On the mechanism of the direct acid catalyzed formation of 2,3-disubstituted imidazo[1,2-a]pyridines from 2-aminopyridines and acetophenones. Concurrence between ketimine and Ortoleva–King type reaction intermediated transformations†‡
Vanya B.
Kurteva
*,
Lubomir A.
Lubenov
and
Daniela V.
Antonova
Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str., bl. 9, 1113 Sofia, Bulgaria. E-mail: vkurteva@orgchm.bas.bg; Fax: +359 2 8700225; Tel: +359 2 9606156
First published on 31st October 2013
Abstract
Direct acid catalyzed formation of 2,3-disubstituted imidazo[1,2-a]pyridines from 2-aminopyridines and acetophenones was studied in order to gain insight into the reaction mechanism. The formation of differently substituted products was explained by a concurrent ketimine and Ortoleva–King type reaction intermediated transformations. The dependence of the reaction output on the catalyst used and on acetophenone and pyridine substituents was discussed.
Introduction
Imidazo[1,2-a]pyridines are isosters of indoles and azaindoles of great importance due to their remarkable biological properties.1 These profiles are strongly dependent on the substitution pattern. For instance, several available on the market drugs with imidazopyridine skeleton possess p-substituted aromatic group at 2-position. Among them, the anticancer agent zolimidine is a monosubstituted compound, while the group of 2,3-disubstituted products includes the sedative and anxiolytic drugs alpidem, saripidem, necopidem, and the agent for treatment of insomnia and some brain disorders zolpidem.Several protocols for the synthesis of this important framework have been developed.2 The main part starts from commercially available 2-aminopyridines and can be classified in two general groups depending on the mechanism: initial coupling reaction with endocyclic nitrogen followed by intramolecular cyclization with the exocyclic amino group, or reversed sequence, initial attack on the exocyclic nitrogen followed by cyclization with the participation of the endocyclic. The first group involves interaction with various reagents, like halocarbonyl compounds, diazoketones, diols, oxothioamides, etc., and goes via N1-substituted intermediates possessing carbonyl functionality capable to react further with the exocyclic amino group.3 Recently, an efficient tandem one-pot protocol has been achieved;4 formation of β-ketoalkyl-2-amino pyridinium iodides via Ortoleva–King reaction and subsequent cyclization in basic conditions.
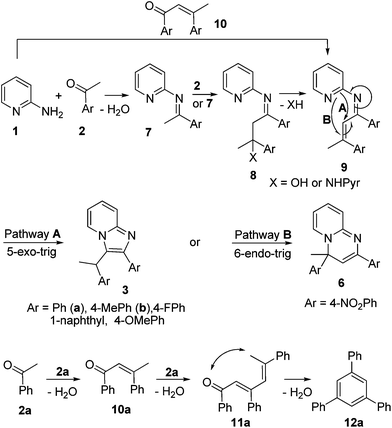
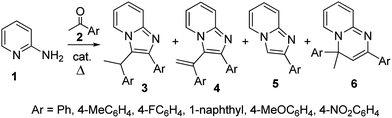
The second series contains initial formation of imine intermediate followed by intramolecular cyclization.5 The multicomponent reactions with aldehydes and various reagents are intensively exploited,6 while the transformations with ketones leading to 2,3-disubstituted compounds are poorly studied.7 Recently, we reported on the direct formation of differently substituted imidazo[1,2-a]pyridines from 2-aminopyridine and acetophenones by heating in solventless conditions.8 Three types of imidazopyridines were obtained; 3-(1-arylethane)-2-aryl (3), 3-(1-arylethene)-2-aryl (4) and 2-aryl (5) substituted compounds (Scheme 1), and was shown that the products substitution pattern is strongly dependent both on the catalyst used and on the acetophenone aromatic substituent.
 | ||
| Scheme 1 Direct formation of imidazo[1,2-a]pyridines from 2-aminopyridine and acetophenones.8 | ||
The formation of these compounds, however, cannot be explained by a common mechanism. A concurrence between simultaneously operating reactions, described herein, serves a reliable solution. The influence of acetophenone and pyridine substituent on the reaction output is also studied.
Results and discussion
The general concept of our study was to achieve one-pot conversion of 2-aminopyridine into 2,3-disubstituted imidazo[1,2-a]pyridines via initial formation of ketimine with acetophenone aldol product. So, the transformation was carried out with 5-fold excess of ketone in the presence of acid catalyst, which catalyzes the aldol condensation as well. However, the strategy was successful only in few cases, while other products were also isolated in the rest, which formation cannot be achieved from the corresponding ketimine intermediates.As reported,8 imidazopyridines 3 were isolated as sole products in good yields from p-toluenesulfonic acid catalyzed reaction of acetophenones without or with electrodonating inductively or through resonance substituents (acetophenone, 4-methylacetophenone, 4-fluoroacetophenone, and aceto-1-naphthone). Contrary, the sulfuric acid catalyzed transformation with acetophenone led to excellent conversion but an easy separable mixture of 3 and 4 was obtained. The reaction of 4-methoxyacetophenone was the only exception where both catalysts generated a mixture of products 3, 4 and 5; conversion being better with p-toluenesulfonic acid. With ketones possessing an electron withdrawing substituent (−M, −I), 4-nitroacetophenone and 2-nitroacetophenone, traces of products were isolated only in the first case; 6via p-toluenesulfonic acid catalyzed reaction and 4 in the presence of sulfuric acid.
The formation of 3-(1-arylethane)-2-aryl-imidazo[1,2-a]pyridines (3) and pyrido[1,2-a]pyrimidine (6) can be explain by 5-exo-trig or 6-endo-trig cyclization of enimines 9, respectively (Scheme 2). Intermediate 9 can be generated by dimerization of ketimine 7 or its interaction with acetophenone followed by elimination of the leaving group in 8 or by direct interaction of 1 with acetophenone aldol product 10. The latter was detected in the nonpolar fractions of the reaction mixture by NMR spectra. Additionally, the reference experiment was carried out, refluxing acetophenone with catalytic amount of p-toluenesulfonic acid in the same conditions. After 1 h heating the mixture contained mainly 2a, 10a and 1,3,5-triphenylbenzene 12a,9 detected by NMR and GC-MS of the crude reaction mixture. With the prolongation of the reaction, the amount of 10a decreased and that of 12a increased, which is an indication that the latter is formed from 10a; most probably by subsequent intermolecular and intramolecular aldol condensation as a small peak of dienone 11a was also found. Analogues acid catalyzed cyclotrimerization of acetophenone is well known.10
The participation of a ketimine intermediate was confirmed by refluxing a sample of the previously isolated 7a with acetophenone in the presence of p-toluenesulphonic acid, where almost complete conversion to 3a was detected by TLC and proton NMR of the crude reaction mixture within 30 min.
The cyclization in 9 can be achieved by attack on one of the olefinic carbons depending on the electronic density distribution. As seen, an electron donating group (X, Me, OMe) accelerates the 5-exo-trig cyclization to 3 (pathway A), while an electron withdrawing (NO2) favours the formation of 6via 6-endo-trig ring-closure (pathway B). The latter was isolated in very poor yield most probably due to reduced reactivity of the ketone or/and low stability of the intermediate.
The intramolecular cyclization of 7 has to be accomplished via disfavoured by Baldwin's rules 4-endo-trig ring-closure to the corresponding 2-methyl-1,2-dihydro-1,3-diazetepyridines, which can explain the fact that these products were not detected in the reaction mixtures.
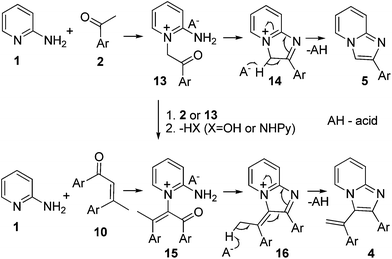
From the other side, imidazopyridines 4 and 5 cannot be formed by the proposed ketimine mechanism. Compound 5a has been obtained via tandem one-pot transformation, Ortoleva–King reaction followed by cyclization in basic conditions.4 This pathway explains the generation of 5 from Ortoleva–King type intermediate 13 (Scheme 3), formed probably by the attack of the endocyclic nitrogen on an aldol intermediate. The dimerization of the latter or aldol reaction with acetophenone followed by elimination of the leaving group or direct reaction between 1 and 10 lead to intermediate 15, the precursor of vinylated products 4.
The formation of 4 in our case is due to the higher catalytic activity of p-toluenesulfonic and sulfuric acid in aldol reactions in respect to iodine. This suggestion as well as the mechanism of the formation of 4 and 5 was confirmed by performing the transformation in the presence of iodine, where 5a was the only product after heating at 110 °C in 20% yield without alkaline treatment and 43% after heating with sodium hydroxide according to the reported protocol. When the transformation was carried out at 210 °C without alkaline step, both 4a and 5a were isolated in 10% and 22% yields, respectively.
Based on these results, it can be concluded that ketimine and Ortoleva–King type reaction intermediated transformations operate simultaneously in the reaction studied leading to the formation of 3-(1-arylethane)-2-aryl (3) and 3-(1-arylethene)-2-aryl (4) imidazopyridines, respectively. To the best of our knowledge, the Ortoleva–King reaction has been never achieved in acid catalysis conditions.
The influence of acetophenone and pyridine substituents on the reaction output was further studied on the example of the most efficient in our previous work acetophenone substituents, Me (+I) and X (−I, +M). The initial idea was to carry out the reaction at constant conditions, heating at 210 °C for 1 h. However, very low conversion was achieved with 4-methyl- and 4-chloroacetophenone; almost quantitative recovering of aminopyridine. So, the transformation was performed with both catalysts under gentle reflux, i.e. at the boiling point of the corresponding acetophenone, which was used in 5-fold excess; acetophenone 202 °C, 4-methylacetophenone 226 °C, 4-chloroacetophenone 232 °C. The prolongation of the reaction was 1 h in an attempt to achieve complete conversion of aminopyridine in all experiments. The products were easily separated by flash chromatography on silica gel. The results are summarized on Table 1.
| Entry | Ar | R | Catalyst | Imidazopyridine 3a | Imidazopyridine 4a |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio 4 ratio |
||
|---|---|---|---|---|---|---|---|---|
| Product | Yield % | Product | Yield % | |||||
| a Isolated yields by flash chromatography. | ||||||||
| 1 | Ph | H | p-TSA | 3a | 72 | 4a | 4 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 2 | H2SO4 | 50 | 11 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
||||
| 3 | 4-MeC6H4 | H | p-TSA | 3b | 91 | 4b | 5 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 4 | H2SO4 | 65 | 13 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
||||
| 5 | 4-ClC6H4 | H | p-TSA | 3c | 71 | 4c | 12 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 6 | H2SO4 | 68 | 15 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
||||
| 7 | Ph | Me | p-TSA | 3d | 86 | 4d | 6 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 8 | H2SO4 | 63 | 9 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
||||
| 9 | Ph | Cl | p-TSA | 3e | 66 | 4e | 15 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 10 | H2SO4 | 56 | 21 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
||||
| 11 | Ph | Br | p-TSA | 3f | 63 | 4f | 18 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 12 | H2SO4 | 50 | 22 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
||||
| 13 | 4-MeC6H4 | Me | p-TSA | 3g | 77 | 4g | 9 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 14 | H2SO4 | 69 | 11 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
||||
| 15 | 4-MeC6H4 | Cl | p-TSA | 3h | 46 | 4h | 20 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 16 | H2SO4 | 42 | 26 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
||||
| 17 | 4-ClC6H4 | Me | p-TSA | 3i | 75 | 4i | 15 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| 18 | H2SO4 | 71 | 17 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
||||
| 19 | 4-ClC6H4 | Cl | p-TSA | 3j | 64 | 4j | 16 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 20 | H2SO4 | 58 | 25 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
||||
Good to excellent conversions were achieved in general. Imidazopyridines 3 and 4 were the only products formed, as detected by TLC, which were isolated in good to excellent overall yields in all cases except 3 h and 4 h (entries 15 and 16), where side-products formation was observed with both catalysts and the products were isolated in 66% and 68% with p-TSA and H2SO4, respectively. The data show that p-toluenesulfonic acid tolerates ketimine formation, while sulfuric acid catalyzes the generation of vinyl substituted product 4 as well. The latter is most probably due to the higher acidity of sulfuric acid in respect to p-toluenesulfonic acid, making it capable to form easier the pyridinium salts or/and leading to an increased part of the imine tautomer.11
The influence of the substituent on the products distribution is clearly demonstrated on the case of aminopyridine's variation as the transformations were performed at the same reaction conditions (temperature). As seen, almost the same proportions 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were obtained with 2-aminopyridine and 2-amino-5-methylpyridine (entries 1–6 vs. 7, 8, 13, 14, 17 and 18). The latter is an indication that an electron donating by inductive effect substituent (Me) does not influence the reaction output significantly. Contrary, the reactions with 2-amino-5-halogenopyridines led to the increased percentage of vinylated products 4 (entries 9–12, 15, 16, 19 and 20). So, it can be concluded that an electron withdrawing inductively but electron donating through resonance (Cl, Br) aminopyridine substituent accelerates the Ortoleva–King type intermediated transformation; the effect being more significant in the presence of sulfuric acid. The same pattern was observed when acetophenone substituent was varied (entries 1, 2, 7–12 vs. 3, 4, 13–16 vs. 5, 6, 17–20). A comparison between products possessing chlorine substituent in pyridine and in acetophenone fragment (entries 9, 10 vs. 5, 6) shows that the effect is stronger when the substituent belongs to pyridine unit.
4 were obtained with 2-aminopyridine and 2-amino-5-methylpyridine (entries 1–6 vs. 7, 8, 13, 14, 17 and 18). The latter is an indication that an electron donating by inductive effect substituent (Me) does not influence the reaction output significantly. Contrary, the reactions with 2-amino-5-halogenopyridines led to the increased percentage of vinylated products 4 (entries 9–12, 15, 16, 19 and 20). So, it can be concluded that an electron withdrawing inductively but electron donating through resonance (Cl, Br) aminopyridine substituent accelerates the Ortoleva–King type intermediated transformation; the effect being more significant in the presence of sulfuric acid. The same pattern was observed when acetophenone substituent was varied (entries 1, 2, 7–12 vs. 3, 4, 13–16 vs. 5, 6, 17–20). A comparison between products possessing chlorine substituent in pyridine and in acetophenone fragment (entries 9, 10 vs. 5, 6) shows that the effect is stronger when the substituent belongs to pyridine unit.
Conclusions
In summary, the direct formation of 2,3-disubstituted imidazo[1,2-a]pyridines from 2-aminopyridines and acetophenones was studied and the products substitution pattern was explained by a concurrent ketimine and Ortoleva–King type reaction intermediated transformations. It was shown that p-toluenesulfonic acid tolerates ketimine formation, while sulfuric acid catalyzes both reactions. From the other side, an electron donating by inductive effect (Me; +I) acetophenone or pyridine substituent does not influence the reaction output significantly, whereas an electron withdrawing inductively but electron donating through resonance (X; −I, +M) substituent accelerates the formation of Ortoleva–King type intermediate.Experimental
General procedures
All reagents were purchased from Aldrich, Merck and Fluka and were used without any further purification. Fluka silica gel/TLC-cards 60778 with fluorescent indicator 254 nm were used for TLC chromatography and Rf-values determination. Merck Silica gel 60 (0.040–0.063 mm) was used for flash chromatography purification of the products. The melting points were determined in capillary tubes on SRS MPA100 OptiMelt (Sunnyvale, CA, USA) automated melting point system. The NMR spectra were recorded on a Bruker Avance II+ 600 spectrometer (Rheinstetten, Germany) at 25 °C; the chemical shifts were quoted in ppm in δ-values against tetramethylsilane (TMS) as an internal standard and the coupling constants were calculated in Hz. The assignment of the signals was confirmed by applying 2D techniques. The spectra were recorded as 10−1 M solutions in deuterochloroform except NOESY experiments where 3 × 10−2 M solutions were used in order to avoid transmolecular interactions. The low resolution mass spectra were taken on a HP 5973 Mass Selective Detector, the high resolution mass spectra on a DFS High Resolution Magnetic Sector MS, Thermo Scientific.Typical procedure
A mixture of 2-aminopyridine (1 mmol), acetophenone (5 mmol), and p-toluenesulphonic acid (17 mg, 10 mol %) or sulfuric acid (10 mg, 10 mol %) was refluxed for 1 h. The products were purified by flash chromatography on silica gel by using mobile phase with a gradient of polarity from CH2Cl2 to acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 5
CH2Cl2 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95. The results are summarized on Table 1. The characterization of compounds 3a, 3b and 4a is published in ref. 8.
95. The results are summarized on Table 1. The characterization of compounds 3a, 3b and 4a is published in ref. 8.
R
f 0.42 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 5
CH2Cl2 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95); mp 134.6–135.7 °C; 1H NMR 1.712 (d, 3H, J 7.4, CH3), 4.820 (q, 1H, J 7.4, CH–CH3), 6.537 (t, 1H, J 6.8, CH-6), 7.070 (m, 3H, CH-7 and CH-2 and CH-6 of 4-ClPh-3), 7.194 (d, 2H, J 8.3, CH-3 and CH-5 of 4-ClPh-3), 7.342 (d, 2H, J 8.5, CH-3 and CH-5 of 4-ClPh-2), 7.481 (dt, 1H, J 1.1, 7.0, CH-5), 7.562 (d, 2H, J 8.5, CH-2 and CH-6 of 4-ClPh-2), 7.751 (1H, overlapped, CH-8); COSY cross peaks 1.712/4.820, 6.537/7.070, 6.537/7.481, 7.070/7.194, 7.070/7.751, 7.342/7.562; NOESY cross peaks 1.712/4.820, 1.712/7.070, 1.712/7.481, 4.820/7.562, 6.537/7.070, 6.537/7.481, 7.070/7.194, 7.070/7.751, 7.342/7.562; 13C NMR 16.82 (CH3), 33.42 (CH–CH3), 112.08 (CH-6), 117.94 (CH-8), 122.57 (Cquat-3), 124.34 (CH-5), 124.40 (CH-7), 128.22 (CH-2 and CH-6 of 4-ClPh-3), 128.78 (CH-3 and CH-5 of 4-ClPh-2), 129.07 (CH-3 and CH-5 of 4-ClPh-3), 130.15 (CH-2 and CH-6 of 4-ClPh-2), 132.68 (Cquat-4 of 4-ClPh-3), 133.33 (Cquat-1 of 4-ClPh-2), 133.89 (Cquat-4 of 4-ClPh-2), 139.41 (Cquat-1 of 4-ClPh-3), 142.67 (Cquat-2), 145.01 (Cquat-9); HSQC cross peaks 1.712/16.82, 4.820/33.42, 6.537/112.08, 7.070/124.40, 7.070/128.22, 7.194/129.07, 7.342/128.78, 7.481/124.34, 7.562/130.15, 7.751/117.94; HMBC cross peaks 1.712/33.42, 1.712/122.57, 1.712/139.41, 4.820/16.82, 4.820/122.57, 4.820/128.22, 4.820/139.41, 4.820/142.67 (weak), 6.537/117.94, 6.537/124.34, 6.537/124.40, 7.070/33.42, 7.070/124.34, 7.070/128.22, 7.070/132.68, 7.070/145.01, 7.194/129.07, 7.194/132.68 (weak), 7.194/139.41, 7.342/128.78, 7.342/133.33, 7.481/112.08, 7.481/124.40, 7.481/145.01, 7.562/130.15, 7.562/133.33, 7.562/142.67, 7.751/112.08, 7.751/145.01 (weak); MS (ESI, pos. mode) m/z 367.17 [M + H+]; HRMS (EI): calcd for C21H16.Cl2N2 (M+) 366.0685, found 366.0656, −7.19 ppm error.
95); mp 134.6–135.7 °C; 1H NMR 1.712 (d, 3H, J 7.4, CH3), 4.820 (q, 1H, J 7.4, CH–CH3), 6.537 (t, 1H, J 6.8, CH-6), 7.070 (m, 3H, CH-7 and CH-2 and CH-6 of 4-ClPh-3), 7.194 (d, 2H, J 8.3, CH-3 and CH-5 of 4-ClPh-3), 7.342 (d, 2H, J 8.5, CH-3 and CH-5 of 4-ClPh-2), 7.481 (dt, 1H, J 1.1, 7.0, CH-5), 7.562 (d, 2H, J 8.5, CH-2 and CH-6 of 4-ClPh-2), 7.751 (1H, overlapped, CH-8); COSY cross peaks 1.712/4.820, 6.537/7.070, 6.537/7.481, 7.070/7.194, 7.070/7.751, 7.342/7.562; NOESY cross peaks 1.712/4.820, 1.712/7.070, 1.712/7.481, 4.820/7.562, 6.537/7.070, 6.537/7.481, 7.070/7.194, 7.070/7.751, 7.342/7.562; 13C NMR 16.82 (CH3), 33.42 (CH–CH3), 112.08 (CH-6), 117.94 (CH-8), 122.57 (Cquat-3), 124.34 (CH-5), 124.40 (CH-7), 128.22 (CH-2 and CH-6 of 4-ClPh-3), 128.78 (CH-3 and CH-5 of 4-ClPh-2), 129.07 (CH-3 and CH-5 of 4-ClPh-3), 130.15 (CH-2 and CH-6 of 4-ClPh-2), 132.68 (Cquat-4 of 4-ClPh-3), 133.33 (Cquat-1 of 4-ClPh-2), 133.89 (Cquat-4 of 4-ClPh-2), 139.41 (Cquat-1 of 4-ClPh-3), 142.67 (Cquat-2), 145.01 (Cquat-9); HSQC cross peaks 1.712/16.82, 4.820/33.42, 6.537/112.08, 7.070/124.40, 7.070/128.22, 7.194/129.07, 7.342/128.78, 7.481/124.34, 7.562/130.15, 7.751/117.94; HMBC cross peaks 1.712/33.42, 1.712/122.57, 1.712/139.41, 4.820/16.82, 4.820/122.57, 4.820/128.22, 4.820/139.41, 4.820/142.67 (weak), 6.537/117.94, 6.537/124.34, 6.537/124.40, 7.070/33.42, 7.070/124.34, 7.070/128.22, 7.070/132.68, 7.070/145.01, 7.194/129.07, 7.194/132.68 (weak), 7.194/139.41, 7.342/128.78, 7.342/133.33, 7.481/112.08, 7.481/124.40, 7.481/145.01, 7.562/130.15, 7.562/133.33, 7.562/142.67, 7.751/112.08, 7.751/145.01 (weak); MS (ESI, pos. mode) m/z 367.17 [M + H+]; HRMS (EI): calcd for C21H16.Cl2N2 (M+) 366.0685, found 366.0656, −7.19 ppm error.
R
f 0.16 (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 1
CH2Cl2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99); mp 111.3–112.5 °C; 1H NMR 1.799 (d, 3H, J 7.4, CH3–CH), 2.150 (d, 3H, J 0.9, CH3-6), 4.977 (q, 1H, J 7.4, CH–CH3), 6.973 (dd, 1H, J 1.6, 9.2, CH-7), 7.246 (m, 3H, CH-2, CH-4 and CH-6 of Ph-3), 7.315 (ddt, 2H, J 1.7, 7.2, 7.9, CH-3 and CH-5 of Ph-3), 7.357 (tt, 1H, J 1.3, 7.4, CH-4 of Ph-2), 7.387 (dd, 1H, J 0.9, 1.6, CH-5), 7.436 (tt, 2H, J 1.6, 7.6, CH-3 and CH-5 of Ph-2), 7.559 (d, 1H, J 9.2, CH-8), 7.719 (dt, 2H, J 1.3, 7.8, CH-2 and CH-6 of Ph-2); COSY cross peaks 1.799/4.977, 2.150/7.387, 6.973/7.387 (weak), 6.973/7.559, 7.246/7.315, 7.357/7.436, 7.436/7.719; NOESY cross peaks 1.799/4.977, 1.799/7.246, 1.799/7.387, 1.799/7.719 (weak), 2.150/6.973, 2.150/7.387, 4.977/7.246, 4.977/7.719, 6.973/7.559, 7.246/7.387, 7.246/7.315, 7.357/7.436, 7.436/7.719; 13C NMR 16.84 (CH3–CH), 18.50 (CH3-6), 33.86 (CH–CH3), 117.09 (CH-8), 121.24 (Cquat-6), 122.20 (CH-5), 122.68 (Cquat-3), 126.63 (CH-7), 126.88 (CH-2 and CH-6 of Ph-3), 127.11 (CH-4 of Ph-3), 127.65 (CH-4 of Ph-2), 128.46 (CH-3 and CH-5 of Ph-2), 128.84 (CH-3 and CH-5 of Ph-3), 128.93 (CH-2 and CH-6 of Ph-2), 135.11 (Cquat-1 of Ph-2), 141.32 (Cquat-1 of Ph-3), 143.48 (Cquat-2), 143.94 (Cquat-9); HSQC cross peaks 1.799/16.84, 2.150/18.50, 4.977/33.86, 6.973/126.63, 7.246/126.88, 7.246/127.11, 7.315/128.84, 7.357/127.65, 7.387/122.20, 7.436/128.46, 7.559/117.09, 7.719/128.93; HMBC cross peaks 1.799/33.86, 1.799/122.68, 1.799/141.32, 2.150/121.24, 2.150/122.20, 2.150/126.63, 4.977/16.84, 4.977/122.68, 4.977/126.88 (weak), 4.977/141.32, 6.973/18.50, 6.973/122.20, 6.973/143.94, 7.246/33.86, 7.246/122.68 (weak), 7.246/126.88, 7.246/127.11, 7.315/128.84, 7.315/141.32, 7.357/128.93, 7.387/121.24 (weak), 7.387/126.63, 7.387/143.94, 7.436/128.46, 7.436/135.11, 7.559/121.24, 7.559/143.94 (weak), 7.719/127.65, 7.719/128.93, 7.719/143.48; MS (EI) m/z 312 [M+]; HRMS (EI): calcd for C22H20N2 (M+) 312.1621, found 312.1616, −1.4 ppm error.
99); mp 111.3–112.5 °C; 1H NMR 1.799 (d, 3H, J 7.4, CH3–CH), 2.150 (d, 3H, J 0.9, CH3-6), 4.977 (q, 1H, J 7.4, CH–CH3), 6.973 (dd, 1H, J 1.6, 9.2, CH-7), 7.246 (m, 3H, CH-2, CH-4 and CH-6 of Ph-3), 7.315 (ddt, 2H, J 1.7, 7.2, 7.9, CH-3 and CH-5 of Ph-3), 7.357 (tt, 1H, J 1.3, 7.4, CH-4 of Ph-2), 7.387 (dd, 1H, J 0.9, 1.6, CH-5), 7.436 (tt, 2H, J 1.6, 7.6, CH-3 and CH-5 of Ph-2), 7.559 (d, 1H, J 9.2, CH-8), 7.719 (dt, 2H, J 1.3, 7.8, CH-2 and CH-6 of Ph-2); COSY cross peaks 1.799/4.977, 2.150/7.387, 6.973/7.387 (weak), 6.973/7.559, 7.246/7.315, 7.357/7.436, 7.436/7.719; NOESY cross peaks 1.799/4.977, 1.799/7.246, 1.799/7.387, 1.799/7.719 (weak), 2.150/6.973, 2.150/7.387, 4.977/7.246, 4.977/7.719, 6.973/7.559, 7.246/7.387, 7.246/7.315, 7.357/7.436, 7.436/7.719; 13C NMR 16.84 (CH3–CH), 18.50 (CH3-6), 33.86 (CH–CH3), 117.09 (CH-8), 121.24 (Cquat-6), 122.20 (CH-5), 122.68 (Cquat-3), 126.63 (CH-7), 126.88 (CH-2 and CH-6 of Ph-3), 127.11 (CH-4 of Ph-3), 127.65 (CH-4 of Ph-2), 128.46 (CH-3 and CH-5 of Ph-2), 128.84 (CH-3 and CH-5 of Ph-3), 128.93 (CH-2 and CH-6 of Ph-2), 135.11 (Cquat-1 of Ph-2), 141.32 (Cquat-1 of Ph-3), 143.48 (Cquat-2), 143.94 (Cquat-9); HSQC cross peaks 1.799/16.84, 2.150/18.50, 4.977/33.86, 6.973/126.63, 7.246/126.88, 7.246/127.11, 7.315/128.84, 7.357/127.65, 7.387/122.20, 7.436/128.46, 7.559/117.09, 7.719/128.93; HMBC cross peaks 1.799/33.86, 1.799/122.68, 1.799/141.32, 2.150/121.24, 2.150/122.20, 2.150/126.63, 4.977/16.84, 4.977/122.68, 4.977/126.88 (weak), 4.977/141.32, 6.973/18.50, 6.973/122.20, 6.973/143.94, 7.246/33.86, 7.246/122.68 (weak), 7.246/126.88, 7.246/127.11, 7.315/128.84, 7.315/141.32, 7.357/128.93, 7.387/121.24 (weak), 7.387/126.63, 7.387/143.94, 7.436/128.46, 7.436/135.11, 7.559/121.24, 7.559/143.94 (weak), 7.719/127.65, 7.719/128.93, 7.719/143.48; MS (EI) m/z 312 [M+]; HRMS (EI): calcd for C22H20N2 (M+) 312.1621, found 312.1616, −1.4 ppm error.
R
f 0.33 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 2
CH2Cl2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98); mp 149.8–150.1 °C; 1H NMR 1.802 (d, 3H, J 7.4, CH3–CH), 4.992 (q, 1H, J 7.4, CH–CH3), 7.090 (dd, 1H, J 1.9, 9.5, CH-7), 7.235 (dd, 2H, J 0.9, 7.3, CH-2 and CH-6 of Ph-3), 7.260 (tt, 1H, J 0.9, 7.5, CH-4 of Ph-3), 7.336 (tt, 2H, J 2.1, 7.7, CH-3 and CH-5 of Ph-3), 7.389 (ddt, 1H, J 1.1, 7.1, 7.6, CH-4 of Ph-2), 7.458 (tt, 2H, J 0.9, 7.7, CH-3 and CH-5 of Ph-2), 7.601 (dd, 1H, J 0.7, 9.5, CH-8), 7.635 (dd, 1H, J 0.7, 1.9, CH-5), 7.720 (dd, 2H, J 1.1, 7.1, CH-2 and CH-6 of Ph-2); COSY cross peaks 1.802/4.992, 7.090/7.601, 7.235/7.336, 7.260/7.336, 7.389/7.458, 7.458/7.720; NOESY cross peaks 1.802/4.992, 1.802/7.235, 1.802/7.635, 4.992/7.235, 4.992/7.635 (weak), 4.992/7.720, 7.090/7.601, 7.090/7.720 (weak), 7.235/7.336, 7.235/7.635, 7.260/7.336, 7.389/7.458, 7.458/7.720; 13C NMR 16.75 (CH3–CH), 33.88 (CH–CH3), 118.18 (CH-8), 119.95 (Cquat-6), 122.36 (CH-5), 123.84 (Cquat-3), 125.40 (CH-7), 126.88 (CH-2 and CH-6 of Ph-3), 127.07 (CH-4 of Ph-3), 128.17 (CH-4 of Ph-2), 128.70 (CH-3 and CH-5 of Ph-2), 128.99 (CH-3 and CH-5 of Ph-3), 129.15 (CH-2 and CH-6 of Ph-2), 134.50 (Cquat-1 of Ph-2), 140.51 (Cquat-1 of Ph-3), 143.27 (Cquat-9), 144.77 (Cquat-2); HSQC cross peaks 1.802/16.75, 4.992/33.88, 7.090/125.40, 7.235/126.88, 7.260/127.07, 7.336/128.99, 7.389/128.17, 7.458/128.70, 7.601/118.18, 7.635/122.36, 7.720/129.15; HMBC cross peaks 1.802/33.88, 1.802/123.84, 1.802/140.51, 4.992/16.75, 4.992/123.84, 4.992/126.88, 4.992/140.51, 4.992/144.77 (weak), 7.090/119.95 (weak), 7.090/122.36, 7.090/143.27, 7.235/126.88, 7.235/127.07, 7.260/126.88, 7.336/128.99, 7.336/140.51, 7.389/129.15, 7.458/128.17, 7.458/128.70, 7.458/134.50, 7.601/119.95, 7.601/143.27 (weak), 7.635/119.95 (weak), 7.635/125.40, 7.635/143.27, 7.720/129.15, 7.720/144.77; MS (EI) m/z 332 [M+]; HRMS (EI): calcd for C21H17ClN2 (M+) 332.1075, found 332.1084, 2.7 ppm error.
98); mp 149.8–150.1 °C; 1H NMR 1.802 (d, 3H, J 7.4, CH3–CH), 4.992 (q, 1H, J 7.4, CH–CH3), 7.090 (dd, 1H, J 1.9, 9.5, CH-7), 7.235 (dd, 2H, J 0.9, 7.3, CH-2 and CH-6 of Ph-3), 7.260 (tt, 1H, J 0.9, 7.5, CH-4 of Ph-3), 7.336 (tt, 2H, J 2.1, 7.7, CH-3 and CH-5 of Ph-3), 7.389 (ddt, 1H, J 1.1, 7.1, 7.6, CH-4 of Ph-2), 7.458 (tt, 2H, J 0.9, 7.7, CH-3 and CH-5 of Ph-2), 7.601 (dd, 1H, J 0.7, 9.5, CH-8), 7.635 (dd, 1H, J 0.7, 1.9, CH-5), 7.720 (dd, 2H, J 1.1, 7.1, CH-2 and CH-6 of Ph-2); COSY cross peaks 1.802/4.992, 7.090/7.601, 7.235/7.336, 7.260/7.336, 7.389/7.458, 7.458/7.720; NOESY cross peaks 1.802/4.992, 1.802/7.235, 1.802/7.635, 4.992/7.235, 4.992/7.635 (weak), 4.992/7.720, 7.090/7.601, 7.090/7.720 (weak), 7.235/7.336, 7.235/7.635, 7.260/7.336, 7.389/7.458, 7.458/7.720; 13C NMR 16.75 (CH3–CH), 33.88 (CH–CH3), 118.18 (CH-8), 119.95 (Cquat-6), 122.36 (CH-5), 123.84 (Cquat-3), 125.40 (CH-7), 126.88 (CH-2 and CH-6 of Ph-3), 127.07 (CH-4 of Ph-3), 128.17 (CH-4 of Ph-2), 128.70 (CH-3 and CH-5 of Ph-2), 128.99 (CH-3 and CH-5 of Ph-3), 129.15 (CH-2 and CH-6 of Ph-2), 134.50 (Cquat-1 of Ph-2), 140.51 (Cquat-1 of Ph-3), 143.27 (Cquat-9), 144.77 (Cquat-2); HSQC cross peaks 1.802/16.75, 4.992/33.88, 7.090/125.40, 7.235/126.88, 7.260/127.07, 7.336/128.99, 7.389/128.17, 7.458/128.70, 7.601/118.18, 7.635/122.36, 7.720/129.15; HMBC cross peaks 1.802/33.88, 1.802/123.84, 1.802/140.51, 4.992/16.75, 4.992/123.84, 4.992/126.88, 4.992/140.51, 4.992/144.77 (weak), 7.090/119.95 (weak), 7.090/122.36, 7.090/143.27, 7.235/126.88, 7.235/127.07, 7.260/126.88, 7.336/128.99, 7.336/140.51, 7.389/129.15, 7.458/128.17, 7.458/128.70, 7.458/134.50, 7.601/119.95, 7.601/143.27 (weak), 7.635/119.95 (weak), 7.635/125.40, 7.635/143.27, 7.720/129.15, 7.720/144.77; MS (EI) m/z 332 [M+]; HRMS (EI): calcd for C21H17ClN2 (M+) 332.1075, found 332.1084, 2.7 ppm error.
R
f 0.33 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 2
CH2Cl2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98); mp 173.6–174.4 °C; 1H NMR 1.802 (d, 3H, J 7.4, CH3–CH), 4.989 (q, 1H, J 7.4, CH–CH3), 7.182 (dd, 1H, J 1.8, 9.5, CH-7), 7.232 (dd, 2H, J 1.1, 7.3, CH-2 and CH-6 of Ph-3), 7.263 (tt, 1H, J 0.7, 7.0, CH-4 of Ph-3), 7.339 (tt, 2H, J 1.6, 7.4, CH-3 and CH-5 of Ph-3), 7.390 (tt, 1H, J 1.9, 7.4, CH-4 of Ph-2), 7.457 (tt, 2H, J 1.6, 7.7, CH-3 and CH-5 of Ph-2), 7.553 (dd, 1H, J 0.6, 9.5, CH-8), 7.716 (ddd, 2H, J 1.4, 1.9, 7.0, CH-2 and CH-6 of Ph-2), 7.738 (dd, 1H, J 0.6, 1.8, CH-5); COSY cross peaks 1.802/4.989, 7.182/7.553, 7.182/7.738 (weak), 7.232/7.339, 7.263/7.339, 7.390/7.457, 7.457/7.716; NOESY cross peaks 1.802/4.989, 1.802/7.232, 1.802/7.738 (weak), 4.989/7.232, 4.989/7.716, 7.182/7.553, 7.232/7.339, 7.232/7.738 (weak), 7.263/7.339, 7.390/7.457, 7.457/7.716, 7.553/7.738 (weak); 13C NMR 16.73 (CH3–CH), 33.81 (CH–CH3), 106.44 (Cquat-6), 118.39 (CH-8), 123.61 (Cquat-3), 124.48 (CH-5), 126.79 (CH-2 and CH-6 of Ph-3), 126.99 (CH-4 of Ph-3), 127.37 (CH-7), 128.09 (CH-4 of Ph-2), 128.61 (CH-3 and CH-5 of Ph-2), 128.92 (CH-3 and CH-5 of Ph-3), 129.05 (CH-2 and CH-6 of Ph-2), 134.37 (Cquat-1 of Ph-2), 140.44 (Cquat-1 of Ph-3), 143.23 (Cquat-9), 144.46 (Cquat-2); HSQC cross peaks 1.802/16.73, 4.989/33.81, 7.182/127.37, 7.232/126.79, 7.263/126.99, 7.339/128.92, 7.390/128.09, 7.457/128.61, 7.553/118.39, 7.716/129.05, 7.738/124.48; HMBC cross peaks 1.802/33.81, 1.802/123.61, 1.802/140.44, 4.989/16.73, 4.989/123.61, 4.989/126.79, 4.989/140.44, 4.989/144.46 (weak), 7.182/106.44 (weak), 7.182/124.48, 7.182/143.23, 7.232/126.79, 7.232/126.99, 7.263/126.79, 7.339/128.92, 7.339/140.44, 7.390/128.61 (weak), 7.390/129.05, 7.457/128.61, 7.457/134.37, 7.553/106.44, 7.553/124.48 (weak), 7.553/127.37 (weak), 7.553/143.23, 7.716/129.05, 7.716/144.46, 7.738/118.39 (weak), 7.738/127.37, 7.738/143.23; MS (EI) m/z 376 [M+]; HRMS (EI): calcd for C21H17BrN2 (M+) 376.0570, found 376.0570, 0.1 ppm error.
98); mp 173.6–174.4 °C; 1H NMR 1.802 (d, 3H, J 7.4, CH3–CH), 4.989 (q, 1H, J 7.4, CH–CH3), 7.182 (dd, 1H, J 1.8, 9.5, CH-7), 7.232 (dd, 2H, J 1.1, 7.3, CH-2 and CH-6 of Ph-3), 7.263 (tt, 1H, J 0.7, 7.0, CH-4 of Ph-3), 7.339 (tt, 2H, J 1.6, 7.4, CH-3 and CH-5 of Ph-3), 7.390 (tt, 1H, J 1.9, 7.4, CH-4 of Ph-2), 7.457 (tt, 2H, J 1.6, 7.7, CH-3 and CH-5 of Ph-2), 7.553 (dd, 1H, J 0.6, 9.5, CH-8), 7.716 (ddd, 2H, J 1.4, 1.9, 7.0, CH-2 and CH-6 of Ph-2), 7.738 (dd, 1H, J 0.6, 1.8, CH-5); COSY cross peaks 1.802/4.989, 7.182/7.553, 7.182/7.738 (weak), 7.232/7.339, 7.263/7.339, 7.390/7.457, 7.457/7.716; NOESY cross peaks 1.802/4.989, 1.802/7.232, 1.802/7.738 (weak), 4.989/7.232, 4.989/7.716, 7.182/7.553, 7.232/7.339, 7.232/7.738 (weak), 7.263/7.339, 7.390/7.457, 7.457/7.716, 7.553/7.738 (weak); 13C NMR 16.73 (CH3–CH), 33.81 (CH–CH3), 106.44 (Cquat-6), 118.39 (CH-8), 123.61 (Cquat-3), 124.48 (CH-5), 126.79 (CH-2 and CH-6 of Ph-3), 126.99 (CH-4 of Ph-3), 127.37 (CH-7), 128.09 (CH-4 of Ph-2), 128.61 (CH-3 and CH-5 of Ph-2), 128.92 (CH-3 and CH-5 of Ph-3), 129.05 (CH-2 and CH-6 of Ph-2), 134.37 (Cquat-1 of Ph-2), 140.44 (Cquat-1 of Ph-3), 143.23 (Cquat-9), 144.46 (Cquat-2); HSQC cross peaks 1.802/16.73, 4.989/33.81, 7.182/127.37, 7.232/126.79, 7.263/126.99, 7.339/128.92, 7.390/128.09, 7.457/128.61, 7.553/118.39, 7.716/129.05, 7.738/124.48; HMBC cross peaks 1.802/33.81, 1.802/123.61, 1.802/140.44, 4.989/16.73, 4.989/123.61, 4.989/126.79, 4.989/140.44, 4.989/144.46 (weak), 7.182/106.44 (weak), 7.182/124.48, 7.182/143.23, 7.232/126.79, 7.232/126.99, 7.263/126.79, 7.339/128.92, 7.339/140.44, 7.390/128.61 (weak), 7.390/129.05, 7.457/128.61, 7.457/134.37, 7.553/106.44, 7.553/124.48 (weak), 7.553/127.37 (weak), 7.553/143.23, 7.716/129.05, 7.716/144.46, 7.738/118.39 (weak), 7.738/127.37, 7.738/143.23; MS (EI) m/z 376 [M+]; HRMS (EI): calcd for C21H17BrN2 (M+) 376.0570, found 376.0570, 0.1 ppm error.
R
f 0.43 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 10
CH2Cl2 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90); mp 178.8–179.8 °C; 1H NMR 1.793 (d, 3H, J 7.4, CH3–CH), 2.186 (d, 3H, J 0.7, CH3-6), 2.356 (s, 3H, CH3 of 4-MePh-3), 2.421 (s, 3H, CH3 of 4-MePh-2), 4.957 (q, 1H, J 7.4, CH–CH3), 6.990 (dd, 1H, J 1.6, 9.2, CH-7), 7.152 (m, 4H, CH of 4-MePh-3), 7.270 (d, 2H, J 8.1, CH-3 and CH-5 of 4-MePh-2), 7.431 (dd, 1H, J 0.7, 1.6, CH-5), 7.579 (d, 1H, J 9.2, CH-8), 7.638 (d, 2H, J 8.1, CH-2 and CH-6 of 4-MePh-2); COSY cross peaks 1.793/4.957, 2.186/7.431, 6.990/7.431, 6.990/7.579, 7.270/7.638; NOESY cross peaks 1.793/4.957, 1.793/7.152, 1.793/7.431, 2.186/6.990, 2.186/7.431, 2.356/7.152, 2.421/7.270, 4.957/7.152 (weak), 4.957/7.638, 6.990/7.579, 7.152/7.431 (weak), 7.270/7.638; 13C NMR 16.84 (CH3–CH), 18.52 (CH3-6), 21.01 (CH3 of 4-MePh-3), 21.33 (CH3 of 4-MePh-2), 33.53 (CH–CH3), 116.97 (CH-8), 121.06 (Cquat-6), 122.27 (CH-5), 122.62 (Cquat-3), 126.76 (CH-2 and CH-6 of 4-MePh-3), 126.94 (CH-7), 128.77 (CH-2 and CH-6 of 4-MePh-2), 129.16 (CH-3 and CH-5 of 4-MePh-2), 129.50 (CH-3 and CH-5 of 4-MePh-3), 132.18 (Cquat-1 of 4-MePh-2), 136.11 (Cquat-4 of 4-MePh-3), 137.35 (Cquat-4 of 4-MePh-2), 138.30 (Cquat-1 of 4-MePh-3), 143.38 (Cquat-2), 143.84 (Cquat-9); HSQC cross peaks 1.793/16.84, 2.186/18.52, 2.356/21.01, 2.421/21.33, 4.957/33.53, 6.990/126.94, 7.152/126.76, 7.152/129.50, 7.270/129.16, 7.431/122.27, 7.579/116.97, 7.638/128.77; HMBC cross peaks 1.793/33.53, 1.793/122.62, 1.793/138.30, 2.186/121.06, 2.186/126.94, 2.356/129.50, 2.356/136.11, 2.421/129.16, 2.421/137.35, 4.957/16.84, 4.957/122.62, 4.957/126.76, 4.957/138.30, 6.990/18.52, 6.990/122.27, 6.990/143.84, 7.152/21.01, 7.152/33.53, 7.152/126.76, 7.152/128.77, 7.152/129.16, 7.152/129.50, 136.11, 7.152/138.30, 7.270/21.33, 7.270/128.77, 7.270/132.18, 7.431/18.52, 7.431/121.06 (weak), 7.431/126.94, 7.431/143.84, 7.579/121.06, 7.579/143.84 (weak), 7.638/128.77, 7.638/137.35, 7.638/143.38; 15N (N-1 and N-4) 198.31; HMBC cross peaks 198.31/4.957, 198.31/7.431, 198.31/7.579; MS (EI) m/z 340 [M+]; HRMS (EI): calcd for C24H24N2 (M+) 340.1934, found 340.1931, −0.9 ppm error.
90); mp 178.8–179.8 °C; 1H NMR 1.793 (d, 3H, J 7.4, CH3–CH), 2.186 (d, 3H, J 0.7, CH3-6), 2.356 (s, 3H, CH3 of 4-MePh-3), 2.421 (s, 3H, CH3 of 4-MePh-2), 4.957 (q, 1H, J 7.4, CH–CH3), 6.990 (dd, 1H, J 1.6, 9.2, CH-7), 7.152 (m, 4H, CH of 4-MePh-3), 7.270 (d, 2H, J 8.1, CH-3 and CH-5 of 4-MePh-2), 7.431 (dd, 1H, J 0.7, 1.6, CH-5), 7.579 (d, 1H, J 9.2, CH-8), 7.638 (d, 2H, J 8.1, CH-2 and CH-6 of 4-MePh-2); COSY cross peaks 1.793/4.957, 2.186/7.431, 6.990/7.431, 6.990/7.579, 7.270/7.638; NOESY cross peaks 1.793/4.957, 1.793/7.152, 1.793/7.431, 2.186/6.990, 2.186/7.431, 2.356/7.152, 2.421/7.270, 4.957/7.152 (weak), 4.957/7.638, 6.990/7.579, 7.152/7.431 (weak), 7.270/7.638; 13C NMR 16.84 (CH3–CH), 18.52 (CH3-6), 21.01 (CH3 of 4-MePh-3), 21.33 (CH3 of 4-MePh-2), 33.53 (CH–CH3), 116.97 (CH-8), 121.06 (Cquat-6), 122.27 (CH-5), 122.62 (Cquat-3), 126.76 (CH-2 and CH-6 of 4-MePh-3), 126.94 (CH-7), 128.77 (CH-2 and CH-6 of 4-MePh-2), 129.16 (CH-3 and CH-5 of 4-MePh-2), 129.50 (CH-3 and CH-5 of 4-MePh-3), 132.18 (Cquat-1 of 4-MePh-2), 136.11 (Cquat-4 of 4-MePh-3), 137.35 (Cquat-4 of 4-MePh-2), 138.30 (Cquat-1 of 4-MePh-3), 143.38 (Cquat-2), 143.84 (Cquat-9); HSQC cross peaks 1.793/16.84, 2.186/18.52, 2.356/21.01, 2.421/21.33, 4.957/33.53, 6.990/126.94, 7.152/126.76, 7.152/129.50, 7.270/129.16, 7.431/122.27, 7.579/116.97, 7.638/128.77; HMBC cross peaks 1.793/33.53, 1.793/122.62, 1.793/138.30, 2.186/121.06, 2.186/126.94, 2.356/129.50, 2.356/136.11, 2.421/129.16, 2.421/137.35, 4.957/16.84, 4.957/122.62, 4.957/126.76, 4.957/138.30, 6.990/18.52, 6.990/122.27, 6.990/143.84, 7.152/21.01, 7.152/33.53, 7.152/126.76, 7.152/128.77, 7.152/129.16, 7.152/129.50, 136.11, 7.152/138.30, 7.270/21.33, 7.270/128.77, 7.270/132.18, 7.431/18.52, 7.431/121.06 (weak), 7.431/126.94, 7.431/143.84, 7.579/121.06, 7.579/143.84 (weak), 7.638/128.77, 7.638/137.35, 7.638/143.38; 15N (N-1 and N-4) 198.31; HMBC cross peaks 198.31/4.957, 198.31/7.431, 198.31/7.579; MS (EI) m/z 340 [M+]; HRMS (EI): calcd for C24H24N2 (M+) 340.1934, found 340.1931, −0.9 ppm error.
R
f 0.28 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 2
CH2Cl2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98); mp 164.0–164.8 °C; 1H NMR 1.794 (d, 3H, J 7.4, CH3–CH), 2.361 (s, 3H, CH3 of 4-MePh-3), 2.430 (s, 3H, CH3 of 4-MePh-2), 4.969 (q, 1H, J 7.4, CH–CH3), 7.103 (dd, 1H, J 1.8, 9.5, CH-7), 7.150 (m, 4H, CH of 4-MePh-3), 7.283 (d, 2H, J 8.2, CH-3 and CH-5 of 4-MePh-2), 7.600 (d, 1H, J 9.25, CH-8), 7.636 (d, 2H, J 8.2, CH-2 and CH-6 of 4-MePh-2), 7.673 (d, 1H, J 1.8, CH-5); COSY cross peaks 1.794/4.969, 7.103/7.600, 7.103/7.673, 7.283/7.636; NOESY cross peaks 1.794/4.969, 1.794/7.150, 1.794/7.673 (weak), 2.361/7.150, 2.430/7.283, 4.969/7.636, 7.103/7.600, 7.283/7.636; 13C NMR 16.63 (CH3–CH), 21.02 (CH3 of 4-MePh-3), 21.35 (CH3 of 4-MePh-2), 33.45 (CH–CH3), 117.94 (CH-8), 119.70 (Cquat-6), 122.33 (CH-5), 123.71 (Cquat-3), 125.15 (CH-7), 126.66 (CH-2 and CH-6 of 4-MePh-3), 128.75 (CH-2 and CH-6 of 4-MePh-2), 129.31 (CH-3 and CH-5 of 4-MePh-2), 129.68 (CH-3 and CH-5 of 4-MePh-3), 131.47 (Cquat-1 of 4-MePh-2), 136.52 (Cquat-4 of 4-MePh-3), 137.37 (Cquat-1 of 4-MePh-3), 137.87 (Cquat-4 of 4-MePh-2), 143.08 (Cquat-9), 144.57 (Cquat-2); HSQC cross peaks 1.794/16.63, 2.361/21.02, 2.430/21.35, 4.969/33.45, 7.103/125.15, 7.150/126.66, 7.150/129.68, 7.283/129.31, 7.600/117.94, 7.636/128.75, 7.673/122.33; HMBC cross peaks 1.794/33.45, 1.794/123.71, 1.794/137.37, 1.794/129.68, 1.794/136.52, 2.430/129.31, 2.430/137.87, 4.969/16.63, 4.969/123.71, 4.969/126.66 (weak), 4.969/137.37, 7.103/122.33 (weak), 7.103/143.08, 7.150/16.63, 7.150/21.02, 7.150/126.66, 7.150/129.68, 7.150/136.52, 7.150/137.37, 7.283/21.35, 7.283/129.31, 7.283/131.47, 7.600/119.70, 7.600/143.08 (weak), 7.636/128.75, 7.636/137.87, 7.636/144.57, 7.673/125.15, 7.636/143.08; 15N 198.54 (N-4 and N-1); HMBC cross peaks 198.54/7.600; MS (EI) m/z 360 [M+]; HRMS (EI): calcd for C23H21ClN2 (M+) 360.1388, found 360.1391, 1.0 ppm error.
98); mp 164.0–164.8 °C; 1H NMR 1.794 (d, 3H, J 7.4, CH3–CH), 2.361 (s, 3H, CH3 of 4-MePh-3), 2.430 (s, 3H, CH3 of 4-MePh-2), 4.969 (q, 1H, J 7.4, CH–CH3), 7.103 (dd, 1H, J 1.8, 9.5, CH-7), 7.150 (m, 4H, CH of 4-MePh-3), 7.283 (d, 2H, J 8.2, CH-3 and CH-5 of 4-MePh-2), 7.600 (d, 1H, J 9.25, CH-8), 7.636 (d, 2H, J 8.2, CH-2 and CH-6 of 4-MePh-2), 7.673 (d, 1H, J 1.8, CH-5); COSY cross peaks 1.794/4.969, 7.103/7.600, 7.103/7.673, 7.283/7.636; NOESY cross peaks 1.794/4.969, 1.794/7.150, 1.794/7.673 (weak), 2.361/7.150, 2.430/7.283, 4.969/7.636, 7.103/7.600, 7.283/7.636; 13C NMR 16.63 (CH3–CH), 21.02 (CH3 of 4-MePh-3), 21.35 (CH3 of 4-MePh-2), 33.45 (CH–CH3), 117.94 (CH-8), 119.70 (Cquat-6), 122.33 (CH-5), 123.71 (Cquat-3), 125.15 (CH-7), 126.66 (CH-2 and CH-6 of 4-MePh-3), 128.75 (CH-2 and CH-6 of 4-MePh-2), 129.31 (CH-3 and CH-5 of 4-MePh-2), 129.68 (CH-3 and CH-5 of 4-MePh-3), 131.47 (Cquat-1 of 4-MePh-2), 136.52 (Cquat-4 of 4-MePh-3), 137.37 (Cquat-1 of 4-MePh-3), 137.87 (Cquat-4 of 4-MePh-2), 143.08 (Cquat-9), 144.57 (Cquat-2); HSQC cross peaks 1.794/16.63, 2.361/21.02, 2.430/21.35, 4.969/33.45, 7.103/125.15, 7.150/126.66, 7.150/129.68, 7.283/129.31, 7.600/117.94, 7.636/128.75, 7.673/122.33; HMBC cross peaks 1.794/33.45, 1.794/123.71, 1.794/137.37, 1.794/129.68, 1.794/136.52, 2.430/129.31, 2.430/137.87, 4.969/16.63, 4.969/123.71, 4.969/126.66 (weak), 4.969/137.37, 7.103/122.33 (weak), 7.103/143.08, 7.150/16.63, 7.150/21.02, 7.150/126.66, 7.150/129.68, 7.150/136.52, 7.150/137.37, 7.283/21.35, 7.283/129.31, 7.283/131.47, 7.600/119.70, 7.600/143.08 (weak), 7.636/128.75, 7.636/137.87, 7.636/144.57, 7.673/125.15, 7.636/143.08; 15N 198.54 (N-4 and N-1); HMBC cross peaks 198.54/7.600; MS (EI) m/z 360 [M+]; HRMS (EI): calcd for C23H21ClN2 (M+) 360.1388, found 360.1391, 1.0 ppm error.
R
f 0.37 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 5
CH2Cl2 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95); mp 187.6–188.7 °C; 1H NMR 1.703 (d, 3H, J 7.4, CH3–CH), 2.109 (d, 3H, J 0.8, CH3-6), 4.780 (q, 1H, J 7.4, CH–CH3), 6.933 (dd, 1H, J 1.6, 9.2, CH-7), 7.069 (dd, 2H, J 0.9, 8.6, CH-2 and CH-6 of 4-ClPh-3), 7.206 (d, 2H, J 8.6, CH-3 and CH-5 of 4-ClPh-3), 7.272 (dd, 1H, J 0.8, 1.6, CH-5), 7.328 (d, 2H, J 8.6, CH-3 and CH-5 of 4-ClPh-2), 7.477 (d, 1H, J 9.2, CH-8), 7.532 (d, 2H, J 8.6, CH-2 and CH-6 of 4-ClPh-2); COSY cross peaks 1.712/4.820, 6.537/7.070, 6.537/7.481, 7.070/7.194, 7.070/7.751, 7.342/7.562; 1.703/4.780, 2.109/7.272, 6.933/7.272 (weak), 6.933/7.477, 7.069/7.206, 7.328/7.532; NOESY cross peaks 1.703/4.780, 1.703/7.272, 2.109/6.933, 2.109/7.272, 4.780/7.532, 6.933/7.477, 7.069/7.206, 7.328/7.532; 13C NMR 16.90 (CH3–CH), 18.55 (CH3-6), 33.49 (CH–CH3), 117.23 (CH-8), 121.71 (Cquat-6), 121.93 (CH-5), 122.21 (Cquat-3), 127.53 (CH-7), 128.23 (CH-2 and CH-6 of 4-ClPh-3), 128.71 (CH-3 and CH-5 of 4-ClPh-2), 129.03 (CH-3 and CH-5 of 4-ClPh-3), 130.12 (CH-2 and CH-6 of 4-ClPh-2), 132.60 (Cquat-4 of 4-ClPh-3), 133.48 (Cquat-1 of 4-ClPh-2), 133.74 (Cquat-4 of 4-ClPh-2), 139.65 (Cquat-1 of 4-ClPh-3), 142.47 (Cquat-2), 144.08 (Cquat-9); HSQC cross peaks 1.703/16.90, 2.109/18.55, 4.780/33.49, 6.933/127.53, 7.069/128.23, 7.206/129.03, 7.272/121.93, 7.328/128.71, 7.477/117.23, 7.532/130.12; HMBC cross peaks 1.703/33.49, 1.703/122.21, 1.703/139.65, 2.109/121.71, 2.109/121.93, 2.109/127.53, 4.780/16.90, 4.780/122.21, 4.780/128.23, 4.780/139.65, 4.780/142.47 (weak), 6.933/18.55, 6.933/121.93, 6.933/144.08, 7.069/33.49, 7.069/128.23, 7.069/132.60, 7.206/129.03, 7.206/132.60 (weak), 7.206/139.65, 7.272/18.55, 7.272/121.71 (weak), 7.272/127.53, 7.272/144.08, 7.328/128.71, 7.328/133.48, 7.477/121.71, 7.477/144.08, 7.532/130.12, 7.532/133.74, 7.532/142.47; MS (ESI, pos. mode) m/z 381.20 [M + H+]; HRMS (EI): calcd for C22H18Cl2N2 (M+) 380.0842, found 380.0859, 1.7 ppm error.
95); mp 187.6–188.7 °C; 1H NMR 1.703 (d, 3H, J 7.4, CH3–CH), 2.109 (d, 3H, J 0.8, CH3-6), 4.780 (q, 1H, J 7.4, CH–CH3), 6.933 (dd, 1H, J 1.6, 9.2, CH-7), 7.069 (dd, 2H, J 0.9, 8.6, CH-2 and CH-6 of 4-ClPh-3), 7.206 (d, 2H, J 8.6, CH-3 and CH-5 of 4-ClPh-3), 7.272 (dd, 1H, J 0.8, 1.6, CH-5), 7.328 (d, 2H, J 8.6, CH-3 and CH-5 of 4-ClPh-2), 7.477 (d, 1H, J 9.2, CH-8), 7.532 (d, 2H, J 8.6, CH-2 and CH-6 of 4-ClPh-2); COSY cross peaks 1.712/4.820, 6.537/7.070, 6.537/7.481, 7.070/7.194, 7.070/7.751, 7.342/7.562; 1.703/4.780, 2.109/7.272, 6.933/7.272 (weak), 6.933/7.477, 7.069/7.206, 7.328/7.532; NOESY cross peaks 1.703/4.780, 1.703/7.272, 2.109/6.933, 2.109/7.272, 4.780/7.532, 6.933/7.477, 7.069/7.206, 7.328/7.532; 13C NMR 16.90 (CH3–CH), 18.55 (CH3-6), 33.49 (CH–CH3), 117.23 (CH-8), 121.71 (Cquat-6), 121.93 (CH-5), 122.21 (Cquat-3), 127.53 (CH-7), 128.23 (CH-2 and CH-6 of 4-ClPh-3), 128.71 (CH-3 and CH-5 of 4-ClPh-2), 129.03 (CH-3 and CH-5 of 4-ClPh-3), 130.12 (CH-2 and CH-6 of 4-ClPh-2), 132.60 (Cquat-4 of 4-ClPh-3), 133.48 (Cquat-1 of 4-ClPh-2), 133.74 (Cquat-4 of 4-ClPh-2), 139.65 (Cquat-1 of 4-ClPh-3), 142.47 (Cquat-2), 144.08 (Cquat-9); HSQC cross peaks 1.703/16.90, 2.109/18.55, 4.780/33.49, 6.933/127.53, 7.069/128.23, 7.206/129.03, 7.272/121.93, 7.328/128.71, 7.477/117.23, 7.532/130.12; HMBC cross peaks 1.703/33.49, 1.703/122.21, 1.703/139.65, 2.109/121.71, 2.109/121.93, 2.109/127.53, 4.780/16.90, 4.780/122.21, 4.780/128.23, 4.780/139.65, 4.780/142.47 (weak), 6.933/18.55, 6.933/121.93, 6.933/144.08, 7.069/33.49, 7.069/128.23, 7.069/132.60, 7.206/129.03, 7.206/132.60 (weak), 7.206/139.65, 7.272/18.55, 7.272/121.71 (weak), 7.272/127.53, 7.272/144.08, 7.328/128.71, 7.328/133.48, 7.477/121.71, 7.477/144.08, 7.532/130.12, 7.532/133.74, 7.532/142.47; MS (ESI, pos. mode) m/z 381.20 [M + H+]; HRMS (EI): calcd for C22H18Cl2N2 (M+) 380.0842, found 380.0859, 1.7 ppm error.
R
f 0.41 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 2
CH2Cl2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98); mp 175.4–175.9 °C; 1H NMR 1.718 (d, 3H, J 7.4, CH3–CH), 4.796 (q, 1H, J 7.4, CH–CH3), 7.058 (m, 3H, CH-7 and CH-2 and CH-6 of 4-ClPh-3), 7.232 (d, 2H, J 8.6, CH-3 and CH-5 of 4-ClPh-3), 7.352 (d, 2H, J 8.6, CH-3 and CH-5 of 4-ClPh-2), 7.532 (m, 4H, CH-5, CH-8 and CH-2 and CH-6 of 4-ClPh-2); COSY cross peaks 1.718/4.796, 7.058/7.232, 7.058/7.532, 7.352/7.532; NOESY cross peaks 1.718/4.796, 1.718/7.058, 1.718/7.532, 4.796/7.058, 4.796/7.532, 7.058/7.232, 7.058/7.532, 7.352/7.532; 13C NMR 16.76 (CH3–CH), 33.44 (CH–CH3), 118.26 (CH-8), 120.31 (Cquat-6), 122.03 (CH-5), 123.24 (Cquat-3), 125.78 (CH-7), 128.14 (CH-2 and CH-6 of 4-ClPh-3), 128.88 (CH-3 and CH-5 of 4-ClPh-2), 129.26 (CH-3 and CH-5 of 4-ClPh-3), 130.11 (CH-2 and CH-6 of 4-ClPh-2), 132.77 (Cquat-1 of 4-ClPh-2), 132.99 (Cquat-4 of 4-ClPh-3), 134.24 (Cquat-4 of 4-ClPh-2), 138.77 (Cquat-1 of 4-ClPh-3), 143.31 (Cquat-2), 143.65 (Cquat-9); HSQC cross peaks 1.718/16.76, 4.796/33.44, 7.058/125.78, 7.058/128.14, 7.232/129.26, 7.352/128.88, 7.532/118.26, 7.532/122.03, 7.532/130.11; HMBC cross peaks 1.718/33.44, 1.718/123.24, 1.718/138.77, 4.796/16.76, 4.796/123.24, 4.796/128.14, 4.796/138.77, 4.796/143.31 (weak), 7.058/33.44, 7.058/120.31 (weak), 7.058/122.03, 7.058/128.14, 7.058/132.99, 7.058/143.65, 7.232/129.26, 7.232/132.99, 7.232/138.77, 7.352/128.88, 7.352/132.77, 7.352/134.24, 7.532/120.31, 7.532/125.78 (weak), 7.532/130.11, 7.532/134.24, 7.532/143.31 (weak), 7.532/143.65; MS (ESI, pos. mode) m/z 401.09 [M + H+]; HRMS (EI): calcd for C21H15Cl3N2 (M+) 400.0295, found 400.0292, −0.9 ppm error.
98); mp 175.4–175.9 °C; 1H NMR 1.718 (d, 3H, J 7.4, CH3–CH), 4.796 (q, 1H, J 7.4, CH–CH3), 7.058 (m, 3H, CH-7 and CH-2 and CH-6 of 4-ClPh-3), 7.232 (d, 2H, J 8.6, CH-3 and CH-5 of 4-ClPh-3), 7.352 (d, 2H, J 8.6, CH-3 and CH-5 of 4-ClPh-2), 7.532 (m, 4H, CH-5, CH-8 and CH-2 and CH-6 of 4-ClPh-2); COSY cross peaks 1.718/4.796, 7.058/7.232, 7.058/7.532, 7.352/7.532; NOESY cross peaks 1.718/4.796, 1.718/7.058, 1.718/7.532, 4.796/7.058, 4.796/7.532, 7.058/7.232, 7.058/7.532, 7.352/7.532; 13C NMR 16.76 (CH3–CH), 33.44 (CH–CH3), 118.26 (CH-8), 120.31 (Cquat-6), 122.03 (CH-5), 123.24 (Cquat-3), 125.78 (CH-7), 128.14 (CH-2 and CH-6 of 4-ClPh-3), 128.88 (CH-3 and CH-5 of 4-ClPh-2), 129.26 (CH-3 and CH-5 of 4-ClPh-3), 130.11 (CH-2 and CH-6 of 4-ClPh-2), 132.77 (Cquat-1 of 4-ClPh-2), 132.99 (Cquat-4 of 4-ClPh-3), 134.24 (Cquat-4 of 4-ClPh-2), 138.77 (Cquat-1 of 4-ClPh-3), 143.31 (Cquat-2), 143.65 (Cquat-9); HSQC cross peaks 1.718/16.76, 4.796/33.44, 7.058/125.78, 7.058/128.14, 7.232/129.26, 7.352/128.88, 7.532/118.26, 7.532/122.03, 7.532/130.11; HMBC cross peaks 1.718/33.44, 1.718/123.24, 1.718/138.77, 4.796/16.76, 4.796/123.24, 4.796/128.14, 4.796/138.77, 4.796/143.31 (weak), 7.058/33.44, 7.058/120.31 (weak), 7.058/122.03, 7.058/128.14, 7.058/132.99, 7.058/143.65, 7.232/129.26, 7.232/132.99, 7.232/138.77, 7.352/128.88, 7.352/132.77, 7.352/134.24, 7.532/120.31, 7.532/125.78 (weak), 7.532/130.11, 7.532/134.24, 7.532/143.31 (weak), 7.532/143.65; MS (ESI, pos. mode) m/z 401.09 [M + H+]; HRMS (EI): calcd for C21H15Cl3N2 (M+) 400.0295, found 400.0292, −0.9 ppm error.
R
f 0.36 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 5
CH2Cl2 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95); mp 73.5–74.7 °C; 1H NMR 2.365 (s, 3H, CH3 of 4-MePh-2), 2.370 (s, 3H, CH3 of 4-MePh-3), 5.541 (d, 1H, J 0.8, ½ of CH2
95); mp 73.5–74.7 °C; 1H NMR 2.365 (s, 3H, CH3 of 4-MePh-2), 2.370 (s, 3H, CH3 of 4-MePh-3), 5.541 (d, 1H, J 0.8, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.154 (d, 1H, J 0.8, ½ of CH2
), 6.154 (d, 1H, J 0.8, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.652 (td, 1H, J 1.1, 6.8, CH-6), 7.171 (m, 5H, CH-7, CH-3 and CH-5 of 4-MePh-2, CH-3 and CH-5 of 4-MePh-3), 7.309 (dt, 2H, J 1.6, 8.2, CH-2 and CH-6 of 4-MePh-3), 7.646 (dt, 1H, J 1.0, 6.9, CH-5), 7.694 (dt, 1H, J 0.9, 9.0, CH-8), 7.880 (dt, 2H, J 1.7, 8.1, CH-2 and CH-6 of 4-MePh-2); COSY cross peaks 5.541/6.154, 6.652/7.171, 6.652/7.646, 7.171/7.309, 7.171/7.694, 7.171/7.880; NOESY cross peaks 2.365/7.171, 2.370/7.171, 5.541/6.154, 6.154/7.309, 6.652/7.171, 6.652/7.646, 7.171/7.309, 7.171/7.694, 7.171/7.880; 13C NMR 21.24 (CH3 of 4-MePh), 21.31 (CH3 of 4-MePh), 112.07 (CH-6), 117.26 (CH-8), 120.00 (Cquat-3), 120.42 (CH2
), 6.652 (td, 1H, J 1.1, 6.8, CH-6), 7.171 (m, 5H, CH-7, CH-3 and CH-5 of 4-MePh-2, CH-3 and CH-5 of 4-MePh-3), 7.309 (dt, 2H, J 1.6, 8.2, CH-2 and CH-6 of 4-MePh-3), 7.646 (dt, 1H, J 1.0, 6.9, CH-5), 7.694 (dt, 1H, J 0.9, 9.0, CH-8), 7.880 (dt, 2H, J 1.7, 8.1, CH-2 and CH-6 of 4-MePh-2); COSY cross peaks 5.541/6.154, 6.652/7.171, 6.652/7.646, 7.171/7.309, 7.171/7.694, 7.171/7.880; NOESY cross peaks 2.365/7.171, 2.370/7.171, 5.541/6.154, 6.154/7.309, 6.652/7.171, 6.652/7.646, 7.171/7.309, 7.171/7.694, 7.171/7.880; 13C NMR 21.24 (CH3 of 4-MePh), 21.31 (CH3 of 4-MePh), 112.07 (CH-6), 117.26 (CH-8), 120.00 (Cquat-3), 120.42 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 124.29 (CH-5), 124.49 (CH-7), 126.10 (CH-2 and CH-6 of 4-MePh-3), 127.75 (CH-2 and CH-6 of 4-MePh-2), 129.10 (CH-3 and CH-5 of 4-MePh-2), 129.80 (CH-3 and CH-5 of 4-MePh-3), 131.24 (Cquat-1 of 4-MePh-2), 134.80 (Cquat-1 of 4-MePh-3), 137.36 (Cquat-4 of 4-MePh-2), 137.58 (Cquat
), 124.29 (CH-5), 124.49 (CH-7), 126.10 (CH-2 and CH-6 of 4-MePh-3), 127.75 (CH-2 and CH-6 of 4-MePh-2), 129.10 (CH-3 and CH-5 of 4-MePh-2), 129.80 (CH-3 and CH-5 of 4-MePh-3), 131.24 (Cquat-1 of 4-MePh-2), 134.80 (Cquat-1 of 4-MePh-3), 137.36 (Cquat-4 of 4-MePh-2), 137.58 (Cquat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 138.79 (Cquat-4 of 4-MePh-3), 143.40 (Cquat-2), 144.79 (Cquat-9); HSQC cross peaks 2.365 and 2.370/21.24 and 21.31, 5.541/120.42, 6.154/120.42, 6.652/112.07, 7.171/124.49, 7.171/129.10, 7.171/129.80, 7.309/126.10, 7.646/124.29, 7.694/117.26, 7.880/127.75; HMBC cross peaks 2.365/129.10, 2.365/137.36, 2.370/129.80, 2.370/138.79, 5.541/120.00, 5.541/134.80, 6.154/120.00, 6.154/134.80, 6.652/117.26, 6.652/124.29 (weak), 6.652/124.49 (weak), 7.171/21.24, 7.171/21.31, 7.171/124.29, 7.171/126.10, 7.171/127.75, 7.171/129.10, 7.171/129.80, 7.171/131.24, 7.171/134.80, 7.171/144.79, 7.309/126.10, 7.309/129.80 (weak), 7.309/137.58, 7.309/138.79, 7.646/112.07 (weak), 7.646/124.49, 7.646/144.79, 7.694/112.07, 7.694/144.79 (weak), 7.880/127.75, 7.880/137.36, 7.880/143.40; MS (EI) m/z 324 [M+]; HRMS (EI): calcd for C23H20N2 (M+) 324.1621, found 324.1630, 2.9 ppm error.
), 138.79 (Cquat-4 of 4-MePh-3), 143.40 (Cquat-2), 144.79 (Cquat-9); HSQC cross peaks 2.365 and 2.370/21.24 and 21.31, 5.541/120.42, 6.154/120.42, 6.652/112.07, 7.171/124.49, 7.171/129.10, 7.171/129.80, 7.309/126.10, 7.646/124.29, 7.694/117.26, 7.880/127.75; HMBC cross peaks 2.365/129.10, 2.365/137.36, 2.370/129.80, 2.370/138.79, 5.541/120.00, 5.541/134.80, 6.154/120.00, 6.154/134.80, 6.652/117.26, 6.652/124.29 (weak), 6.652/124.49 (weak), 7.171/21.24, 7.171/21.31, 7.171/124.29, 7.171/126.10, 7.171/127.75, 7.171/129.10, 7.171/129.80, 7.171/131.24, 7.171/134.80, 7.171/144.79, 7.309/126.10, 7.309/129.80 (weak), 7.309/137.58, 7.309/138.79, 7.646/112.07 (weak), 7.646/124.49, 7.646/144.79, 7.694/112.07, 7.694/144.79 (weak), 7.880/127.75, 7.880/137.36, 7.880/143.40; MS (EI) m/z 324 [M+]; HRMS (EI): calcd for C23H20N2 (M+) 324.1621, found 324.1630, 2.9 ppm error.
R
f 0.42 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 2
CH2Cl2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98); mp 132.1–133.2 °C; 1H NMR 5.526 (d, 1H, J 0.7, ½ of CH2
98); mp 132.1–133.2 °C; 1H NMR 5.526 (d, 1H, J 0.7, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.107 (d, 1H, J 0.7, ½ of CH2
), 6.107 (d, 1H, J 0.7, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.628 (td, 1H, J 1.2, 6.7, CH-6), 7.134 (ddd, 1H, J 1.3, 6.7, 8.9, CH-7), 7.214 (m, 6H, 4-ClPh-3 and CH-3 and CH-5 of 4-ClPh-2), 7.585 (m, 2H, CH-5 and CH-8), 7.751 (d, 2H, J 8.6, CH-2 and CH-6 of 4-ClPh-2); COSY cross peaks 5.526/6.107, 6.628/7.134, 6.628/7.585, 7.134/7.585, 7.214/7.751; NOESY cross peaks 5.526/6.107, 6.107/7.214, 6.628/7.134, 6.628/7.585, 7.134/7.585, 7.214/7.751; 13C NMR 112.60 (CH-6), 117.53 (CH-8), 119.67 (Cquat-3), 121.84 (CH2
), 6.628 (td, 1H, J 1.2, 6.7, CH-6), 7.134 (ddd, 1H, J 1.3, 6.7, 8.9, CH-7), 7.214 (m, 6H, 4-ClPh-3 and CH-3 and CH-5 of 4-ClPh-2), 7.585 (m, 2H, CH-5 and CH-8), 7.751 (d, 2H, J 8.6, CH-2 and CH-6 of 4-ClPh-2); COSY cross peaks 5.526/6.107, 6.628/7.134, 6.628/7.585, 7.134/7.585, 7.214/7.751; NOESY cross peaks 5.526/6.107, 6.107/7.214, 6.628/7.134, 6.628/7.585, 7.134/7.585, 7.214/7.751; 13C NMR 112.60 (CH-6), 117.53 (CH-8), 119.67 (Cquat-3), 121.84 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 124.11 (CH-5), 125.12 (CH-7), 127.45 (2CH of 4-ClPh), 128.61 (2CH of 4-ClPh), 129.05 (CH-2 and CH-6 of 4-ClPh-2), 129.34 (2CH of 4-ClPh), 132.40 (Cquat of 4-ClPh), 133.63 (Cquat-4 of 4-ClPh-2), 134.89 (Cquat of 4-ClPh), 135.89 (Cquat
), 124.11 (CH-5), 125.12 (CH-7), 127.45 (2CH of 4-ClPh), 128.61 (2CH of 4-ClPh), 129.05 (CH-2 and CH-6 of 4-ClPh-2), 129.34 (2CH of 4-ClPh), 132.40 (Cquat of 4-ClPh), 133.63 (Cquat-4 of 4-ClPh-2), 134.89 (Cquat of 4-ClPh), 135.89 (Cquat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 136.53 (Cquat of 4-ClPh), 142.42 (Cquat-2), 145.05 (Cquat-9); HSQC cross peaks 5.526/121.84, 6.107/121.84, 6.628/112.60, 7.134/125.12, 7.214/127.45, 7.214/128.61, 7.214/129.34, 7.585/117.53, 7.585/124.11, 7.751/129.05; HMBC cross peaks 5.526/119.67, 5.526/135.89, 6.107/119.67, 6.107/135.89, 6.628/117.53, 6.628/124.11, 7.134/124.11, 7.134/145.05, 7.214/127.45, 7.214/128.61, 7.214/129.34, 7.214/132.40, 7.214/134.89, 7.214/136.53, 7.585/112.60, 7.585/125.12, 7.585/145.05, 7.751/129.05, 7.751/136.53, 7.751/142.42; MS (ESI, pos. mode) m/z 365.14 [M + H+]; HRMS (EI): calcd for C21H14Cl2N2 (M+) 364.0529, found 364.0521, −2.0 ppm error.
), 136.53 (Cquat of 4-ClPh), 142.42 (Cquat-2), 145.05 (Cquat-9); HSQC cross peaks 5.526/121.84, 6.107/121.84, 6.628/112.60, 7.134/125.12, 7.214/127.45, 7.214/128.61, 7.214/129.34, 7.585/117.53, 7.585/124.11, 7.751/129.05; HMBC cross peaks 5.526/119.67, 5.526/135.89, 6.107/119.67, 6.107/135.89, 6.628/117.53, 6.628/124.11, 7.134/124.11, 7.134/145.05, 7.214/127.45, 7.214/128.61, 7.214/129.34, 7.214/132.40, 7.214/134.89, 7.214/136.53, 7.585/112.60, 7.585/125.12, 7.585/145.05, 7.751/129.05, 7.751/136.53, 7.751/142.42; MS (ESI, pos. mode) m/z 365.14 [M + H+]; HRMS (EI): calcd for C21H14Cl2N2 (M+) 364.0529, found 364.0521, −2.0 ppm error.
R
f 0.25 (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 1
CH2Cl2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99); mp 86.8–88.1 °C; 1H NMR 2.103 (d, 3H, J 0.7, CH3-6), 5.485 (d, 1H, J 0.9, ½ of CH2
99); mp 86.8–88.1 °C; 1H NMR 2.103 (d, 3H, J 0.7, CH3-6), 5.485 (d, 1H, J 0.9, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.107 (d, 1H, J 0.9, ½ of CH2
), 6.107 (d, 1H, J 0.9, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.940 (dd, 1H, J 1.6, 9.2, CH-7), 7.157 (t, 1H, J 17.4, CH-4 of Ph-2), 7.234 (m, 5H, CH-3 and CH-5 of Ph-2 and CH-3, CH-4 and CH-5 of Ph-3), 7.311 (d, 2H, J 7.1, CH-2 and CH-6 of Ph-3), 7.354 (dd, 1H, J 0.7, 1.6, CH-5), 7.496 (d, 1H, J 9.2, CH-8), 7.835 (d, 2H, J 1.9, 7.1, CH-2 and CH-6 of Ph-2); COSY cross peaks 2.103/7.354, 5.485/6.107, 6.940/7.354, 6.940/7.496, 7.157/7.234, 7.234/7.311, 7.234/7.835; NOESY cross peaks 2.103/6.940, 2.103/7.354, 5.485/6.107, 6.940/7.496, 7.157/7.234, 7.234/7.311, 7.234/7.835; 13C NMR 18.43 (CH3-6), 116.79 (CH-8), 119.95 (Cquat-6), 121.32 (CH2
), 6.940 (dd, 1H, J 1.6, 9.2, CH-7), 7.157 (t, 1H, J 17.4, CH-4 of Ph-2), 7.234 (m, 5H, CH-3 and CH-5 of Ph-2 and CH-3, CH-4 and CH-5 of Ph-3), 7.311 (d, 2H, J 7.1, CH-2 and CH-6 of Ph-3), 7.354 (dd, 1H, J 0.7, 1.6, CH-5), 7.496 (d, 1H, J 9.2, CH-8), 7.835 (d, 2H, J 1.9, 7.1, CH-2 and CH-6 of Ph-2); COSY cross peaks 2.103/7.354, 5.485/6.107, 6.940/7.354, 6.940/7.496, 7.157/7.234, 7.234/7.311, 7.234/7.835; NOESY cross peaks 2.103/6.940, 2.103/7.354, 5.485/6.107, 6.940/7.496, 7.157/7.234, 7.234/7.311, 7.234/7.835; 13C NMR 18.43 (CH3-6), 116.79 (CH-8), 119.95 (Cquat-6), 121.32 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 121.89 (CH-5), 121.93 (Cquat-3), 126.26 (CH-2 and CH-6 of Ph-3), 127.53 (CH-4 of Ph-2), 127.80 (CH-2 and CH-6 of Ph-2), 127.93 (CH-7), 128.37 (CH-3 and CH-5 of Ph), 128.82 (CH-4 of Ph-3), 129.11 (CH-3 and CH-5 of Ph), 134.26 (Cquat-1 of Ph-2), 137.75 (Cquat
), 121.89 (CH-5), 121.93 (Cquat-3), 126.26 (CH-2 and CH-6 of Ph-3), 127.53 (CH-4 of Ph-2), 127.80 (CH-2 and CH-6 of Ph-2), 127.93 (CH-7), 128.37 (CH-3 and CH-5 of Ph), 128.82 (CH-4 of Ph-3), 129.11 (CH-3 and CH-5 of Ph), 134.26 (Cquat-1 of Ph-2), 137.75 (Cquat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 137.94 (Cquat-1 of Ph-3), 143.14 (Cquat-2), 144.04 (Cquat-9); HSQC cross peaks 2.103/18.43, 5.485/121.32, 6.107/121.32, 6.940/127.93, 7.157/127.53, 7.234/128.37, 7.234/128.82, 7.234/129.11, 7.311/126.26, 7.354/121.89, 7.496/116.79, 7.835/127.80; HMBC cross peaks 2.103/119.95, 2.103/121.89, 2.103/127.93, 5.485/121.93, 5.485/137.75, 5.485/137.94, 6.107/121.93, 6.107/137.75, 6.107/137.94, 6.940/18.43, 6.940/121.89, 6.940/144.04, 7.157/127.80, 7.234/126.26, 7.234/128.37, 7.234/129.11, 7.234/134.26, 7.234/137.94, 7.311/126.26, 7.311/128.82, 7.354/18.43, 7.354/127.93, 7.354/144.04, 7.496/119.95, 7.835/127.53, 7.835/127.80, 7.835/143.14; MS (EI) m/z 310 [M+]; HRMS (EI): calcd for C22H18N2 (M+) 310.1464, found 310.1471, 2.2 ppm error.
), 137.94 (Cquat-1 of Ph-3), 143.14 (Cquat-2), 144.04 (Cquat-9); HSQC cross peaks 2.103/18.43, 5.485/121.32, 6.107/121.32, 6.940/127.93, 7.157/127.53, 7.234/128.37, 7.234/128.82, 7.234/129.11, 7.311/126.26, 7.354/121.89, 7.496/116.79, 7.835/127.80; HMBC cross peaks 2.103/119.95, 2.103/121.89, 2.103/127.93, 5.485/121.93, 5.485/137.75, 5.485/137.94, 6.107/121.93, 6.107/137.75, 6.107/137.94, 6.940/18.43, 6.940/121.89, 6.940/144.04, 7.157/127.80, 7.234/126.26, 7.234/128.37, 7.234/129.11, 7.234/134.26, 7.234/137.94, 7.311/126.26, 7.311/128.82, 7.354/18.43, 7.354/127.93, 7.354/144.04, 7.496/119.95, 7.835/127.53, 7.835/127.80, 7.835/143.14; MS (EI) m/z 310 [M+]; HRMS (EI): calcd for C22H18N2 (M+) 310.1464, found 310.1471, 2.2 ppm error.
R
f 0.42 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 2
CH2Cl2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98); mp 159.6–161.0 °C; 1H NMR 5.591 (d, 1H, J 0.6, ½ of CH2
98); mp 159.6–161.0 °C; 1H NMR 5.591 (d, 1H, J 0.6, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.224 (d, 1H, J 0.6, ½ of CH2
), 6.224 (d, 1H, J 0.6, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.144 (dd, 1H, J 1.9, 9.5, CH-7), 7.269 (tt, 1H, J 1.9, 7.3, CH-4 of Ph-2), 7.344 (m, 7H, 5 CH of Ph-3, CH-3 and CH-5 of Ph-2), 7.621 (dd, 1H, J 0.6, 9.5, CH-8), 7.699 (dd, 1H, J 0.6, 1.9, CH-5), 7.905 (ddd, 2H, J 1.3, 1.9, 7.2, CH-2 and CH-6 of Ph-2); COSY cross peaks 5.591/6.224, 7.144/7.621, 7.144/7.699, 7.269/7.344, 7.344/7.905; NOESY cross peaks 5.591/6.224, 5.591/7.699, 6.224/7.344, 7.144/7.621, 7.269/7.344, 7.344/7.905; 13C NMR 117.87 (CH-8), 120.60 (Cquat-6), 120.76 (Cquat-3), 121.87 (CH2
), 7.144 (dd, 1H, J 1.9, 9.5, CH-7), 7.269 (tt, 1H, J 1.9, 7.3, CH-4 of Ph-2), 7.344 (m, 7H, 5 CH of Ph-3, CH-3 and CH-5 of Ph-2), 7.621 (dd, 1H, J 0.6, 9.5, CH-8), 7.699 (dd, 1H, J 0.6, 1.9, CH-5), 7.905 (ddd, 2H, J 1.3, 1.9, 7.2, CH-2 and CH-6 of Ph-2); COSY cross peaks 5.591/6.224, 7.144/7.621, 7.144/7.699, 7.269/7.344, 7.344/7.905; NOESY cross peaks 5.591/6.224, 5.591/7.699, 6.224/7.344, 7.144/7.621, 7.269/7.344, 7.344/7.905; 13C NMR 117.87 (CH-8), 120.60 (Cquat-6), 120.76 (Cquat-3), 121.87 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 122.08 (CH-5), 126.17 (CH-7), 126.21 (CH-2 and CH-6 of Ph-3), 127.88 (CH-2 and CH-6 of Ph-2), 128.03 (CH-4 of Ph-2), 128.52 (CH-3 and CH-5 of Ph), 128.03 (CH-4 of Ph-3), 129.29 (CH-3 and CH-5 of Ph), 133.60 (Cquat
), 122.08 (CH-5), 126.17 (CH-7), 126.21 (CH-2 and CH-6 of Ph-3), 127.88 (CH-2 and CH-6 of Ph-2), 128.03 (CH-4 of Ph-2), 128.52 (CH-3 and CH-5 of Ph), 128.03 (CH-4 of Ph-3), 129.29 (CH-3 and CH-5 of Ph), 133.60 (Cquat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 137.16 (Cquat-1 of Ph-3), 137.33 (Cquat-1 of Ph-2), 143.30 (Cquat-9), 144.33 (Cquat-2); HSQC cross peaks 5.591/121.87, 6.224/121.87, 7.144/126.17, 7.269/128.03, 7.344/126.21, 7.344/128.52, 7.344/128.03, 7.344/129.29, 7.621/117.87, 7.699/122.08, 7.905/127.88; HMBC cross peaks 5.591/120.76, 5.591/137.16, 6.224/120.76, 6.224/137.16, 7.144/122.08, 7.144/143.30, 7.269/7.905, 7.344/126.21, 7.344/128.52, 7.344/128.03, 7.344/129.29, 7.344/133.60, 7.344/137.16, 7.344/137.33, 7.621/120.60, 7.621/143.30 (weak), 7.699/126.17, 7.699/143.30, 7.905/127.88, 7.905/128.03, 7.905/144.33; MS (EI) m/z 330 [M+]; HRMS (EI): calcd for C21H15ClN2 (M+) 330.0918, found 330.0916, −0.7 ppm error.
), 137.16 (Cquat-1 of Ph-3), 137.33 (Cquat-1 of Ph-2), 143.30 (Cquat-9), 144.33 (Cquat-2); HSQC cross peaks 5.591/121.87, 6.224/121.87, 7.144/126.17, 7.269/128.03, 7.344/126.21, 7.344/128.52, 7.344/128.03, 7.344/129.29, 7.621/117.87, 7.699/122.08, 7.905/127.88; HMBC cross peaks 5.591/120.76, 5.591/137.16, 6.224/120.76, 6.224/137.16, 7.144/122.08, 7.144/143.30, 7.269/7.905, 7.344/126.21, 7.344/128.52, 7.344/128.03, 7.344/129.29, 7.344/133.60, 7.344/137.16, 7.344/137.33, 7.621/120.60, 7.621/143.30 (weak), 7.699/126.17, 7.699/143.30, 7.905/127.88, 7.905/128.03, 7.905/144.33; MS (EI) m/z 330 [M+]; HRMS (EI): calcd for C21H15ClN2 (M+) 330.0918, found 330.0916, −0.7 ppm error.
R
f 0.45 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 2
CH2Cl2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98); mp 128.2–128.8 °C; 1H NMR 5.594 (d, 1H, J 0.6, ½ of CH2
98); mp 128.2–128.8 °C; 1H NMR 5.594 (d, 1H, J 0.6, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.233 (d, 1H, J 0.6, ½ of CH2
), 6.233 (d, 1H, J 0.6, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.238 (dd, 1H, J 1.9, 9.5, CH-7), 7.267 (tt, 1H, J 2.1, 7.4, CH-4 of Ph-2), 7.343 (m, 7H, 5 CH of Ph-3, CH-3 and CH-5 of Ph-2), 7.572 (dd, 1H, J 0.7, 9.5, CH-8), 7.806 (dd, 1H, J 0.7, 1.9, CH-5), 7.906 (ddd, 2H, J 1.4, 2.0, 7.1, CH-2 and CH-6 of Ph-2); COSY cross peaks 5.594/6.233, 7.238/7.572, 7.238/7.806 (weak), 7.267/7.343, 7.343/7.906; NOESY cross peaks 5.594/6.233, 5.594/7.806, 6.233/7.343, 7.238/7.572, 7.267/7.343, 7.343/7.806 (weak), 7.343/7.906; 13C NMR 107.01 (Cquat-6), 118.07 (CH-8), 120.50 (Cquat-3), 121.77 (CH2
), 7.238 (dd, 1H, J 1.9, 9.5, CH-7), 7.267 (tt, 1H, J 2.1, 7.4, CH-4 of Ph-2), 7.343 (m, 7H, 5 CH of Ph-3, CH-3 and CH-5 of Ph-2), 7.572 (dd, 1H, J 0.7, 9.5, CH-8), 7.806 (dd, 1H, J 0.7, 1.9, CH-5), 7.906 (ddd, 2H, J 1.4, 2.0, 7.1, CH-2 and CH-6 of Ph-2); COSY cross peaks 5.594/6.233, 7.238/7.572, 7.238/7.806 (weak), 7.267/7.343, 7.343/7.906; NOESY cross peaks 5.594/6.233, 5.594/7.806, 6.233/7.343, 7.238/7.572, 7.267/7.343, 7.343/7.806 (weak), 7.343/7.906; 13C NMR 107.01 (Cquat-6), 118.07 (CH-8), 120.50 (Cquat-3), 121.77 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 124.17 (CH-5), 126.11 (CH-2 and CH-6 of Ph-3), 127.79 (CH-2 and CH-6 of Ph-2), 127.94 (CH-4 of Ph-2), 128.12 (CH-7), 128.43 (CH-3 and CH-5 of Ph), 129.05 (CH-4 of Ph-3), 129.19 (CH-3 and CH-5 of Ph), 133.48 (Cquat-1 of Ph-2), 137.07 (Cquat-1 of Ph-3), 137.24 (Cquat
), 124.17 (CH-5), 126.11 (CH-2 and CH-6 of Ph-3), 127.79 (CH-2 and CH-6 of Ph-2), 127.94 (CH-4 of Ph-2), 128.12 (CH-7), 128.43 (CH-3 and CH-5 of Ph), 129.05 (CH-4 of Ph-3), 129.19 (CH-3 and CH-5 of Ph), 133.48 (Cquat-1 of Ph-2), 137.07 (Cquat-1 of Ph-3), 137.24 (Cquat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 143.28 (Cquat-9), 144.02 (Cquat-2); HSQC cross peaks 5.594/121.77, 6.233/121.77, 7.238/128.12, 7.267/127.94, 7.343/126.11, 7.343/128.43, 7.343/129.05, 7.343/129.19, 7.572/118.07, 7.806/124.17, 7.906/127.79; HMBC cross peaks 5.594/120.50, 5.594/137.24, 6.233/120.50, 6.233/137.24, 7.238/107.01 (weak), 7.238/124.17, 7.238/143.28, 7.267/127.79, 7.343/126.11, 7.343/128.43, 7.343/129.05, 7.343/129.19, 7.343/133.48, 7.343/137.07, 7.572/107.01, 7.572/143.28 (weak), 7.806/128.12, 7.806/143.28, 7.906/127.79, 7.906/127.94, 7.906/144.02; MS (EI) m/z 374 [M+]; HRMS (EI): calcd for C21H15BrN2 (M+) 374.0413, found 374.0411, −0.6 ppm error.
), 143.28 (Cquat-9), 144.02 (Cquat-2); HSQC cross peaks 5.594/121.77, 6.233/121.77, 7.238/128.12, 7.267/127.94, 7.343/126.11, 7.343/128.43, 7.343/129.05, 7.343/129.19, 7.572/118.07, 7.806/124.17, 7.906/127.79; HMBC cross peaks 5.594/120.50, 5.594/137.24, 6.233/120.50, 6.233/137.24, 7.238/107.01 (weak), 7.238/124.17, 7.238/143.28, 7.267/127.79, 7.343/126.11, 7.343/128.43, 7.343/129.05, 7.343/129.19, 7.343/133.48, 7.343/137.07, 7.572/107.01, 7.572/143.28 (weak), 7.806/128.12, 7.806/143.28, 7.906/127.79, 7.906/127.94, 7.906/144.02; MS (EI) m/z 374 [M+]; HRMS (EI): calcd for C21H15BrN2 (M+) 374.0413, found 374.0411, −0.6 ppm error.
R
f 0.66 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 10
CH2Cl2 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90); mp 126.1–126.8 °C; 1H NMR 2.218 (d, 3H, J 0.7, CH3-6), 2.354 (s, 3H, CH3 of 4-MePh-2), 2.375 (s, 3H, CH3 of 4-MePh-3), 5.529 (d, 1H, J 0.9, ½ of CH2
90); mp 126.1–126.8 °C; 1H NMR 2.218 (d, 3H, J 0.7, CH3-6), 2.354 (s, 3H, CH3 of 4-MePh-2), 2.375 (s, 3H, CH3 of 4-MePh-3), 5.529 (d, 1H, J 0.9, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.180 (d, 1H, J 0.9, ½ of CH2
), 6.180 (d, 1H, J 0.9, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.040 (dd, 1H, J 1.6, 9.2, CH-7), 7.157 (m, 4H, CH-3 and CH-5 of 4-MePh-2 and 4-MePh-3), 7.312 (d, 2H, J 8.2, CH-2 and CH-6 of 4-MePh-3), 7.453 (dd, 1H, J 0.7, 1.6, CH-5), 7.595 (d, 1H, J 9.2, CH-8), 7.849 (d, 2H, J 8.2, CH-2 and CH-6 of 4-MePh-2); COSY cross peaks 2.218/7.453, 5.529/6.180, 7.040/7.453 (weak), 7.040/7.595, 7.157/7.312, 7.157/7.849; NOESY cross peaks 2.218/6.040, 2.218/7.453, 2.354/7.157, 2.375/7.157, 5.529/6.180, 6.180/7.312 (weak), 7.040/7.595, 7.157/7.312, 7.157/7.849; 13C NMR 18.37 (CH3-6), 21.25 (CH3 of 4-MePh-2), 21.28 (CH3 of 4-MePh-3), 116.57 (CH-8), 119.71 (Cquat-3), 120.26 (CH2
), 7.040 (dd, 1H, J 1.6, 9.2, CH-7), 7.157 (m, 4H, CH-3 and CH-5 of 4-MePh-2 and 4-MePh-3), 7.312 (d, 2H, J 8.2, CH-2 and CH-6 of 4-MePh-3), 7.453 (dd, 1H, J 0.7, 1.6, CH-5), 7.595 (d, 1H, J 9.2, CH-8), 7.849 (d, 2H, J 8.2, CH-2 and CH-6 of 4-MePh-2); COSY cross peaks 2.218/7.453, 5.529/6.180, 7.040/7.453 (weak), 7.040/7.595, 7.157/7.312, 7.157/7.849; NOESY cross peaks 2.218/6.040, 2.218/7.453, 2.354/7.157, 2.375/7.157, 5.529/6.180, 6.180/7.312 (weak), 7.040/7.595, 7.157/7.312, 7.157/7.849; 13C NMR 18.37 (CH3-6), 21.25 (CH3 of 4-MePh-2), 21.28 (CH3 of 4-MePh-3), 116.57 (CH-8), 119.71 (Cquat-3), 120.26 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 121.68 (Cquat-6), 121.79 (CH-5), 126.05 (CH-2 and CH-6 of 4-MePh-3), 127.53 (CH-2 and CH-6 of 4-MePh-2), 127.67 (CH-7), 129.04 (CH-3 and CH-5 of 4-MePh-2), 129.74 (CH-3 and CH-5 of 4-MePh-3), 131.32 (Cquat-1 of 4-MePh-2), 134.82 (Cquat-1 of 4-MePh-3), 137.17 (Cquat-4 of 4-MePh-2), 137.77 (Cquat
), 121.68 (Cquat-6), 121.79 (CH-5), 126.05 (CH-2 and CH-6 of 4-MePh-3), 127.53 (CH-2 and CH-6 of 4-MePh-2), 127.67 (CH-7), 129.04 (CH-3 and CH-5 of 4-MePh-2), 129.74 (CH-3 and CH-5 of 4-MePh-3), 131.32 (Cquat-1 of 4-MePh-2), 134.82 (Cquat-1 of 4-MePh-3), 137.17 (Cquat-4 of 4-MePh-2), 137.77 (Cquat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 138.71 (Cquat-4 of 4-MePh-3), 142.92 (Cquat-2), 143.83 (Cquat-9); HSQC cross peaks 2.218/18.37, 2.354/21.25, 2.375/21.28, 5.529/120.26, 6.180/120.26, 7.040/127.67, 7.157/129.04, 7.157/129.74, 7.312/126.05, 7.453/121.79, 7.595/116.57, 7.849/127.53; HMBC cross peaks 2.218/121.68, 2.218/121.79, 2.218/127.67, 2.354/129.04, 2.354/137.17, 2.375/129.74, 2.375/138.71, 5.529/119.71, 5.529/134.82, 5.529/137.77 (weak), 6.180/119.71, 6.180/134.82, 6.180/137.77 (weak), 7.040/18.37, 7.040/121.79, 7.040/143.83, 7.157/21.25, 7.157/21.28, 7.157/126.05 (weak), 7.157/127.53 (weak), 7.157/129.04, 7.157/129.74, 7.157/131.32, 7.157/134.82, 7.312/119.71 (weak), 7.312/126.05, 7.312/138.71, 7.453/121.68 (weak), 7.453/127.67, 7.453/143.83, 7.595/121.68, 7.595/143.83 (weak), 7.849/127.53, 7.849/137.17, 7.849/142.92; 15N 202.28 (N-4 and N-1); HMBC cross peaks 202.28/7.453, 202.28/7.595; MS (EI) m/z 338 [M+]; HRMS (EI): calcd for C24H22N2 (M+) 338.1778, found 338.1762, −1.6 ppm error.
), 138.71 (Cquat-4 of 4-MePh-3), 142.92 (Cquat-2), 143.83 (Cquat-9); HSQC cross peaks 2.218/18.37, 2.354/21.25, 2.375/21.28, 5.529/120.26, 6.180/120.26, 7.040/127.67, 7.157/129.04, 7.157/129.74, 7.312/126.05, 7.453/121.79, 7.595/116.57, 7.849/127.53; HMBC cross peaks 2.218/121.68, 2.218/121.79, 2.218/127.67, 2.354/129.04, 2.354/137.17, 2.375/129.74, 2.375/138.71, 5.529/119.71, 5.529/134.82, 5.529/137.77 (weak), 6.180/119.71, 6.180/134.82, 6.180/137.77 (weak), 7.040/18.37, 7.040/121.79, 7.040/143.83, 7.157/21.25, 7.157/21.28, 7.157/126.05 (weak), 7.157/127.53 (weak), 7.157/129.04, 7.157/129.74, 7.157/131.32, 7.157/134.82, 7.312/119.71 (weak), 7.312/126.05, 7.312/138.71, 7.453/121.68 (weak), 7.453/127.67, 7.453/143.83, 7.595/121.68, 7.595/143.83 (weak), 7.849/127.53, 7.849/137.17, 7.849/142.92; 15N 202.28 (N-4 and N-1); HMBC cross peaks 202.28/7.453, 202.28/7.595; MS (EI) m/z 338 [M+]; HRMS (EI): calcd for C24H22N2 (M+) 338.1778, found 338.1762, −1.6 ppm error.
R
f 0.41 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 2
CH2Cl2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98); mp 160.3–160.8 °C; 1H NMR 2.361 (s, 3H, CH3 of 4-MePh-2), 2.382 (s, 3H, CH3 of 4-MePh-3), 5.549 (d, 1H, J 0.8, ½ of CH2
98); mp 160.3–160.8 °C; 1H NMR 2.361 (s, 3H, CH3 of 4-MePh-2), 2.382 (s, 3H, CH3 of 4-MePh-3), 5.549 (d, 1H, J 0.8, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.208 (d, 1H, J 0.8, ½ of CH2
), 6.208 (d, 1H, J 0.8, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.155 (dd, 1H, J 1.9, 9.5, CH-7), 7.171 (m, 4H, CH-3 and CH-5 of 4-MePh-2 and 4-MePh-3), 7.288 (d, 2H, J 8.2, CH-2 and CH-6 of 4-MePh-3), 7.637 (dd, 1H, J 0.7, 9.5, CH-8), 7.709 (dd, 1H, J 0.7, 1.9, CH-5), 7.841 (d, 2H, J 8.2, CH-2 and CH-6 of 4-MePh-2); COSY cross peaks 5.549/6.208, 7.155/7.637, 7.155/7.709, 7.171/7.288, 7.171/7.841, 7.637/7.709; NOESY cross peaks 2.361/7.171, 2.382/7.171, 5.549/6.208, 6.208/7.288 (weak), 7.155/7.637, 7.171/7.288, 7.171/7.841; 13C NMR 21.26 (CH3 of 4-MePh-2), 21.31 (CH3 of 4-MePh-3), 117.60 (CH-8), 120.33 (Cquat-6), 120.51 (Cquat-3), 120.77 (CH2
), 7.155 (dd, 1H, J 1.9, 9.5, CH-7), 7.171 (m, 4H, CH-3 and CH-5 of 4-MePh-2 and 4-MePh-3), 7.288 (d, 2H, J 8.2, CH-2 and CH-6 of 4-MePh-3), 7.637 (dd, 1H, J 0.7, 9.5, CH-8), 7.709 (dd, 1H, J 0.7, 1.9, CH-5), 7.841 (d, 2H, J 8.2, CH-2 and CH-6 of 4-MePh-2); COSY cross peaks 5.549/6.208, 7.155/7.637, 7.155/7.709, 7.171/7.288, 7.171/7.841, 7.637/7.709; NOESY cross peaks 2.361/7.171, 2.382/7.171, 5.549/6.208, 6.208/7.288 (weak), 7.155/7.637, 7.171/7.288, 7.171/7.841; 13C NMR 21.26 (CH3 of 4-MePh-2), 21.31 (CH3 of 4-MePh-3), 117.60 (CH-8), 120.33 (Cquat-6), 120.51 (Cquat-3), 120.77 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 121.95 (CH-5), 125.89 (CH-7), 125.98 (CH-2 and CH-6 of 4-MePh-3), 127.61 (CH-2 and CH-6 of 4-MePh-2), 129.17 (CH-3 and CH-5 of 4-MePh-2), 129.90 (CH-3 and CH-5 of 4-MePh-3), 130.64 (Cquat-1 of 4-MePh-2), 134.20 (Cquat-1 of 4-MePh-3), 137.13 (Cquat
), 121.95 (CH-5), 125.89 (CH-7), 125.98 (CH-2 and CH-6 of 4-MePh-3), 127.61 (CH-2 and CH-6 of 4-MePh-2), 129.17 (CH-3 and CH-5 of 4-MePh-2), 129.90 (CH-3 and CH-5 of 4-MePh-3), 130.64 (Cquat-1 of 4-MePh-2), 134.20 (Cquat-1 of 4-MePh-3), 137.13 (Cquat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 137.75 (Cquat-4 of 4-MePh-2), 139.08 (Cquat-4 of 4-MePh-3), 143.07 (Cquat-9), 144.11 (Cquat-2); HSQC cross peaks 2.361/21.26, 2.382/21.31, 5.549/120.77, 6.208/120.77, 7.155/125.89, 7.171/129.17, 7.171/129.90, 7.288/125.98, 7.637/117.60, 7.709/121.95, 7.841/127.61; HMBC cross peaks 2.361/129.17, 2.361/137.75, 2.382/129.90, 2.382/139.08, 5.549/120.51, 5.549/134.20, 5.549/137.13 (weak), 6.208/120.51, 6.208/134.20, 7.155/121.95 (weak), 7.155/143.07, 7.171/129.17, 7.171/129.90, 7.171/130.64, 7.171/134.20, 7.288/125.98, 7.288/137.13, 7.288/139.08, 7.637/120.33, 7.637/143.07 (weak), 7.709/125.89, 7.709/143.07, 7.841/127.61, 7.841/137.75, 7.841/144.11; 15N 202.48 (N-4 and N-1); HMBC cross peaks 202.48/7.637, 202.48/7.709; MS (EI) m/z 358 [M+]; HRMS (EI): calcd for C23H19ClN2 (M+) 358.1231, found 358.1243, 3.2 ppm error.
), 137.75 (Cquat-4 of 4-MePh-2), 139.08 (Cquat-4 of 4-MePh-3), 143.07 (Cquat-9), 144.11 (Cquat-2); HSQC cross peaks 2.361/21.26, 2.382/21.31, 5.549/120.77, 6.208/120.77, 7.155/125.89, 7.171/129.17, 7.171/129.90, 7.288/125.98, 7.637/117.60, 7.709/121.95, 7.841/127.61; HMBC cross peaks 2.361/129.17, 2.361/137.75, 2.382/129.90, 2.382/139.08, 5.549/120.51, 5.549/134.20, 5.549/137.13 (weak), 6.208/120.51, 6.208/134.20, 7.155/121.95 (weak), 7.155/143.07, 7.171/129.17, 7.171/129.90, 7.171/130.64, 7.171/134.20, 7.288/125.98, 7.288/137.13, 7.288/139.08, 7.637/120.33, 7.637/143.07 (weak), 7.709/125.89, 7.709/143.07, 7.841/127.61, 7.841/137.75, 7.841/144.11; 15N 202.48 (N-4 and N-1); HMBC cross peaks 202.48/7.637, 202.48/7.709; MS (EI) m/z 358 [M+]; HRMS (EI): calcd for C23H19ClN2 (M+) 358.1231, found 358.1243, 3.2 ppm error.
R
f 0.61 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 5
CH2Cl2 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95); mp 153.6–153.9 °C; 1H NMR 2.249 (d, 3H, J 0.8, CH3-6), 5.617 (d, 1H, J 0.7, ½ of CH2
95); mp 153.6–153.9 °C; 1H NMR 2.249 (d, 3H, J 0.8, CH3-6), 5.617 (d, 1H, J 0.7, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.228 (d, 1H, J 0.7, ½ of CH2
), 6.228 (d, 1H, J 0.7, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.091 (dd, 1H, J 1.6, 9.2, CH-7), 7.314 (m, 6H, 4-ClPh-3 and CH-3 and CH-5 of 4-ClPh-2), 7.474 (dd, 1H, J 0.8, 1.6, CH-5), 7.604 (d, 1H, J 9.2, CH-8), 7.833 (d, 2H, J 8.7, CH-2 and CH-6 of 4-ClPh-2); COSY cross peaks 2.249/7.474, 5.617/6.228, 7.091/7.474 (weak), 7.091/7.604, 7.314/7.833; NOESY cross peaks 2.249/7.091, 2.249/7.474, 5.617/6.228, 6.228/7.314, 7.091/7.604, 7.314/7.833; 13C NMR 18.39 (CH3-6), 116.80 (CH-8), 119.38 (Cquat-3), 121.61 (CH-5), 121.73 (CH2
), 7.091 (dd, 1H, J 1.6, 9.2, CH-7), 7.314 (m, 6H, 4-ClPh-3 and CH-3 and CH-5 of 4-ClPh-2), 7.474 (dd, 1H, J 0.8, 1.6, CH-5), 7.604 (d, 1H, J 9.2, CH-8), 7.833 (d, 2H, J 8.7, CH-2 and CH-6 of 4-ClPh-2); COSY cross peaks 2.249/7.474, 5.617/6.228, 7.091/7.474 (weak), 7.091/7.604, 7.314/7.833; NOESY cross peaks 2.249/7.091, 2.249/7.474, 5.617/6.228, 6.228/7.314, 7.091/7.604, 7.314/7.833; 13C NMR 18.39 (CH3-6), 116.80 (CH-8), 119.38 (Cquat-3), 121.61 (CH-5), 121.73 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 122.37 (Cquat-6), 127.42 (2CH of 4-ClPh), 128.38 (CH-7), 128.57 (2CH of 4-ClPh), 128.87 (CH-2 and CH-6 of 4-ClPh-2), 129.30 (2CH of 4-ClPh), 132.45 (Cquat of 4-ClPh), 133.46 (Cquat-4 of 4-ClPh-2), 134.83 (Cquat of 4-ClPh), 135.91 (Cquat-1 of 4-ClPh-3), 136.68 (Cquat
), 122.37 (Cquat-6), 127.42 (2CH of 4-ClPh), 128.38 (CH-7), 128.57 (2CH of 4-ClPh), 128.87 (CH-2 and CH-6 of 4-ClPh-2), 129.30 (2CH of 4-ClPh), 132.45 (Cquat of 4-ClPh), 133.46 (Cquat-4 of 4-ClPh-2), 134.83 (Cquat of 4-ClPh), 135.91 (Cquat-1 of 4-ClPh-3), 136.68 (Cquat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 141.92 (Cquat-2), 144.06 (Cquat-9); HSQC cross peaks 2.249/18.39, 5.617/121.73, 6.228/121.73, 7.091/128.38, 7.314/127.42, 7.314/128.57, 7.314/129.30, 7.474/121.61, 7.604/116.80, 7.833/128.87; HMBC cross peaks 2.249/121.61, 2.249/122.37, 2.249/128.38, 5.617/119.38, 5.617/135.91, 6.228/119.38, 6.228/135.91, 7.091/121.61, 7.091/128.38, 7.314/121.73 (weak), 7.314/127.42, 7.314/128.57, 7.314/129.30, 132.45, 7.314/133.46 (weak), 7.314/134.83, 7.314/135.91, 7.314/136.68, 7.474/116.80 (weak), 7.474/122.37 (weak), 7.474/128.38, 7.474/144.06, 7.604/122.37, 7.604/144.06 (weak), 7.833/128.87, 7.833/133.46, 7.833/141.92; MS (ESI, pos. mode) m/z 379.13 [M + H+]; HRMS (EI): calcd for C22H16Cl2N2 (M+) 378.0685, found 378.0673, −3.1 ppm error.
), 141.92 (Cquat-2), 144.06 (Cquat-9); HSQC cross peaks 2.249/18.39, 5.617/121.73, 6.228/121.73, 7.091/128.38, 7.314/127.42, 7.314/128.57, 7.314/129.30, 7.474/121.61, 7.604/116.80, 7.833/128.87; HMBC cross peaks 2.249/121.61, 2.249/122.37, 2.249/128.38, 5.617/119.38, 5.617/135.91, 6.228/119.38, 6.228/135.91, 7.091/121.61, 7.091/128.38, 7.314/121.73 (weak), 7.314/127.42, 7.314/128.57, 7.314/129.30, 132.45, 7.314/133.46 (weak), 7.314/134.83, 7.314/135.91, 7.314/136.68, 7.474/116.80 (weak), 7.474/122.37 (weak), 7.474/128.38, 7.474/144.06, 7.604/122.37, 7.604/144.06 (weak), 7.833/128.87, 7.833/133.46, 7.833/141.92; MS (ESI, pos. mode) m/z 379.13 [M + H+]; HRMS (EI): calcd for C22H16Cl2N2 (M+) 378.0685, found 378.0673, −3.1 ppm error.
R
f 0.42 (acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 2
CH2Cl2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98); mp 189.1–190.3 °C; 1H NMR 5.545 (d, 1H, J 0.5, ½ of CH2
98); mp 189.1–190.3 °C; 1H NMR 5.545 (d, 1H, J 0.5, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.167 (d, 1H, J 0.5, ½ of CH2
), 6.167 (d, 1H, J 0.5, ½ of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.104 (dd, 1H, J 1.9, 9.5, CH-7), 7.210 (m, 6H, 4-ClPh-3 and CH-3 and CH-5 of 4-ClPh-2), 7.541 (dd, 1H, J 0.7, 9.5, CH-8), 7.651 (dd, 1H, J 0.7, 1.9, CH-5), 7.724 (d, 2H, J 8.7, CH-2 and CH-6 of 4-ClPh-2); COSY cross peaks 5.545/6.167, 7.104/7.541, 7.104/7.651, 7.210/7.724; NOESY cross peaks 5.545/6.167, 6.167/7.210, 7.104/7.541, 7.210/7.724; 13C NMR 117.91 (CH-8), 120.14 (Cquat-3), 120.92 (Cquat-6), 121.78 (CH-5), 122.19 (CH2
), 7.104 (dd, 1H, J 1.9, 9.5, CH-7), 7.210 (m, 6H, 4-ClPh-3 and CH-3 and CH-5 of 4-ClPh-2), 7.541 (dd, 1H, J 0.7, 9.5, CH-8), 7.651 (dd, 1H, J 0.7, 1.9, CH-5), 7.724 (d, 2H, J 8.7, CH-2 and CH-6 of 4-ClPh-2); COSY cross peaks 5.545/6.167, 7.104/7.541, 7.104/7.651, 7.210/7.724; NOESY cross peaks 5.545/6.167, 6.167/7.210, 7.104/7.541, 7.210/7.724; 13C NMR 117.91 (CH-8), 120.14 (Cquat-3), 120.92 (Cquat-6), 121.78 (CH-5), 122.19 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 126.54 (CH-7), 127.35 (2CH of 4-ClPh), 128.70 (2CH of 4-ClPh), 128.92 (CH-2 and CH-6 of 4-ClPh-2), 129.45 (2CH of 4-ClPh), 131.86 (Cquat of 4-ClPh), 133.97 (Cquat-4 of 4-ClPh-2), 135.16 (Cquat of 4-ClPh), 135.34 (Cquat
), 126.54 (CH-7), 127.35 (2CH of 4-ClPh), 128.70 (2CH of 4-ClPh), 128.92 (CH-2 and CH-6 of 4-ClPh-2), 129.45 (2CH of 4-ClPh), 131.86 (Cquat of 4-ClPh), 133.97 (Cquat-4 of 4-ClPh-2), 135.16 (Cquat of 4-ClPh), 135.34 (Cquat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 136.13 (Cquat of 4-ClPh), 143.16 (Cquat-2), 143.36 (Cquat-9); HSQC cross peaks 5.545/122.1, 6.167/122.19, 7.104/126.54, 7.210/127.35, 7.210/128.70, 7.210/129.45, 7.541/117.91, 7.651/121.78, 7.724/128.92; HMBC cross peaks 5.545/120.14, 5.545/135.34, 6.167/120.14, 6.167/135.34, 7.104/121.78, 7.104/143.36, 7.210/127.35, 7.210/128.70, 7.210/129.45, 7.210/131.86, 7.210/135.16, 7.210/135.34, 7.210/136.13, 7.541/120.92, 7.541/143.36 (weak), 7.651/126.54, 7.651/143.36, 7.724/128.92, 7.724/133.97, 7.724/143.16; MS (ESI, pos. mode) m/z 399.20 [M + H+]; HRMS (EI): calcd for C21H13Cl3N2 (M+) 398.0139, found 398.0123, −4.0 ppm error.
), 136.13 (Cquat of 4-ClPh), 143.16 (Cquat-2), 143.36 (Cquat-9); HSQC cross peaks 5.545/122.1, 6.167/122.19, 7.104/126.54, 7.210/127.35, 7.210/128.70, 7.210/129.45, 7.541/117.91, 7.651/121.78, 7.724/128.92; HMBC cross peaks 5.545/120.14, 5.545/135.34, 6.167/120.14, 6.167/135.34, 7.104/121.78, 7.104/143.36, 7.210/127.35, 7.210/128.70, 7.210/129.45, 7.210/131.86, 7.210/135.16, 7.210/135.34, 7.210/136.13, 7.541/120.92, 7.541/143.36 (weak), 7.651/126.54, 7.651/143.36, 7.724/128.92, 7.724/133.97, 7.724/143.16; MS (ESI, pos. mode) m/z 399.20 [M + H+]; HRMS (EI): calcd for C21H13Cl3N2 (M+) 398.0139, found 398.0123, −4.0 ppm error.
Acknowledgements
The financial support by The Bulgarian Science Fund, project “Development of Advanced and Effective Research Infrastructure for NMR Analysis of Bio- and Nanomaterials” and infrastructure projects UNA-17/2005 and DRNF-02-13/2009, is gratefully acknowledged. We also thank Prof. DSc Svetlana Simova from IOCCP-BAS for carrying out a significant part of the NMR experiments.Notes and references
- (a) A. Durand, J. P. Thénot, G. Bianchetti and P. L. Morselli, Drug Metab. Rev., 1992, 24, 239 CrossRef CAS PubMed; (b) M. Lhassani, O. Chavignon, J. M. Chezal, J. C. Teulade, J. P. Chapat, R. Snoeck, G. Andrei, J. Balzarini, E. De Clercq and A. Gueiffier, Eur. J. Med. Chem., 1999, 34, 271 CrossRef CAS; (c) K. Mizushige, T. Ueda, K. Yukiiri and H. Suzuki, Cardiovasc. Drug Rev., 2002, 20, 163 CrossRef CAS PubMed; (d) Z.-P. Zhuang, M.-P. Kung, A. Wilson, C.-W. Lee, K. Plössl, C. Hou, D. M. Holtzman and H. F. Kung, J. Med. Chem., 2003, 46, 237 CrossRef CAS PubMed; (e) C. Enguehard-Gueiffier and A. Gueiffier, Mini-Rev. Med. Chem., 2007, 7, 888 CrossRef CAS PubMed; (f) P. A. Babu, M. L. Narasu and K. Srinivas, ARKIVOC, 2007, 247 CAS; (g) F. Couty and G. Evano, Comprehensive Heterocyclic Chemistry, Elsevier, Oxford, 2008 Search PubMed; (h) N. M. Shukla, D. B. Salunke, E. Yoo, C. A. Mutz, R. Balakrishna and S. A. David, Bioorg. Med.Chem., 2012, 20, 5850 CrossRef CAS PubMed.
- (a) W. L. Mosby, Chem. Heterocycl. Compd., 1961, 15, 460 Search PubMed; (b) J. A. Montgomery and J. A. Secrist, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky, C. W. Rees and K. T. Potts, Pergamon: Oxford, New York, 1984, vol. 5, pp. 607–668 Search PubMed; (c) C. Hulme and Y.-S. Lee, Mol. Diversity, 2008, 12, 1 CrossRef CAS PubMed; (d) E. Kianmehr, M. Ghanbari, M. N. Niri and R. Faramarzi, J. Comb. Chem., 2010, 12, 41 CrossRef CAS PubMed; (e) S. Husinec, R. Markovic, M. Petkovic, V. Nasufovic and V. Savic, Org. Lett., 2011, 13, 2286 CrossRef CAS PubMed; (f) C. He, J. Hao, H. Xu, Y. Mo, H. Liu, J. Han and A. Lei, Chem. Commun., 2012, 48, 11073 RSC; (g) I. B. Rozentsveig, V. Y. Serykh, G. N. Chernysheva, K. A. Chernyshev, E. V. Kondrashov, E. V. Tretyakov and G. V. Romanenko, Eur. J. Org. Chem., 2013, 368 CrossRef CAS; (h) D. C. Mohan, S. N. Rao and S. Adimurthy, J. Org. Chem., 2013, 78, 1266 CrossRef PubMed.
- (a) J. G. Lombardino, J. Org. Chem., 1965, 30, 2403 CrossRef CAS; (b) E. S. Hand and W. W. Paudler, Tetrahedron, 1982, 38, 49 CrossRef CAS; (c) Y. Sumalatha, T. R. Reddy, P. P. Reddy and B. Satyanarayana, ARKIVOC, 2009, 315 CAS; (d) A. Herath, R. Dahl and N. D. P. Cosford, Org. Lett., 2010, 12, 412 CrossRef CAS PubMed; (e) S. El Kazzouli, A. G. du Bellay, S. Berteina-Raboin, P. Delagrange, D.-H. Caignard and G. Guillaumet, Eur. J. Med. Chem., 2011, 46, 4252 CrossRef CAS PubMed; (f) T. Mutai, H. Sawatani, T. Shida, H. Shono and K. Araki, J. Org. Chem., 2013, 78, 2482 CrossRef CAS PubMed.
- A. J. Stasyuk, M. Banasiewicz, M. K. Cyrański and D. T. Gryko, J. Org. Chem., 2012, 77, 5552 CrossRef CAS PubMed.
- (a) D. Sucunza, A. Samadi, M. Chioua, D. B. Silva, C. Yunta, L. Infantes, M. C. Carreiras, E. Soriano and J. Marco-Contelles, Chem. Commun., 2011, 47, 5043 RSC; (b) O. Kim, Y. Jeong, H. Lee, S.-S. Hong and S. Hong, J. Med. Chem., 2011, 54, 2455 CrossRef CAS PubMed; (c) A. Basilio-Lopes, T. M. de Aquino, A. Mongeot, J.-J. Bourguignon and M. Schmitt, Tetrahedron Lett., 2012, 53, 2583 CrossRef CAS; (d) D. K. Nair, S. M. Mobin and I. N. N. Namboothiri, Org. Lett., 2012, 14, 4580 CrossRef CAS PubMed.
- (a) H. Bienaymé and K. Bouzid, Angew. Chem., Int. Ed., 1998, 37, 2234 CrossRef; (b) E. F. DiMauro and J. M. Kennedy, J. Org. Chem., 2007, 72, 1013 CrossRef CAS PubMed; (c) N. Chernyak and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2743 CrossRef CAS PubMed; (d) F. Mert-Balci, J. Conrad and U. Beifuss, ARKIVOC, 2012, 243 CAS; (e) A. B. Ramesha, G. M. Raghavendra, K. N. Nandeesh, K. S. Rangappa and K. Mantelingu, Tetrahedron Lett., 2013, 54, 95 CrossRef CAS.
- (a) Y.-L. Chang, H.-M. Wang, R.-S. Hou, I.-J. Kang and L.-C. Chen, J. Chin. Chem. Soc., 2010, 57, 153 CrossRef CAS; (b) K. C. Chunavala, G. Joshi, E. Suresh and S. Adimurthy, Synthesis, 2011, 42, 635 Search PubMed; (c) Z. Fei, Y.-P. Zhu, M.-C. Liu, F.-C. Jia and A.-X. Wu, Tetrahedron Lett., 2013, 54, 1222 CrossRef CAS.
- V. B. Kurteva, L. A. Lubenov, D. V. Nedeltcheva, R. P. Nikolova and B. L. Shivachev, ARKIVOC, 2012, 282 CAS.
- CAS no. 612-71-5.
- (a) R. E. Lyle, E. J. DeWitt, N. M. Nichols and W. Cleland, J. Am. Chem. Soc., 1953, 75, 5959 CrossRef CAS; (b) R. Ruíz-Guerrero, J. Cárdenas, L. Bautista, M. Vargas, E. Vázquez-Labastida and M. Salmón, J. Mex. Chem. Soc., 2006, 50, 114 Search PubMed; (c) S. Kotha, D. Kashinath and S. Kumar, Tetrahedron Lett., 2008, 49, 5419 CrossRef CAS; (d) A. Kumar, M. Dixit, S. P. Singh, R. Raghunandan, P. R. Maulik and A. Goel, Tetrahedron Lett., 2009, 50, 4335 CrossRef CAS.
- (a) M. M. Karelson, A. R. Katritzky, M. Szafran and M. C. Zerner, J. Org. Chem., 1989, 54, 6030 CrossRef CAS; (b) J. A. Kereselidze and T. S. Zarkua, Chem. Heterocycl. Compd., 2000, 36, 1161 CrossRef CAS; (c) H. I. Abdulla and M. F. El-Bermani, Spectrochim. Acta, Part A, 2001, 57, 2659 CrossRef CAS; (d) N. Akai, K. Ohno and M. Aida, Chem. Phys. Lett., 2005, 413, 306 CrossRef CAS; (e) R. Glaser, J. Yin and S. Miller, J. Org. Chem., 2010, 75, 1132 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR spectra of all new compounds. See DOI: 10.1039/c3ra45005h |
| ‡ Dedicated to our colleague and friend Prof. DSc Svetlana Simova on the occasion of her 60th anniversary. |
| This journal is © The Royal Society of Chemistry 2014 |