A Cu-catalyzed practical approach to α-ketoesters under air: an efficient aerobic oxidative dehydrogenative coupling of alcohols and α-carbonyl aldehydes†
Chun
Zhang
a and
Ning
Jiao
*ab
aState Key Laboratory of Natural and Biomimetic Drugs, Peking University, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191, China. E-mail: jiaoning@bjmu.edu.cn; Fax: (+86)-010-8280-5297; Tel: (+86)01082805297
bShanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, Shanghai 200062, China
First published on 8th January 2014
Abstract
A novel and efficient approach to α-ketoesters has been developed with wide functional group tolerance. This copper-catalyzed oxidative dehydrogenative coupling reaction of alcohols and α-carbonyl aldehydes employs air as the oxidant and generates H2O as the only by-product. Broad substrate scope, high atom economy and mild reaction conditions make this chemistry very practical.
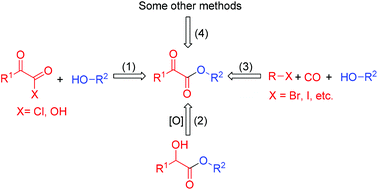
α-Ketoesters are ubiquitous units in many biologically active compounds.1 Furthermore, they have been widely used as useful precursors in organic synthesis.2 Consequently, a lot of effort has been made to construct α-ketoesters. For example, esterification of α-ketoacyl halides and α-ketoacids (1, Scheme 1),3 oxidation of α-hydroxy ester (2, Scheme 1),4 double carbopalladative esterification (3, Scheme 1),5 and other methods have been widely used (4, Scheme 1).6 Despite the numerous efforts toward the synthesis of α-ketoesters, development of mild, efficient, and environmentally friendly methods is still desirable.
In recent years, copper catalyzed aerobic oxidative cross dehydrogenative coupling (CDC) has been demonstrated to be an efficient tool in organic transformations.7,8 Recently, by using Cu-catalysis, we developed a novel approach to construct α-ketoesters through C–C bond cleavage (eqn (1)).9 However, some drawbacks may limit its application: (1) an excess amount of alcohol substrate (3.0 eq.) is required for this transformation; (2) since the fragmentation of the intermediate could couple with alcohol to afford the byproduct, the atom-economy is not high; (3) pure molecular oxygen is required in that method to ensure high efficiency. Based on previous research, we developed an efficient copper catalyzed aerobic oxidative dehydrogenative coupling reaction of α-carbonyl aldehydes with alcohols for the synthesis of α-ketoesters (eqn (2)).10 Broad substrate scope and mild reaction conditions under air make this chemistry very practical.
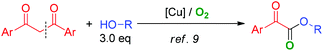 | (1) |
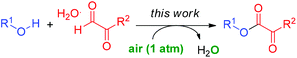 | (2) |
Under air conditions, the CuBr-catalyzed reaction of phenylglyoxal monohydrate (1a) with 2-phenylethanol (2a) afforded phenethyl 2-oxo-2-phenylacetate (3aa) in 88% yield (entry 1, Table 1). Other copper salts, such as Cu(OH)2, Cu(NO3)2·3H2O, CuCl and Cu2O, can also catalyze this reaction affording the desired product in moderate yields (entries 2–5, Table 1). However, CuI did not work well (entry 6, Table 1). We then surveyed the effect of different solvents. The reactions gave low yields in MeCN or DCE respectively (entries 7 and 8, Table 1). This chemistry nearly did not afford the desired product when using 1,4-dioxane, DMF or DMSO as the solvent (entries 9–11, Table 1).
| Entry | Cu | Solvent | Yield of 3aab (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.25 mmol), 2a (0.375 mmol), catalyst (0.025 mmol), pyridine (0.125 mmol), solvent (1.5 mL), Air (1 atm), at 90 °C for 18 h. b Isolated yield. | |||
| 1 | CuBr | Toluene | 88 |
| 2 | Cu(OH)2 | Toluene | 50 |
| 3 | Cu(NO3)2·3H2O | Toluene | 80 |
| 4 | CuCl | Toluene | 75 |
| 5 | Cu2O | Toluene | 72 |
| 6 | CuI | Toluene | Trace |
| 7 | CuBr | MeCN | 35 |
| 8 | CuBr | DCE | 15 |
| 9 | CuBr | 1,4-Dioxane | Trace |
| 10 | CuBr | DMF | Trace |
| 11 | CuBr | DMSO | Trace |
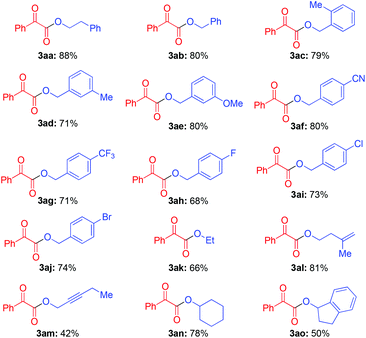
Under optimized conditions, the substrate scope of alcohols (2) was investigated (Table 2). Besides 2-phenylethanol (2a), various primary alcohols also worked well (3ab–3ao, Table 2). When using benzyl alcohol derivatives as starting materials, substrates with electron donating and withdrawing groups at the para-position were examined. The results indicate that the electronic variation of the functional groups did not obviously affect the yield of desired products (3ab–3aj, Table 2). Furthermore, the steric hindrance of the substituent at the ortho-positions of the arene group did not affect the efficiency (3ac, Table 2). In addition, chloro- and bromo-substituted benzyl alcohol afforded the desired product in good yields (3ai and 3aj, Table 2). It is noteworthy that simple alkyl alcohols also worked well under this reaction system (3ak–3am, Table 2). Notably, the alcohol substrate containing an alkenyl group performed well and afforded the desired product in 81% yield (3al, Table 2), which could not be achieved by our recent work (eqn (1)).9 Under the standard conditions, some secondary alcohols, such as cyclohexanol and 1-hydroxyhydrindene, also worked well (3an and 3ao, Table 2). However, tertiary alcohols, such as tertiary butanol, cannot afford the desired product under these reaction conditions.
| a Reaction conditions: 1a (0.25 mmol), 2 (0.375 mmol), CuBr (0.025 mmol), pyridine (0.0125 mmol), toluene (1.5 mL) at 90 °C under air condition for 18 h. Isolated yield. |
|---|
|
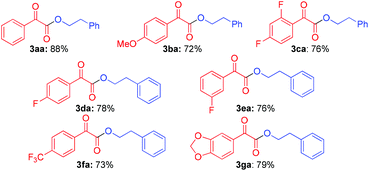
The scope of substituted α-carbonyl aldehydes 1 was also investigated (Table 3). This aromatic ring of α-carbonyl aldehyde derivates was found to be tolerant to both electron-rich groups, such as methoxyl (3ba), and electron-deficient groups, such as the fluoride group (3ca, 3da and 3ea) and the trifluoromethyl group (3fa). Furthermore, heteroaryl substituted α-carbonyl aldehyde performed well under these conditions affording 3ga in 79% yield. However, aliphatic α-carbonyl aldehyde, such as 2-oxopropanal, did not work.
| a Reaction conditions: 1 (0.25 mmol), 2a (0.375 mmol), CuBr (0.025 mmol), pyridine (0.0125 mmol), toluene (1.5 mL) at 90 °C under air condition for 18 h. Isolated yield. |
|---|
|
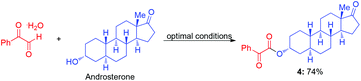
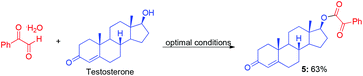
Because complex target molecules are very difficult to obtain, the late-stage modification of bio-active molecule is very important for medical chemistry studies. We selected androsterone, testosterone and cholesterol as starting materials to illustrate the advantage of this method in late-stage esterification to synthesize complex α-ketoester molecules (eqn (3)–(5)). These results indicate that the present Cu-catalyzed aerobic oxidative coupling reaction is very efficient and practical. In contrast to the low yield (33%) by the reported method,9 the reaction of androsterone produced the corresponding α-ketoester in 74% yield (eqn (3)).
 | (3) |
 | (4) |
 | (5) |
Moreover, the control reaction of 1a and 2a in the presence of 18O2 generated showed that no oxygen-atom incorporation is involved in this transformation. Air is proved only as a very efficient oxidant in this case.
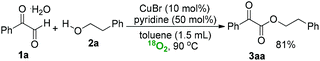 | (6) |
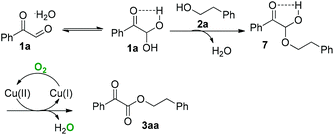
On the basis of the above results and our previous studies, a plausible mechanism for the copper-catalyzed aerobic oxidative coupling reaction is illustrated in Scheme 2. The interconversion between 2-oxo-2-phenylacetaldehyde hydrate (1a) and 2,2-dihydroxy-1-phenylethanone (1a) should be involved in this reaction process.11 Under this reaction system, the addition of alcohol (2a) to arylglyoxal (1a) to afford the hemiacetal intermediate 7.12 The carbonyl group of α-carbonyl aldehyde could facilitate this process.13 Subsequently, copper-catalyzed aerobic oxidation of intermediate 7 occurs to produce α-ketoamide 3aa.14,15
In conclusion, we have demonstrated a practical and efficient approach to α-ketoesters, which are widely spread units in many biologically active compounds. Using air as the oxidant, various commercially available α-carbonyl aldehyde substrates and alcohol derivatives could be smoothly transformed into desired products. Broad substrate scope, high atom economy and mild reaction conditions make this chemistry very practical. Further investigations of the synthetic application are ongoing in our group.
Financial support from the National Science Foundation of China (no. 21325206, 21172006), the National Young Top-notch Talent Support Program, and the Ph.D. Programs Foundation of the Ministry of Education of China (no. 20120001110013) are greatly appreciated. We thank Xiaoyang Wang in this group for reproducing the results of 3am and 6b.
Notes and references
- (a) Y. Nie, R. Xiao, Y. Xu and G. T. Montelione, Org. Biomol. Chem., 2011, 9, 4070 RSC; (b) T. Kawasuji and T. Yoshinaga, PCT Int. Appl., WO 2001017968 A1 20010315, 2001 Search PubMed; (c) Y. Aoki, S. Tanimoto, D. Takahashi and K. Toshima, Chem. Commun., 2013, 49, 1169 RSC; (d) I. Hemeon and A. J. Bennet, Synthesis, 2007, 1899 CAS.
- (a) Y. Gravenfors, J. Viklund, J. Blid, T. Ginman, S. Karlström, J. Kihlström, K. Kolmodin, J. Lindström, S. Berg, F. Kieseritzky, C. Slivo, B.-M. Swahn, L.-L. Olsson, P. Johansson, S. Eketjäll, J. Fälting, F. Jeppsson, K. Strömberg, J. Janson and F. Rahm, J. Med. Chem., 2012, 55, 9297 CrossRef CAS PubMed; (b) B. A. Boughton, L. Hor, J. A. Gerrard and C. A. Hutton, Bioorg. Med. Chem., 2012, 20, 2419 CrossRef CAS PubMed.
- For some selected examples, see: (a) A. De, V. Mauro, D. H. R. Barton, I. Bytheway, J. A. Ferreira, M. B. Hall, W. Liu, D. K. Taylor and L. Thomson, J. Am. Chem. Soc., 1995, 117, 4870 CrossRef; (b) A. Bacchi, M. Costa, C. N. Della, B. Gabriele, G. Salerno and S. Cassoni, J. Org. Chem., 2005, 70, 4971 CrossRef CAS PubMed; (c) P. Wipf and C. R. J. Stephenson, Org. Lett., 2003, 5, 2449 CrossRef CAS PubMed.
- For some selected examples, see: (a) V. Ozawa, N. Kawasaki, T. Yamamoto and A. Yamamoto, Chem. Lett., 1985, 567 CrossRef; (b) T. Sakakura, H. Yamashita, T. Kobayashi, T. Hayashi and M. Tanaka, J. Org. Chem., 1987, 52, 5733 CrossRef CAS.
- For some selected examples, see: (a) S. R. Reddy, S. Stella and A. Chadha, Synth. Commun., 2012, 42, 3493 CrossRef CAS; (b) R. Hosseinzadeh, M. Mohadjerani, M. Tajbakhsh and M. Nouzarian, Synth. Commun., 2011, 41, 1725 CrossRef CAS; (c) E. V. Johnston, E. A. Karlsson, L.-H. Tran, B. Aakermark and J.-E. Baeckvall, Eur. J. Org. Chem., 2010, 1971 CrossRef CAS.
- For some selected examples in recent 3 years, see: (a) C. G. Screttas, B. R. Steele, M. Micha-Screttas and G. A. Heropoulos, Org. Lett., 2012, 14, 5680 CrossRef CAS PubMed; (b) S. H. Kim, K. H. Kim and J. N. Kim, Adv. Synth. Catal., 2011, 353, 3335 CrossRef CAS; (c) Y. Su, L. Zhang and N. Jiao, Org. Lett., 2011, 13, 2168 CrossRef CAS PubMed; (d) M. Hayashi and S. Nakamura, Angew. Chem., Int. Ed., 2011, 50, 2249 CrossRef CAS PubMed; (e) M. Lamani and K. R. Prabhu, Angew. Chem., Int. Ed., 2010, 49, 6622 CrossRef CAS PubMed; (f) K. C. Nicolaou, Q. Kang, T. R. Wu, C. S. Lim and D. Y.-K. Chen, J. Am. Chem. Soc., 2010, 132, 7540 CrossRef CAS PubMed.
- For some selected reviews on cross-dehydrogenative-coupling (CDC) reaction, see: (a) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (b) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed . For some selected examples of (CDC) reactions, see: ; (c) Y. Zhang and C.-J. Li, J. Am. Chem. Soc., 2006, 128, 4242 CrossRef CAS PubMed; (d) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2006, 128, 56 CrossRef CAS PubMed; (e) Y. Zhang and C.-J. Li, Angew. Chem., Int. Ed., 2006, 45, 1949 CrossRef CAS PubMed; (f) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 3672 CrossRef CAS PubMed.
- For some selected reviews about copper catalyzed aerobic oxidative dehydrogenative coupling reaction in recent 3 years, see: (a) A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062 CrossRef CAS PubMed; (b) Z. Shi, C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381 RSC; (c) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464 RSC; (d) A. N. Campbell and S. S. Stahl, Acc. Chem. Res., 2012, 45, 851 CrossRef CAS PubMed . For some selected examples about copper catalyzed aerobic oxidative dehydrogenative coupling reaction in recent 3 years, see: ; (e) S. Chiba, L. Zhang and J.-Y. Lee, J. Am. Chem. Soc., 2010, 132, 7266 CrossRef CAS PubMed; (f) H. Wang, Y. Wang, D. Liang, L. Liu, J. Zhang and Q. Zhu, Angew. Chem., Int. Ed., 2011, 50, 5678 CrossRef CAS PubMed; (g) J. Wang, J. Wang, Y. Zhu, P. Lu and Y. Wang, Chem. Commun., 2011, 47, 3275 RSC; (h) A. Hausser, M. Trautmann and E. Roduner, Chem. Commun., 2011, 47, 6954 RSC; (i) I. Garcia-Bosch, A. Company, J. R. Frisch, M. Torrent-Sucarrat, M. Cardellach, I. Gamba, M. Güell, L. Casella, L. Que, Jr., X. Ribas, J. M. Luis and M. Costas, Angew. Chem., Int. Ed., 2010, 49, 2406 CrossRef CAS PubMed; (j) C. Zhang, L. Zhang and N. Jiao, Adv. Synth. Catal., 2012, 354, 1293 CrossRef CAS; (k) Q. Liu, P. Wu, Y. Yang, Z. Zeng, J. Liu, H. Yi and A. Lei, Angew. Chem., Int. Ed., 2012, 51, 4666 CrossRef CAS PubMed; (l) Z. Xu, C. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2012, 51, 11367 CrossRef CAS PubMed; (m) X. Li, L. Huang, H. Chen, W. Wu, H. Huang and H. Jiang, Chem. Sci., 2012, 3, 3463 RSC; (n) F.-T. Du and J.-X. Ji, Chem. Sci., 2012, 3, 460 RSC.
- C. Zhang, P. Feng and N. Jiao, J. Am. Chem. Soc., 2013, 135, 15257 CrossRef CAS PubMed.
- For some selected examples about oxidative esterification of aldehydes, see: (a) C. B. Kelly, M. A. Mercadante, R. J. Wiles and N. E. Leadbeater, Org. Lett., 2013, 15, 2222 CrossRef CAS PubMed; (b) X.-F. Wu, Tetrahedron Lett., 2012, 53, 3397 CrossRef CAS PubMed; (c) J. Feng, S. Liang, S.-Y. Chen, J. Zhang, S.-S. Fu and X.-Q. Yu, Adv. Synth. Catal., 2012, 354, 1287 CrossRef CAS; (d) K. Suzuki, T. Yamaguchi, K. Matsushita, C. Iitsuka, J. Miura, T. Akaogi and H. Ishida, ACS Catal., 2013, 3, 1845 CrossRef CAS; (e) Y. Zhu and Y. Wei, RSC Adv., 2013, 3, 13668 RSC; (f) B. R. Travis, M. Sivakumar, G. O. Hollist and B. Borhan, Org. Lett., 2003, 5, 1031 CrossRef CAS PubMed . For some reviews, see: ; (g) K. Ekoue-Kovi and C. Wolf, Chem.–Eur. J., 2008, 14, 6302 CrossRef CAS PubMed; (h) R. Samanta, K. Matcha and A. P. Antonchick, Eur. J. Org. Chem., 2013, 5769 CrossRef CAS.
- A.-K. C. Schmidt and C. B. W. Stark, Org. Lett., 2011, 13, 4164 CrossRef CAS PubMed.
- (a) C. Liu, J. Wang, L. Meng, Y. Deng, Y. Li and A. Lei, Angew. Chem., Int. Ed., 2011, 50, 5144 CrossRef CAS PubMed; (b) S. Gowrisankar, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 5139 CrossRef CAS PubMed; (c) M. Zhang, S. Zhang, G. Zhang, F. Chen and J. Cheng, Tetrahedron Lett., 2011, 52, 2480 CrossRef CAS PubMed; (d) P. R. Likhar and A. K. Bandyopadhyay, Synlett, 2000, 538 CAS; (e) S. J. Jin, P. K. Arora and L. M. Sayre, J. Org. Chem., 1990, 55, 3011 CrossRef CAS.
- C. Zhang, X. Zong, L. Zhang and N. Jiao, Org. Lett., 2012, 14, 3280 CrossRef CAS PubMed.
- C. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2010, 49, 6174 CrossRef CAS PubMed.
- S. Murata, K. Suzuki, M. Miura and M. Nomura, J. Chem. Soc., Perkin Trans. 1, 1990, 361 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3qo00041a |
| This journal is © the Partner Organisations 2014 |