO-67 leads to an enlargement of the pores and pore windows in UiO-67. Thus, the diameter of the triangular pore window increases from 4.16 Å in UiO-66 to 6.52 Å in UiO-67 allowing for a wider range of adsorption applications. Comparison of powder X-ray diffraction data of UiO-67 and UiO-67(bipy) confirms the expected assumption of the structural analogy (Fig. S1†).
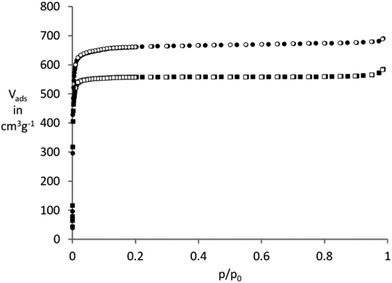
The porosity of the investigated compounds was studied by nitrogen physisorption at 77 K. It was observed that the porosity of UiO-67(bipy) depends on the type of the modulator used during the synthesis, which is in agreement with the findings of Behrens et al.24 They could increase the specific surface area for UiO-67 by changing the modulator from acetic acid to benzoic acid. The BET surface area for UiO-67(bipy) derived from nitrogen physisorption isotherms (Fig. 2) is 2150 m2 g−1 when acetic acid is used as a modulator and 2500 m2 g−1 when acetic acid is replaced by benzoic acid.
For the desired application, the stability of the adsorbent under ambient conditions and under increased humidity is crucial; therefore the stability of MOF was investigated in details. Storage under ambient conditions (laboratory air for 3 months) does not affect the crystallinity according to powder X-ray diffraction (PXRD) experiments. More important, the nitrogen uptake is constant. To prove the moisture tolerance of UiO-67(bipy), a sample was soaked in water at room temperature for 24 h. PXRD measurements also show no significant changes after such treatment (Fig. S2†). This finding is in contrast to the work recently published by Walton et al.22 reporting a loss of crystallinity for the material suspended in water for 24 h. However, their synthesis procedure does not include the use of a modulator, potentially leading to a somehow different parent material. The highly crystalline UiO-67(bipy) exhibits reasonable stability, which opens the opportunity for application under humid conditions. In addition, loading of the framework with metal salts from aqueous solutions is feasible making UiO-67(bipy) a very attractive material.
The stability of UiO-67(bipy) against the toxic gases hydrogen sulfide and ammonia was also tested. UiO-67(bipy) remains intact after contact with hydrogen sulfide, retaining crystallinity as well as porosity (Fig. S3†). Similar to UiO-67, UiO-67(bipy) also shows no specific affinity towards hydrogen sulfide. In contrast, the exposure of UiO-67(bipy) to ammonia leads to the loss of crystallinity and porosity (Fig. S3†). Nevertheless, UiO-67(bipy) can capture 2.4 wt% ammonia during the framework collapse.
In order to increase the adsorption capacities for hydrogen sulfide, pristine UiO-67(bipy) was loaded with metal salts from the liquid phase. Copper, cobalt and nickel were chosen as metals due to their ability to form stable metal sulfides.
Loading was realized by adding desolvated UiO-67(bipy) samples to an aqueous or ethanolic solution of the corresponding metal salt containing two equivalents of metal ions per linker. Ethanol as a solvent is more favorable, because it can be removed after loading under dynamic vacuum already at room temperature. It should be noted that metal acetylacetonates were loaded using tetrahydrofuran as a solvent due to the low solubility of the salts in ethanol. During the metal loading process, the originally white powder of UiO-67(bipy) changes the color to blue for copper salts, orange for cobalt salts, and pale green for nickel salts (Fig. 3).
 | ||
| Fig. 3 The originally white UiO-67(bipy) turns blue after loading with copper salt (A), orange after loading with cobalt salt (B) and pale green after loading with nickel salt (C). | ||
The metal salt loading process was followed by UV/Vis spectroscopy monitoring the metal ion concentration in the supernatant solution. Kinetic investigation of the copper(II) chloride adsorption from an aqueous solution on UiO-67(bipy) reveals a rapid uptake until a saturation level, which is reached after 20 hours (Fig. 4). To study the leaching behavior of the copper(II) chloride loaded material, the sample was placed in fresh water in a quartz cuvette and left there for 24 h. During this time no leaching of copper ions from the UiO-67(bipy) framework into the supernatant could be detected by UV/Vis spectroscopy, indicating a very strong interaction between UiO-67(bipy) and the metal salt (chemisorption).
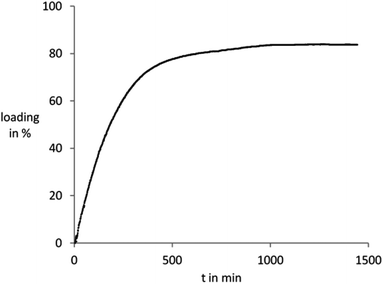 | ||
| Fig. 4 Loading of UiO-67(bipy) with copper(II) chloride from aqueous solution. A loading of 100% corresponds to one metal ion per linker molecule of the MOF. | ||
In addition, the absorption maximum of a sample loaded with an aqueous copper(II) chloride solution is shifted to 750 nm compared to the peak maximum of 815 nm for the aqueous solution of copper(II) chloride itself. This is an indication for the change in the chemical environment of the copper atom and supports the assumption of the formation of a new complex within the framework rather than the sole presence of hydrated copper(II) chloride in the pores (Fig. S4†).
To investigate the influence of the salt-forming anion, UiO-67(bipy) was loaded with a range of different copper salts including copper(II) chloride, copper(I) chloride, copper(II) nitrate, copper(II) sulfate, copper(II) acetylacetonate and copper(II) tetrafluoroborate. The investigation shows that the type of anion has a great influence on the stability of the resulting metal loaded UiO-67(bipy) material. According to the XRD analysis, all materials loaded with copper salts from ethanolic solution are crystalline (Fig. S5†).
Additional activation at room temperature yields crystalline products for copper(II) nitrate@UiO-67(bipy), copper(II) sulfate@UiO-67(bipy) and copper(II) acetylacetonate@UiO-67(bipy). Samples of copper(I) chloride@UiO-67(bipy) and copper(II) chloride@UiO-67(bipy) give amorphous products after activation (Fig. 5). Specific BET surface areas of the loaded samples decrease to 2036 m2 g−1 for copper(II) sulfate@UiO-67(bipy), 1541 m2 g−1 for copper(II) acetylacetonate@UiO-67(bipy) and 549 m2 g−1 for copper(II) nitrate@UiO-67(bipy) (Fig. S6†).
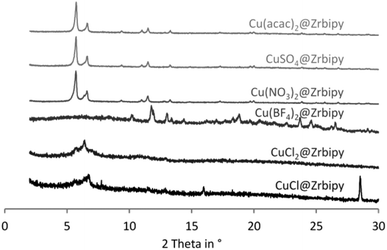 | ||
| Fig. 5 PXRD patterns of UiO-67(bipy) loaded with different copper salts from ethanolic solutions and additionally activated at room temperature under dynamic vacuum. | ||
Not only the stability of the loaded MOF is affected by the anion, but also the amount of metal ions (according to the EDX measurements), which could be incorporated into the MOF. In an ideal case, a metal to 2,2′-bipyridine-5,5′-dicarboxylate ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 should be achieved, which is regarded as 100% loading. EDX measurements reveal that the adsorbed amount of metal strongly depends on the used metal salt. The highest loading from ethanolic solution was observed for copper(II) nitrate reaching around 60%. In contrast, the utilisation of copper(II) sulfate and copper(II) acetylacetonate yielded materials with a much lower metal content of only 32% and 23% loading, respectively.
1 should be achieved, which is regarded as 100% loading. EDX measurements reveal that the adsorbed amount of metal strongly depends on the used metal salt. The highest loading from ethanolic solution was observed for copper(II) nitrate reaching around 60%. In contrast, the utilisation of copper(II) sulfate and copper(II) acetylacetonate yielded materials with a much lower metal content of only 32% and 23% loading, respectively.
Hydrogen sulfide breakthrough measurements show an increased adsorption capacity of 0.9 wt% for copper(II) sulfate@UiO-67(bipy), 2.3 wt% for copper(II) acetylacetonate@UiO-67(bipy) and 3.8 wt% for copper(II) nitrate@UiO-67(bipy). Apparently the adsorption capacity does not only depend on the amount of metal ions inserted into the framework but also on the anion of the used copper salt.
UiO-67(bipy) was also loaded with metal salts from aqueous solutions, which is advantageous from an economic and ecologic point of view. In addition, for an application as a respiratory device, water is also favored over ethanol. After loading from aqueous solutions the samples were not activated in vacuum, but shortly dried in an argon stream, allowing the removal of water stuck on the particles’ outer surface, but leaving the pores filled with water. UiO-67(bipy) materials were loaded with copper(II) nitrate, copper(II) sulfate and also with copper(II) chloride, respectively, from aqueous solutions, while loading with copper(II) acetylacetonate was not performed due to the insolubility of copper(II) acetylacetonate in water. In this case, the copper(II) chloride sample stays crystalline, because the activation step under dynamic vacuum is omitted (Fig. S7†). EDX measurements reveal loadings much higher compared to the values obtained for the adsorption from ethanolic solutions. In addition, the loading is apparently not affected by the anion as the amounts of incorporated metal (87% for copper(II) chloride and copper(II) nitrate, 90% for copper(II) sulfate) are all very similar. In contrast, the loading of UiO-67, not possessing the chelating bipyridine moiety, with copper(II) nitrate from an aqueous solution leads only to an integrated metal amount of below 1%. The hydrogen sulfide uptake for UiO-67(bipy) loaded via the aqueous route is considerably increased compared to the activated samples.
The comparison between the two samples loaded with copper(II) nitrate shows the influence of the sample preparation procedure (Table 1). While the metal content of the wet sample (loaded from aqueous solution) is only 22.6% higher compared to the metal content of the activated sample (loaded from ethanolic solution), the capacity for hydrogen sulfide per gram of material is twice as high. Considering that the sample with higher performance contains water (and consequently is heavier), the value for the adsorption, for example per metal (or per formula unit of MOF), is even higher.
| Precursor | Solvent | Metal loadinga (%) | Capacity for H2Sb (wt%) |
|---|---|---|---|
| a Metal loading (%) of the maximum loading, which is one metal ion per linker. b Testing conditions: 1000 ppm hydrogen sulfide in humid air (70% rel. humidity), gas flow 118 ml min−1 (20 °C, 1 atm). | |||
| CuCl2 | H2O | 86.8 | 7.6 |
| Cu(NO3)2 | H2O | 86.8 | 7.8 |
| Cu(NO3)2 | EtOH | 60.2 | 3.8 |
| CuSO4 | H2O | 90.4 | 0.9 |
| CuSO4 | EtOH | 32.0 | 1.0 |
| Cu(acac)2 | THF | 23.4 | 2.3 |
| CoCl2 | H2O | 23.0 | 0 |
| Co(NO3)2 | EtOH | 24.4 | 1.0 |
| CoSO4 | EtOH | 10.8 | 0 |
| Co(acac)2 | THF | 13.4 | 0.4 |
| NiCl2 | H2O | 29.8 | 0 |
| Ni(NO3)2 | EtOH | 4.6 | 0 |
| Ni(acac)2 | THF | 4.3 | 0 |
Copper(II) chloride·zH2O@UiO-67(bipy) and copper(II) nitrate·zH2O@UiO-67(bipy) loaded samples show a comparable metal content and consequently a comparable adsorption capacity of 7.6 and 7.8 wt%, respectively. However, although the copper(II) sulfate loaded sample shows a higher uptake of metal out of aqueous solutions the capacity for hydrogen sulfide stays at the same level. The capacity of 7.8 wt% for copper(II) nitrate·zH2O@UiO-67(bipy) is in the range of HKUST-1 adsorbing 8 wt%10 and somewhat lower than the capacity of 13 wt% observed for CPO-27(Ni).25 Copper(II) nitrate·zH2O@UiO-67 shows only an uptake of 0.2 wt%. Under the same test conditions, industrially used zinc oxide has a capacity of around 3 wt% according to our investigations.26
Cobalt and nickel salts were introduced in the same manner as the copper salts. In all cases the metal salt loadings were significantly lower compared to the trials with copper salts. Investigations of the stability of the loaded samples reveal a similar behavior as for the copper loaded samples (Fig. S8 and S9†). Activation of samples loaded with the metal chloride leads to a framework collapse upon activation, but the use of the corresponding nitrate, sulfate or acetylacetonate as an anion of the metal precursor stabilizes the framework during the activation procedure. However, the H2S sorption capacities are quite low compared to the copper loaded samples. In fact only the materials loaded with cobalt(II) nitrate and cobalt(II) acetylacetonate show an uptake of hydrogen sulfide. None of the nickel loaded samples have a significant capacity for the adsorption of hydrogen sulfide. A better understanding of the ongoing reactions during H2S sorption is currently under investigation. However, the sorption process is irreversible and regeneration of the material was only possible to a limited degree.
Conclusions
In conclusion, we have synthesized a UiO-67(bipy) MOF containing a chelating ligand, which was used for complexation of different metal salts inside the MOF. The metal salts were introduced by a liquid phase adsorption route. Ethanolic and aqueous solutions of different copper, nickel and cobalt salts were used. It was shown that the anion plays a crucial role for the stabilization of the framework during the activation of the loaded materials. Nitrate, sulfate and acetylacetonate seem to have a stabilizing effect whereas metal chloride loaded frameworks collapse upon activation. Hydrogen sulfide adsorption experiments show no capacity for the nickel salt loaded samples and low capacities for the cobalt salt loaded samples. In contrast, samples loaded with copper salts show high hydrogen sulfide uptakes up to 7.8 wt% in the presence of moisture. As the loading was performed from aqueous solution, an application, e.g., in respiratory protection devices seems feasible. The high adsorption capacities for various metal ions make UiO-67(bipy) also a promising candidate for water purification applications. Moreover, the metal_salt@UiO-67(bipy) family represents an example for a modular framework functionalization strategy for trace gas adsorption and has also potential for catalytic applications.Acknowledgements
The authors gratefully acknowledge financial support from the EU Seventh Framework Program (FP7) and nanoMOF (CP-IP 228604-2).Notes and references
- S. T. Meek, J. A. Greathouse and M. D. Allendorf, Adv. Mater., 2011, 23, 249–267 CrossRef CAS PubMed.
- K. Gedrich, M. Heitbaum, A. Notzon, I. Senkovska, R. Fröhlich, J. Getzschmann, U. Mueller, F. Glorius and S. Kaskel, Chem.–Eur. J., 2011, 17, 2099–2106 CrossRef CAS PubMed.
- U. Stoeck, S. Krause, V. Bon, I. Senkovska and S. Kaskel, Chem. Commun., 2012, 48, 10841–10843 RSC.
- T. Lescouet, C. Chizallet and D. Farrusseng, J. Am. Chem. Soc., 2013, 135, 4195–4198 CrossRef PubMed.
- Y. Takashima, V. M. Martinez, S. Furukawa, M. Kondo, S. Shimomura, H. Uehara, M. Nakahama, K. Sugimoto and S. Kitagawa, Nat. Commun., 2011, 2, 1–8 Search PubMed.
- N. A. Khan, Z. Hasan and S. H. Jhung, J. Hazard. Mater., 2013, 244–245, 444–456 CrossRef CAS PubMed.
- L. Hamon, C. Serre, T. Devic, T. Loiseau, F. Millange, G. Férey and G. De Weireld, J. Am. Chem. Soc., 2009, 131, 8775–8777 CrossRef CAS PubMed.
- P. D. C. Dietzel, B. Panella, M. Hirscher, R. Bloma and H. Fjellvåg, Chem. Commun., 2006, 959–961 RSC.
- P. K. Allan, P. S. Wheatley, D. Aldous, M. I. Mohideen, C. Tang, J. A. Hriljac, I. L. Megson, K. W. Chapman, G. De Weireld, S. Vaesen and R. E. Morris, Dalton Trans., 2012, 41, 4060–4066 RSC.
- C. Petit and T. J. Bandosz, Dalton Trans., 2012, 41, 4027–4035 RSC.
- P. Küsgens, M. Rose, I. Senkovska, H. Fröde, A. Henschel, S. Siegle and S. Kaskel, Microporous Mesoporous Mater., 2009, 120, 325–330 CrossRef PubMed.
- B. Jee, D. Himsl, M. Icker, M. Hartmann and A. Pöppl, Chem. Ing. Tech., 2010, 82, 1025–1029 CrossRef.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS.
- V. Guillerm, F. Ragon, M. Dan-Hardi, T. Devic, M. Vishnuvarthan, B. Campo, A. Vimont, G. Clet, Q. Yang, G. Maurin, G. Férey, A. Vittadini, S. Gross and C. Serre, Angew. Chem., Int. Ed., 2012, 51, 9267–9271 CrossRef CAS PubMed.
- V. Bon, I. Senkovska, I. A. Baburin and S. Kaskel, Cryst. Growth Des., 2013, 13, 1231–1237 CAS.
- A. J. Blake, N. R. Champness, T. L. Easun, D. R. Allan, H. Nowell, M. W. George, J. Jia and X.-Z. Sun, Nat. Chem., 2010, 2, 688–694 CrossRef CAS PubMed.
- F. Carson, S. Agrawal, M. Gustafsson, A. Bartoszewicz, F. Moraga, X. Zou and B. Martín-Matute, Chem.–Eur. J., 2012, 18, 15337–15344 CrossRef CAS PubMed.
- E. D. Bloch, D. Britt, C. Lee, C. J. Doonan, F. J. Uribe-Romo, H. Furukawa, J. R. Long and O. M. Yaghi, J. Am. Chem. Soc., 2010, 132, 14382–14384 CrossRef CAS PubMed.
- Z. Min, M. A. Singh-Wilmot, C. L. Cahill, M. Andrews and R. Taylor, Eur. J. Inorg. Chem., 2012, 4419–4426 CrossRef CAS.
- C. Wang, Z. Xie, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 13445–13454 CrossRef CAS PubMed.
- J. B. DeCoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y.-g. Huang and K. S. Walton, J. Mater. Chem. A, 2013, 1, 5642–5650 CAS.
- L. Li, S. Tang, C. Wang, X. Lv, M. Jiang, H. Wu and X. Zhao, Chem. Commun., 2014, 50, 2304–2307 RSC.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Eur. J., 2011, 17, 6643–6651 CrossRef CAS PubMed.
- S. Chavan, F. Bonino, L. Valenzano, B. Civalleri, C. Lamberti, N. Acerbi, J. H. Cavka, M. Leistner and S. Bordiga, J. Phys. Chem. C, 2013, 117, 15615–15622 CAS.
- I. Rosso, C. Galletti, M. Bizzi, G. Saracco and V. Specchia, Ind. Eng. Chem. Res., 2003, 42, 1688–1697 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD pattern, UV/vis spectra, nitrogen physisorption isotherms. CCDC 960486. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3qi00093a |
| This journal is © the Partner Organisations 2014 |