Engineered-membranes and engineered-micelles as efficient tools for purification of halorhodopsin and bacteriorhodopsin
Sansa
Dutta
a,
Divya K.
Nair
b,
Irishi N. N.
Namboothiri
b,
Ellen
Wachtel
c,
Noga
Friedman
a,
Mordechai
Sheves
*a and
Guy
Patchornik
*d
aDepartment of Organic Chemistry, Weizmann Institute of Science, Rehovot, 76100, Israel. E-mail: mudi.sheves@weizmann.ac.il; Fax: +972-8-9344142; Tel: +972-8-9344320
bDepartment of Chemistry, Indian Institute of Technology, Powai, Bombay, 400076, India
cChemical Research Infrastructure Unit, Weizmann Institute of Science, 76100, Rehovot, Israel
dDepartment of Biological Chemistry, Ariel University, Ariel, 40700, Israel. E-mail: guyp@ariel.ac.il; Fax: +972-3-9066634; Tel: +972-3-9755806
First published on 3rd November 2014
Abstract
We describe two alternative and complementary purification methods for halorhodopsin and bacteriorhodopsin. The first relies on a unique form of detergent micelles which we have called engineered-micelles. These are specifically conjugated in the presence of [hydrophobic chelator:Fe2+] complexes and form detergent aggregates into which membrane proteins partition, but hydrophilic water-soluble proteins do not. The approach was tested on the membrane protein, bacteriorhodopsin (bR), with five non-ionic detergents (OG, OTG, NG, DM, and DDM), commonly used in purification and crystallization of membrane proteins, in combination with the commercially available bathophenanthroline or with one of the three synthesized phenanthroline derivatives (Phen-C10, Phen-C8 and Phen-C6). Our results show that bR is extracted efficiently (60–86%) and directly from its native membrane into diverse detergent aggregates with preservation of its native conformation, while 90–95% of an artificial contaminating background is excluded. For implementation of the second method, based on engineered-membranes, the use of detergents, which in some cases may produce protein denaturation, is not required at all. Protein-containing membranes are conjugated via the same hydrophobic [chelator:metal ion] complexes but maintain the membrane protein in its native bilayer environment throughout the process. This method is demonstrated on the membrane protein halorhodopsin from Natronomonas pharaonis (phR) and leads to good recovery yields (74–89%) and removal of >85% of artificial background impurities while preserving the native state of phR. The detailed structure of the hydrophobic chelator used has been found to have a marked effect on the purity and yield of both methods.
Introduction
Isolation of membrane proteins (MPs) in a pure, concentrated and functional state is a precondition for structural determination by X-ray crystallography, electron microscopy or NMR. To obtain sufficient material, MPs are generally expressed in diverse host organisms and once the expression levels are satisfactory, purification is initiated. Ideally, the expressed MP is embedded in the membrane of the host cell and its extraction from the membrane is achieved by addition of detergents above their critical micellar concentration (cmc).1 Under these conditions, the detergent disrupts the membrane and, in parallel, surrounds and covers the hydrophobic domains of the protein, leading to water-soluble [detergent–MP–lipid] ternary complexes. Purification is accomplished either via classical chromatographic methods (e.g. ion exchange chromatography) or by genetically engineered affinity-tags (e.g. His-tag) which can lead to highly pure protein preparations.2 Clearly, exclusion of most of the host proteins by non-chromatographic means prior to the chromatographic step would simplify purification and potentially lead to higher recovery yields and overall greater purity.In 1981, Bordier demonstrated that MPs (being hydrophobic) partition efficiently into detergent rich phases composed of the non-ionic detergent Triton X-114, whereas water-soluble proteins do not.3 This partitioning process, called cloud point extraction, relied on the ability of Triton X-114 to undergo phase separation at ∼22 °C into detergent rich and poor phases.4 Although Triton X-114 provided working conditions that could preserve the functionality of many MPs, the approach was limited by the fact that numerous other detergents, commonly used in the purification of membrane proteins, only reach the cloud point at elevated temperatures that would denature most proteins. Successful attempts to lower the cloud point temperature in the presence of high concentrations of water-soluble polymers (e.g. dextran, PEG-40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–7) or precipitants (e.g. ammonium sulfate8,9) have been reported. However, it is clear that purification would be greatly simplified if neither polymers nor precipitants were required.
5–7) or precipitants (e.g. ammonium sulfate8,9) have been reported. However, it is clear that purification would be greatly simplified if neither polymers nor precipitants were required.
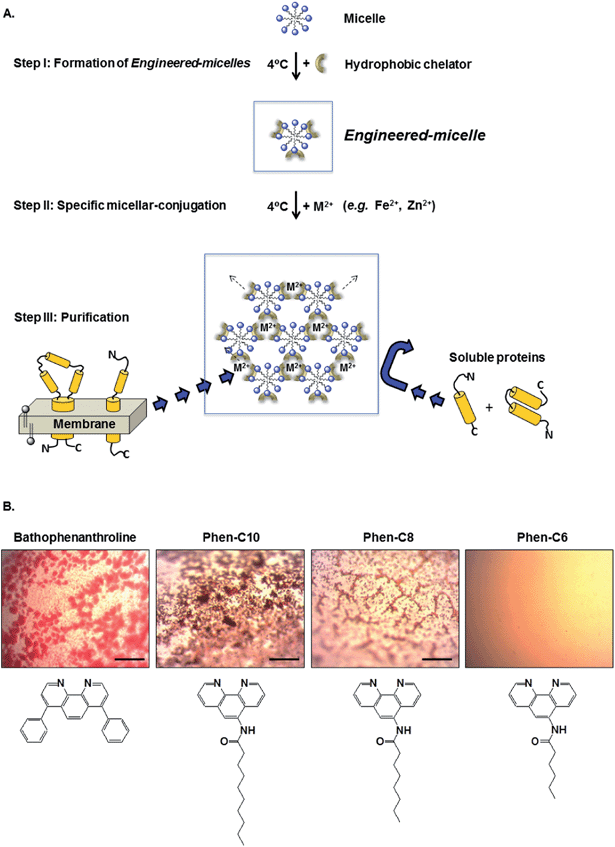
To overcome these limitations and produce detergent rich phases under mild conditions regardless of (a) the cloud point temperature of the detergent used, (b) at low detergent concentration, and (c) without polymers or precipitants, we have developed a new class of micelles which we call engineered-micelles.10 These micelles can be specifically conjugated via strong complexation between hydrophobic metal chelators (embedded within the micelle) and divalent metal ions, particularly Fe2+, which serve as mediators in the aqueous phase (Fig. 1A). We recently demonstrated that conjugated engineered-micelles composed of [octylglucoside (OG)/the hydrophobic chelator, bathophenanthroline/Fe2+] can purify the light driven proton pump, bacteriorhodopsin (bR) from the added E. coli lysate.11 Here we show that four additional non-ionic detergents commonly used in the purification of MPs are also able to produce high levels of bR purification.
Since in some cases membrane disruption by detergents may be accompanied by the denaturation of the membrane-bound proteins, we have, in addition, developed a complementary approach that relies on conjugation of membranes containing the light driven chloride pump, halorhodopsin (phR) (rather than micelles), using the same [chelator:metal] complexes and thereby maintaining the membrane protein within its native environment and circumventing the need for detergents. Synthesized phenanthroline derivatives are shown to be able to produce high levels of both bR and phR purification.
Results
Conjugation of engineered-micelles
Aqueous suspensions containing 200 mM NaCl and the non-ionic detergent OG (22 mM) were incubated at room temperature with either the commercially available hydrophobic chelator bathophenanthroline (1 mM) or each of the three synthesized phenanthroline derivatives (Phen-C10, Phen-C-8 and Phen-C6). Following the addition of Fe2+ (0.5 mM) red oily globules appeared in a light microscope except in the case of Phen-C6 (Fig. 1B). The color of the globules indicates that they contain the red [(phenanthroline)3:Fe2+] hydrophobic complex. No globules appeared in the absence of the chelators, the metal or both (not shown).These findings are consistent with our previous studies demonstrating that conjugation of detergent micelles is a rapid process, occurring within seconds after Fe2+ addition.10 Similar short times were found within the complete temperature range of interest, i.e. 4–23 °C.10 We attribute this temperature independence to the strong binding affinity between Fe2+ and the chelators used, all of which are derivatives of 1,10-phenanthroline, known to generate very strong complexes with Fe2+.12 Cryo-TEM analysis of the cylindrical micelles, stacked-membranes or multilamellar vesicles, formed within seconds following addition of Fe2+ to engineered-micelles, provides strong support for this conclusion.13 Finally, phase separation is accomplished with detergent concentration in the vicinity of the cmc and in the absence of precipitant.
Purification of bacteriorhodopsin (bR) with conjugated engineered-micelles
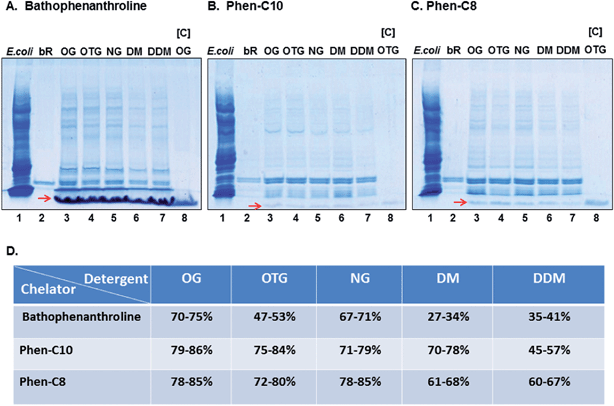
Purification of bR, embedded within purple membranes, from the E. coli lysate added as an artificial background, was accomplished with bathophenanthroline, Phen-C10 or Phen-C8 in combination with each of the non-ionic detergents: OG, OTG, NG, DM or DDM. The procedure was performed in three steps. In the first step, conjugated engineered-micelles, containing one of the above-listed detergents and one of the hydrophobic chelators, were prepared. Conjugation was induced by addition of Fe2+ (at 4 °C) and resulted in the formation of a distinct (red) detergent aggregate. Immediately thereafter, a mixture of bR and the E. coli lysate was added. Gentle agitation at 4 °C in the dark followed by a short (2 min.) low spin centrifugation generated red pellets for all [detergent–chelator–Fe2+] combinations and a clear colorless supernatant.SDS-PAGE analysis of the pellets showed that they all contained bR. However, yield and purity differed among the [detergent:chelator] combinations (Fig. 2A). Highest recovery yields (by densitometry) were obtained with the two synthesized phenanthroline derivatives: Phen-C10 (45–86%) and Phen-C8 (60–85%), whereas the commercial chelator bathophenanthroline was less efficient (27–75%) (Fig. 2A–C). More than 90% of the contaminating background was removed by all [detergent:chelator] combinations as determined by densitometry. The highest purity, on average, was obtained with Phen-C10 and the least with bathophenanthroline (Fig. 2A–C). Analysis of detergent aggregates devoid of protein demonstrated that they migrate at the front of the gels and are also stained with Coomassie brilliant blue (see red arrows in Fig. 2A–C, lanes 3–8).
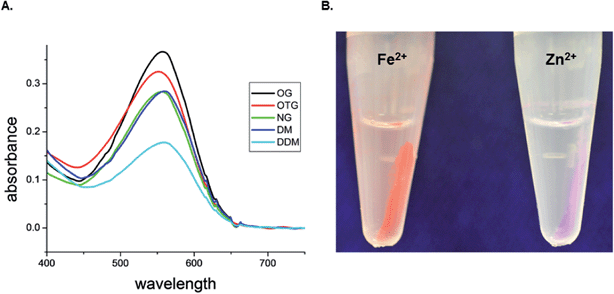
Absorption spectra of bR after partitioning into conjugated engineered-micelles and pellet dissolution
The absorption spectra of bR were recorded after partitioning into different conjugated engineered-micelles and following pellet dissolution (Fig. 3A). Micellar conjugation was carried out with the [(Phen-C10)3:Zn2+] complex to prevent the overlapping absorption of the [(phenanthroline)3:Fe2+] red complex. Efficient pellet dissolution was observed in the presence of EDTA (50 mM) and imidazole (200 mM). Characteristic absorption (at 550–565 nm) of the native bR was observed with all five non-ionic detergents (Fig. 3A).Purification of halorhodopsin from Natronomonas pharaonis (phR) with conjugated engineered-membranes
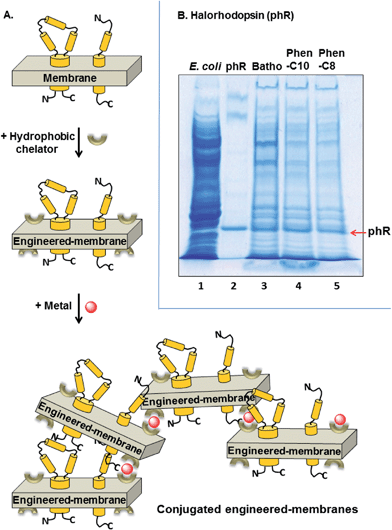
Purification of phR proteins embedded within their native membranes was obtained with three hydrophobic chelators (bathophenanthroline, Phen-C10 or Phen-C8) in the presence of ZnCl2 without any added detergent (Fig. 4A). We found that phR-containing membranes could be efficiently precipitated after a brief incubation (2 min) with one of the above hydrophobic chelators followed by the addition of Zn2+. SDS-PAGE analysis of the resulting purple pellets showed that most of the impurities formed due to the E. coli lysate background (>85% by densitometry) were removed by all three chelators tested. However, the two synthesized chelators (Phen-C10 and Phen-C8) were found to lead to purer samples than the commercially available bathophenanthroline (Fig. 4B, lanes 4 and 5 vs. 3). The presence of EDTA (10–50 mM) after the addition of Zn2+ and during the washing step of the pellet was found to substantially increase the phR purity and therefore was used in all experiments. Similar results were also observed with FeSO4 under identical conditions (not shown). Recovery yields of phR with bathophenanthroline, Phen-C10 and Phen-C8 were: 82–89%, 74–79% and 31–38%, respectively (by densitometry) (Fig. 4B, lanes 3–5).Absorption spectra of phR following pellet dissolution
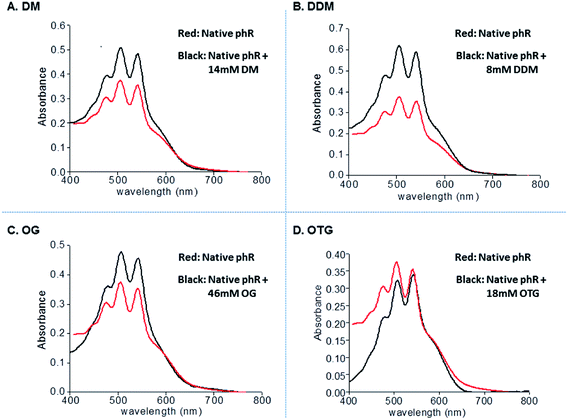
The absorption spectra of phR were recorded in engineered-membranes, conjugated with the [(bathophenanthroline)3:Zn2+] complex, and following pellet dissolution by each of the 4 different nonionic detergents (Fig. 5A–D). Characteristic absorption of the native phR at 578 nm and the absorption of the carotenoid chromophore, including its fine structure, were essentially preserved with all four non-ionic detergents tested (Fig. 5A–D). The purity of bR or phR could not be assessed by measurement of the 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 568 nm or 280
568 nm or 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 578 nm ratios respectively, since all detergent aggregates contain hydrophobic chelators that absorb at 280 nm.
578 nm ratios respectively, since all detergent aggregates contain hydrophobic chelators that absorb at 280 nm.
Discussion
The ability of conjugated engineered-micelles to phase separate at low temperature (4 °C) and under mild conditions that do not require high detergent concentration, water-soluble polymers (e.g. PEG) or precipitants (e.g. ammonium sulfate) motivated us to assess the generality of such a procedure for the purification of bR (Fig. 1A).Micelles composed of five non-ionic detergents, commonly used in the purification of membrane proteins (OG, OTG, NG, DM and DDM), were studied. These were first transformed into the corresponding engineered-micelles by brief incubation with one of the four hydrophobic metal chelators (bathophenanthroline, Phen-C10, Phen-C8 and Phen-C6), followed by specific conjugation with Fe2+. Light microscopy images resulting from such an experiment with OG micelles reveal red oily globules that were observed with all chelators except for Phen-C6 (Fig. 1B). Apparently the alkyl tail of Phen-C6 is too short to support efficient partitioning into OG micelles. Therefore, further investigations did not include Phen-C6. Similar images were observed with the other four detergents (data not shown).
The hydrophobic nature of the red oily globules suggested that bR, being a MP, would partition into them whereas water soluble proteins would not. Moreover, we hypothesized that partitioning could take place while bR is embedded within its native lipid bilayer. This would constitute a single-step method to direct bR from its membrane environment into diverse detergent aggregates. Such a scenario would provide a distinct advantage over the current phase separation methods (i.e. cloud point extraction), which rely on two sequential steps: (i) extraction of MPs from the membrane with appropriate detergents and the formation of stable protein detergent complexes (PDCs), followed by (ii) aggregation of PDCs and formation of a detergent rich phase.14 To test the above hypothesis, conjugated engineered-micelles were incubated with E. coli lysate (serving as an artificial contamination background) and purple membranes containing the membrane protein, bacteriorhodopsin (bR). bR is an intrinsically small (26 kDa) MP that functions as a light driven proton pump in Halobacterium salinarum.15 It is by far the major membrane protein in purple membranes (Fig. 2A–C, lanes 2) and the artificial impurity background was required to demonstrate the process efficiency (i.e. removal of hydrophilic proteins).
We found that gentle agitation at 4 °C of bR-containing purple membranes with different [detergent:chelator:Fe2+] combinations generated red pellets after a short low-spin centrifugation step (985 × g) and that these contained the target membrane protein bR as determined by SDS-PAGE analysis (Fig. 2A–C, lanes 3–7). In addition, most water-soluble proteins (>90%, by densitometry) were excluded from the pellets, regardless of the combination used. These results show that bR partitions directly from its native membrane into conjugated engineered-micelles whereas the large majority of soluble proteins present in the lysate do not. Surprisingly, the chemical structure of the hydrophobic chelator was found to have a marked effect on the efficiency of the purification process. The lowest yields and purity were obtained with the commercially available bathophenanthroline, whereas the two synthesized derivatives, Phen-C10 and Phen-C8, led to higher recovery yields and purity (Fig. 2A–D). In particular, Phen-C10 excluded more than 95% of the impurities (by densitometry) and also appeared to be superior to Phen-C8 with respect to purity (Fig. 2Bvs.C). These results point to the pivotal role of the hydrophobic anchor of the chelator moiety (1,10-phenanthroline). The hydrophobic anchor affects both purity and yield and single aliphatic tails containing 8 or 10 carbons are favored over the two phenyl groups present in bathophenanthroline (Fig. 1B). Therefore, further process optimization may require defining the ideal chain length, regardless of the chelating moiety used.
Phen-C10 was chosen as the preferred chelator since it led to the highest purity (Fig. 2B) and moderate recovery yields (Fig. 2D). Even though micellar conjugation was accomplished near the cmc values of the detergents studied, upon conjugation, the detergent concentration within the aggregates increases significantly. It was thus essential to assess whether bR had experienced a denaturation process, and therefore the absorption spectra of bR were recorded following the purification process. In this case, conjugation was induced by Zn2+ so as to circumvent the overlapping absorption of the [(Phen-C10)3:Fe2+] red complex (Fig. 3B), with that of bR. The results show that the native state of bR was not significantly perturbed during purification and only minor deviations from the absorption maximum (568 nm) in the native membranes were observed (Fig. 3A). These may likely be attributed to the presence of detergents as has been found for example with OG,16 OTG17 and NG.18 Interestingly, efficient pellet dissolution also required the presence of EDTA (50 mM) and imidazole (200 mM), presumably because these can compete with the hydrophobic chelator for chelated Zn2+ and thus promote more efficient disintegration of engineered-micelles.
Current findings demonstrate that the detergent-based membrane protein purification method presented here benefits from the following features. (a) Micellar aggregates are formed at low detergent concentrations, close to the cmc values of the detergent used. (b) The utilization of a single detergent and the absence of polymers or precipitants throughout the process simplifies protein isolation. (c) Purification is conducted at low temperature (4 °C) thus making the approach applicable to heat-sensitive membrane proteins. (d) Exclusion of most impurities (90–95%) in a single step is an advantageous starting point for any additional chromatography. (e) The red color of the [(phenanthroline)3:Fe2+] complex serves as an indicator for the process efficiency, and its presence in the supernatant serves as a marker for engineered-micelles that did not precipitate. The loss of protein after washing the pellet can be determined accordingly. (f) bR partitions directly from its native membrane into the detergent aggregate, thus circumventing an intermediate step required in different phase separation approaches. MPs are first extracted from the membrane with detergents and only then PDCs are induced to aggregate into a detergent rich phase. (g) The process is rapid (∼15–30 min) and requires only simple instrumentation (vortex + table centrifuge). Finally, removal of the hydrophobic chelator from the protein may be accomplished in a similar manner to the way detergents are exchanged for other detergents while the target protein is bound to the chromatographic media.
A significant drawback inherent to the conjugated-micelle method of bR purification is the locally high detergent concentration within the aggregate which can induce irreversible denaturation of some membrane proteins. To overcome this problem, we developed a complementary strategy that does not include the detergent and maintains the target membrane protein within its native bilayer throughout the purification process. This strategy relies on our previous observations that purple membranes can be conjugated in the presence of diverse metals and hydrophobic chelators, while bR remains in its native functional state.19 We therefore applied similar conjugation conditions to aqueous suspensions of native, phR-containing membranes in a background of E. coli lysate. phR has shown itself to be sensitive to high detergent concentrations (not shown).
The phR used in this study derives from Natronomonas pharaonis and is an inward-directed light driven chloride pump.20 It belongs to a subfamily of 7 transmembrane integral membrane proteins (archaeal rhodopsins) and has sequence homology to bR as well as to sensory rhodopsins. phR binds retinal via K242 on helix G as a protonated Schiff base and absorbs green light with a maximum wavelength of 578 nm.20 Photon absorption triggers its photocycle by inducing isomerization of the retinal all-trans isomer to the 13-cis. It was expected that partitioning of the highly hydrophobic chelators: bathophenanthroline, Phen-C10 or Phen-C8 into membranes containing phR would transform the latter into the corresponding engineered-membranes and allow their conjugation with an appropriate metal (Fig. 4A).
Indeed, SDS-PAGE analysis of pellets comprised of conjugated engineered-membranes were found to contain the target phR whereas most of the E. coli impurities (>85%, by densitometry) were excluded (Fig. 4B). These results demonstrate that separation of a membrane protein from soluble proteins can be achieved in the absence of detergents thereby preventing potential denaturation of the target membrane protein. The commercially available bathophenanthroline led to higher recovery yields (82–89%, by densitometry), whereas the two synthesized chelators, Phen-C10 and Phen-C8, led to 74–79% and 31–38% recovery, respectively, but provided greater purity (Fig. 4B, lanes 3–5). The greater purity of the synthesized derivatives is consistent with the results obtained with engineered-micelles and shows that the structure of the chelator used is a major factor in the purification process affecting both purity and yield. We found that the pellets of the conjugated phR-containing membranes could be readily dissolved with relatively low concentrations of several non-ionic detergents (e.g. 8 mM DDM, 14 mM DM, 18 mM OTG and 46 mM OG) while maintaining phR in its native state (Fig. 5A–D). Measurement of the absorption spectra of the dissolved pellets showed that the characteristic absorption of the retinal moiety at 578 nm is preserved as well as the ratio of the beta-carotene and the retinal chromophores (2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively).
1, respectively).
Finally, the finding that precipitation of the membrane proteins embedded within their bilayers takes only a few minutes under mild conditions appears to be a valuable asset for proteomic studies. Samples containing both membrane and soluble proteins could be rapidly and efficiently separated prior to 2D gel electrophoresis, thus reducing the number of spots per gel and simplifying the analysis.
Experimental
Materials
Bathophenanthroline, octyl β-D-glucoside (OG), octyl β-D-1-thioglucoside (OTG), nonyl-β-D-glucoside (NG), decyl-β-D-maltoside (DM), dodecyl-β-D-maltoside (DDM), sodium dodecyl sulfate (SDS), NaCl, and FeSO4 were obtained from Sigma-Aldrich (St. Louis, MO).Synthesis of hydrophobic phenanthroline derivatives (Phen-C6, Phen-C8 and Phen C-10)
To a vigorously stirred saturated aqueous solution of NaHCO3 (18 ml) at 0 °C was added 1,10-phenanthroline-5-amine (400 mg, 2.05 mmol) followed by hexanoyl chloride (0.350 ml, 337 mg, 2.5 mmol) or octanoyl chloride (0.432 ml, 407 mg, 2.5 mmol) or decanoyl chloride (0.515 ml, 477 mg, 2.5 mmol). After continuous stirring until the completion of the reaction (3 h, monitored by TLC), the mixture was extracted with ethyl acetate (3 × 10 ml). The organic layer was then treated with a few drops of pyridine and washed successively with 5% aqueous HCl (10 ml), 5% NaHCO3 (10 ml) and water until neutral pH was achieved. The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo to provide the crude amide which was purified by silica gel column chromatography.Characterization of phenanthroline derivatives
Preparation of conjugated engineered-micelles (general protocol)
Engineered-micelles composed of non-ionic detergents were prepared by addition of 5 μl aliquots of freshly prepared 20 mM bathophenanthroline (in methanol) into aqueous solutions containing 11 μl (200 mM OG), 10 μl (100 mM OTG), 10 μl (80 mM NG), 12.5 μl (20 mM DM), or 10 μl (10 mM DDM) with vigorous vortexing. This was followed by the addition of double distilled water (DDW) to a final volume of 35 μl. Conjugation of engineered-micelles was achieved by further addition of 50 μl of FeSO4 (1 mM) in NaCl (400 mM).Purification of bR with conjugated engineered-micelles (general protocol)
Into 85 μl of freshly prepared conjugated engineered-micelles (85 μl), E. coli lysate (7–15 μl) and purple membranes (1–2 μl) containing bR at 7.5 mg ml−1 were added without vortexing. The system was gently agitated with an orbital shaker (300 rounds per minute) for 15–30 minutes at 4 °C in the dark. Samples were then briefly centrifuged for 2 minutes at 985 × g; the majority of the supernatant (100 μl) was carefully discarded and the resulting pellet was briefly washed with cold 200 mM NaCl (90 μl). An additional spin (1 min at 3935 × g) was applied; the supernatant was removed and the pellet composition was analyzed by SDS-PAGE.Purification of bR with conjugated engineered-micelles containing synthesized phenanthroline derivatives (Phen-C8 and Phen-C10)
Purification was identical to the general protocol described above but Phen-C8 or Phen-C10 (in MeOH) was present rather than bathophenanthroline.Pellet dissolution
Pellets were dissolved by vortexing with 20 μl imidazole (2 M, pH = 7.5) and 20 μl EDTA (0.5 M, pH = 7.5) for 30 seconds. This was followed by further vortexing for 60 seconds with 50 mM OG, 50 mm OTG, 20 mM NG, 10 mM DM, or 20 mM DDM in a final volume of 200 μl. Samples were subjected to a short (3 minutes), low-spin (2236 × g) step which led to a purple supernatant and a very small, off-white precipitate. Essentially, total dissolution was observed with pellets containing OG, OTG, NG and DM, whereas pellets containing DDM led to partial dissolution.Purification of halorhodopsin (phR) with conjugated engineered-membranes
Into an aqueous mixture containing DDW (60 μl), 0.8 M NaCl (50 μl), E. coli lysate (20 μl), hR (30 μl of 0.2 mg ml−1), 10 μl of bathophenanthroline, Phen-C10 or Phen-C8 (all at 20 mM in MeOH) were added with constant vigorous vortexing. After a short incubation (2 min) at room temperature (or 4 °C), 10 μl of 50 mM ZnCl2 and 10 μl of 0.5 M EDTA (pH = 7.5) were added sequentially with constant vortexing. Samples were centrifuged for 3 minutes at 1537 × g and the majority of the supernatant (160 μl) was discarded. Pellets were resuspended by addition of 160 μl of 50 mM EDTA (pH = 7.5), centrifuged at 6150 × g for 2 min and the supernatant was discarded. Washed pellets were then dissolved with sample buffer, by vortexing or by crushing with a pipette tip, and analyzed by SDS-PAGE. For spectral analysis, pellets were dissolved with 50–200 μl of 14 mM DM, 8 mM DDM, 46 mM OG or 18 mM OTG in DDW.Preparation of purple membranes
Halobacterium salinarum was grown from the S9 strain and purple membranes containing bacteriorhodopsin (bR) were isolated as previously described.21Preparation of halorhodopsin (phR)
Halorhodopsin was produced from the mutant KM-1 strain of Natronomonas pharaonis as previously described by Ihara et al.22 In brief, the cells were grown under illumination for two weeks in the culture medium at pH 9 containing 1 g KH2PO4, 1 g KCl, 1 g NH4Cl, 200 mg MgSO4·7H2O, 200 g NaCl, 10 g casamino acids, 5 g Na2CO3, and trace metals (0.1 mg ZnSO4·7H2O, 0.03 mg MnCl2·4H2O, 0.3 mg H3BO3, 0.2 mg CoCl2·6H2O, 0.01 mg CuCl2·2H2O, 0.02 mg NiCl2·6H2O and 0.03 mg Na2MoO4·2H2O). The cell pellets were obtained by centrifugation, suspended in a basal salt medium and frozen overnight. The next day, after thawing, it was then treated with DNase at room temperature for a few hours followed by dialysis for two days. Next the cell debris was discarded by fast centrifugation. The supernatant was collected and washed with 100 mM NaCl several times to remove the excess bacterioruberin. Finally, the phR membranes were suspended in 100 mM NaCl.UV spectroscopy
Absorption measurements were performed using an HP 8453 UV-vis spectrophotometer.Light microscopy
Light microscopy images were obtained using an Olympus CX40 microscope.Densitometry
The density of bands in the gels was quantified using the ImageJ (NIH) image analysis program.Conclusions
Following conjugation, engineered-micelles composed of non-ionic detergents, hydrophobic chelators and divalent cations were found to efficiently extract bacteriorhodopsin from the purple membrane while preserving its native state and functionality; the majority of hydrophilic macromolecules in the contaminating background were excluded. As an alternative procedure, the conjugation of membranes containing halorhodopsin succeeded in purifying the protein while avoiding exposure to high detergent concentrations and potential denaturation. Therefore, depending on the particular membrane protein, either engineered-micelles or engineered-membranes provide a practical route for purification: no sophisticated instrumentation is required (only vortex + table centrifuge); the process is relatively rapid, is performed under mild conditions (4 °C) and is capable of removing the large majority of hydrophilic impurities prior to a final chromatographic step. Further improvements in process efficiency, purity and yield may come from metal chelators, other than hydrophobic 1,10-phenanthroline derivatives (e.g. 8-hydroxyquinoline, catechol), and these are currently being explored.Abbreviations
| NMR | Nuclear magnetic resonance |
| MP | Membrane protein |
| PDC | Protein detergent complex |
| Batho | Bathophenanthroline |
| bR | Bacteriorhodopsin |
| phR | Halorhodopsin |
| cmc | Critical micelle concentration |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
Acknowledgements
We would like to thank the Kimmelman Center for Biomolecular Structure and Assembly at the Weizmann Institute of Science for its generous support (to M.S.). M.S. holds the Katzir-Makineni Chair in Chemistry. INNN thanks IIT Bombay for employment and DKN thanks CSIR India for a senior research fellowship. We would also like to thank Dr Shira Albeck of the Weizmann Institute Proteomics Center for providing us with the E. coli lysate and Mr Allon Adler and Mr Guy Lifshiz for their valuable technical assistance.Notes and references
- R. M. Garavito and S. Ferguson-Miller, J. Biol. Chem., 2001, 276, 32403–32406 CrossRef CAS PubMed.
- P. J. Loll, J. Struct. Biol., 2003, 142, 144–153 CrossRef CAS PubMed.
- C. Bordier, J. Biol. Chem., 1981, 256, 1604–1607 CAS.
- W. L. Hinze and E. Pramauro, Crit. Rev. Anal. Chem., 1993, 24, 133–177 CrossRef CAS.
- P. A. Albertsson, Biochemistry, 1973, 12, 2525–2530 CrossRef CAS PubMed.
- M. A. Lukoyanova and N. M. Petukhova, Biochemistry, 1976, 41, 1468–1475 Search PubMed.
- U. Sivars and F. Tjerneld, Biochim. Biophys. Acta, 2000, 1474, 133–146 CrossRef CAS.
- B. Fricke, Anal. Biochem., 1993, 212, 154–159 CrossRef CAS PubMed.
- C. R. Parish, B. J. Classon, J. Tsaqaratos, I. D. Walker, L. Kirszbaum and I. F. McKenzie, Anal. Biochem., 1986, 156, 495–502 CrossRef CAS PubMed.
- G. Patchornik, I. N. N. Namboothiri, D. K. Nair and R. Persky, Soft Matter, 2012, 8, 8456–8463 RSC.
- G. Patchornik, G. D. Danino, E. Kesselman, E. Wachtel, N. Friedman and M. Sheves, Bioconjugate Chem., 2013, 24, 1270–1275 CrossRef CAS PubMed.
- R. M. Smith and A. E. Martell, in Critical Stability Constants, Plenum Press, New York and London, 1990, vol. 2, pp. 251–253 Search PubMed.
- G. Patchornik, E. Wachtel, E. Kesselman and D. Danino, Soft Matter, 2014, 10, 4922–4928 RSC.
- T. Arnold and D. Linke, Biotechniques, 2007, 43, 427–440 CrossRef CAS PubMed.
- H. G. Khorana, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 1166–1171 CrossRef CAS.
- N. A. Dencher and M. P. Heyn, FEBS Lett., 1978, 96, 322–326 CrossRef CAS PubMed.
- Y. Gohon, T. Dahmane, R. W. Ruiqrok, P. Schuck, D. Charvolin, F. Rappaport, P. Timmins, D. M. Engelman, C. Tribet, J. L. Popot and C. Ebel, Biophys. J., 2008, 94, 3523–3537 CrossRef CAS PubMed.
- G. Q. Chen and E. Gouaux, Biochemistry, 1999, 38, 15380–15387 CrossRef CAS PubMed.
- G. Patchornik, I. N. N. Namboothiri, D. K. Nair, E. Wachtel, S. R. Cohen, N. Friedman and M. Sheves, J. Colloid Interface Sci., 2012, 388, 300–305 CrossRef CAS PubMed.
- M. Kolbe, H. Besir, L. O. Essen and D. Oesterhelt, Science, 2000, 288, 1390–1396 CrossRef CAS PubMed.
- D. Oesterhelt and D. Stoeckenius, Methods Enzymol., 1974, 31, 667–678 CAS.
- K. Ihara, A. Narusawa, K. Maruyama, M. Takeguchi and T. Kouyama, FEBS Lett., 2008, 582, 2931–2936 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |