Dual-functional probes for sequential thiol and redox homeostasis sensing in live cells
Tao
Ma
a,
Hui
Ding
a,
Haijiao
Xu
b,
Yanlin
Lv
a,
Heng
Liu
a,
Hongda
Wang
*b and
Zhiyuan
Tian
*a
aSchool of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences (UCAS), Beijing 100049, P. R. China. E-mail: zytian@ucas.ac.cn
bState Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences (CAS), Changchun 130022, P. R. China. E-mail: hdwang@ciac.jl.cn
First published on 23rd October 2014
Abstract
A new type of resorufin-based dual-functional fluorescent probe whose fluorescence emission features are sensitive to thiol compounds and redox homeostasis was developed. Thiols-triggered nucleophilic substitution of the probes converts the nonfluorescent probe to the highly fluorescent resorufin moiety; the released resorufin not only enables fluorescence signaling specific for thiol compounds but functions as a redox indicator with sensitive colorimetric and fluorescence emission change upon redox variation. Preliminary fluorescence imaging experiments have revealed the biocompatibility of the as-prepared probes and validated their practicability for thiol sensing and redox homeostasis mapping in living cells.
1 Introduction
Thiols-containing biological molecules, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), play crucial roles in many physiological processes, especially in maintaining the biological redox homeostasis through the equilibrium of free thiols (RSH) and disulfides (RSSR). Cysteine (Cys) deficiency is often involved in retarded growth in children, decrease in hematopoiesis, leukocyte loss, skin lesions, liver damage, and weakness.1–3 Homocysteine (Hcy) is a risk factor for cardiovascular and Alzheimer's disease, inflammatory bowel disease, neural tube defect, and osteoporosis.4,5 Glutathione (GSH) is the key factor for the protective and detoxifying functions of the cell in maintaining the proper thiol–disulfide status of proteins, preventing serious damage to DNA, proteins, and lipid membranes by scavenging reactive free-radical species;5–8 and alteration of the optimum cellular thiol–disulfide status has been amalgamated with disturbance of physiological functions resulting in heart disease, stroke, and other neurological disorders. Thus, it is of significant importance to develop efficient methods to detect thiols for investigating their functions in cells and disease diagnosis. It is known that a delicate redox homeostasis plays critical roles in numerous physiological functions of cells – the alterations in the redox homeostasis induced by exogenous stimuli or endogenous stress or both exert significant influence on a host of cell functions, including but not limited to growth, differentiation, metabolism, cell cycle, stress responses, communication, migration, gene transcription, ion channels, and immune responses.9–15 For instance, redox homeostasis acts as an important modulator in the self-renewal and differentiation of stem cells.16–19 Additionally, redox homeostasis is a pivotal index for disease diagnosis because redox imbalance induced by oxidative stress may lead to an oncogenic stimulation, defective cell death and aberrant proliferation, and eventually contribute to the development of cancer.20,21 Beyond doubt, it is critical to monitor the redox state of a cell and its oscillation for an accurate evaluation of the cellular functioning and/or exploration of the relationship between the disturbances in the redox homeostasis and some diseases like cardiovascular diseases, Alzheimer's, and cancers. However, increasing evidence is now supporting the notion that informative measurement of cellular redox state might be more challenging than previously realized and the lack of an effective strategy for measuring the status of intracellular redox homeostasis has been a profound limitation for basic research and practical clinical diagnosis.22 Owing to their apparent advantages in terms of ultrahigh sensitivity, excellent spatiotemporal resolution, simplicity of manipulation and applicability to intracellular detection over other strategies, such as high performance liquid chromatography (HPLC), capillary electrophoresis (CE), electrochemical techniques, and mass spectrometry, fluorescence-based approaches have played active roles at the forefront of thiol detection in recent years.23 Most fluorescent probes for thiols developed so far have been based on a salient feature of thiols, namely their strong nucleophilicity, which inherently bestows high binding affinity to thiols toward specific metal ions, such as Hg2+, and specific reactions between thiols and probes, such as Michael addition, cyclization reaction with aldehyde and thiols-triggered cleavage reactions. The binding of metal ions to thiols and thiols-mediated chemical reactions of the probe result in change in the detectable fluorescence signal of the probes, which forms the basis of fluorescence-based thiol detection.23–26 Redox-sensitive genetically encoded green fluorescent protein (GFP) and yellow fluorescent protein (YFP) were developed, generally by integrating a dithiol–disulfide pair into the structures of YFP and GFP, respectively, and used as probes for imaging of intracellular redox potential.22,27–30 Very recently, Tang and co-workers reported small-molecular-weight fluorescent probes for real-time reversible imaging of redox status changes in vivo and mapping of superoxide anion fluctuations in live cells and in vivo, respectively, which clearly demonstrates the salient features of the synthesized probes in terms of sensitivity for mapping redox homeostasis and molecular events involved in redox regulation.31,32In the present work, a new type of dual-functional probe capable of sequential thiol detection and redox homeostasis evaluation was developed by nucleophilic substitution of hexafluorobenzene with two resorufin units at the para position, as shown in Scheme 1. The as-prepared probes underwent thiol-mediated cleavage of the strong electron-withdrawing tetrafluorobenzene group, which converts the nonfluorescent resorufin-based ether to fluorescent resorufin units and therefore enables thiol sensing.23–26 It deserves mentioning that, in this work, the thiol-mediated release of resorufin not only enables fluorescence signaling specific for thiols but also generates a redox indicator for redox homeostasis evaluation because the fluorescent resorufin serves as an electron acceptor and can be reduced to nonfluorescent dihydroresorufin, which can be reoxidized to resorufin.33 It is known that, as a typical strategy, sulfonate esters and sulfonamide derivatives have been developed as probes for sensing of thiols based on thiol-triggered removal of the strong electron-withdrawing 2,4-dinitrobenzenesulfonyl group from the probes and release of fluorophores such as fluorescein, naphthalimide, benzoxadiazole derivatives, and resorufin for fluorescence signaling.24,34–38 Resorufin derivatives have been used as probes for enzyme activity, glucose oxidase-catalyzed oxidation of glucose, oxygen33 and hydrogen peroxide.39 However, the exploitation of resorufin-based dual-functional probes for sequential thiol detection and redox homeostasis evaluation has been unexplored to date. The applicability of the as-prepared probes for thiol sensing and redox homeostasis mapping in live cells was confirmed, which is the first paradigm where a single probe can be used for both intracellular thiol sensing and redox homeostasis mapping.
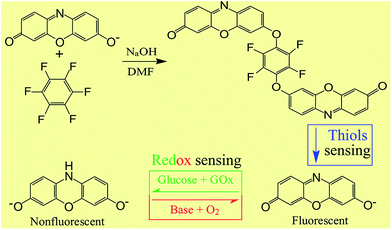 | ||
| Scheme 1 Synthesis of the dual-functional probes and their sequential responses to thiols and redox homeostasis. | ||
2 Experimental
2.1 Chemicals
NaHS and other metal ion inorganic salts used in the experiments were obtained from Shanghai Shenbo Chemical Co., Ltd, China. Acetonitrile (CH3CN) and other organic reagents were purchased from Aladdin Chemistry Co., Ltd, China. Biological reagents were purchased from Acros and Aldrich. All the reagents were analytical reagent grade and used without purification. All solutions were freshly prepared before use. Milli-Q ultrapure water (18.2 MΩ cm) was used in all experiments.2.2 Apparatus
The structural information of the probe was obtained in transmission mode on a Fourier-transform infrared spectrophotometer (FT-IR, American Nicolet Corp. Model 170-SX) using the KBr pellet technique. 1H NMR spectra were measured on a Bruker DMX-400 spectrometer at 400 MHz in CDCl3 with tetramethylsilane as the internal standard. Fluorescence emission spectra were recorded with a Fluoromax-4 spectrofluorometer instrument.2.3 Synthesis
To a mixture of hexafluorobenzene (37.2 mg, 0.2 mmol) and resorufin sodium salt (105.8 mg, 0.45 mmol) in DMF (10 mL) in ice-water bath, NaOH (16 mg, 0.4 mmol) was added and the resulting mixture was stirred at room temperature for 12 h until the completion of the reaction, which was confirmed by TLC. Water (10 mL) was subsequently added and the mixture was extracted with ethyl acetate (30 mL), washed with brine (30 mL), and dried with anhydrous magnesium sulfate and then concentrated in vacuum. After solvent removal, the crude product was purified by silica gel column chromatography to give a yellow product, 78 mg, yield: 68%. The structure of the compound was confirmed by mass spectrometry, IR spectra, and 1H-NMR spectroscopy. FT-IR (KBr) ν (cm−1): 2961, 1726, 1600, 1514, 1284, 1123, 1076, 787. 1H-NMR (300 MHz, CDCl3): δ (ppm) 7.71 (d, J = 1.2, 2H), 7.65 (d, J = 4.8, 2H), 7.63 (s, 2H), 7.57 (s, 2H), 7.53 (d, J = 1.5, 2H), 7.48 (d, J = 4.8, 2H). MALDI-TOF MS: 572.1 (M+).2.4 UV-visible absorption and fluorescence spectral study
Stock solutions of Cys, GSH and NaHS, respectively, in distilled water and the probe in acetonitrile (CH3CN) were prepared before UV-visible absorption and fluorescence emission measurements. In a typical spectral measurement, an aqueous sample of the probe was prepared by adding small aliquots of the probe/CH3CN stock solution into a cuvette with 2 mL of 0.2 M 3-(N-morpholine) propanesulfonic acid (MOPS) buffer (pH 7).2.5 General procedure for cell imaging
Macrophages were cultured in media (GIBCO RPMI 1640 supplemented with 10% FBS, 100 units per mL of penicillin and 100 units per mL of streptomycin) at 37 °C in a humidified incubator, and culture media were replaced with fresh media every day. The cells were treated with 1 mM NEM (N-ethylmaleimide) in culture media for 30 min at 37 °C and then washed with phosphate buffered saline (PBS) before experiment. The cells were further incubated with 5 μM of probe in culture media for 15 min at 37 °C and then washed 3 times with warm PBS buffer before cell fluorescence imaging experiments with confocal laser scanning microscopy.3 Results and discussion
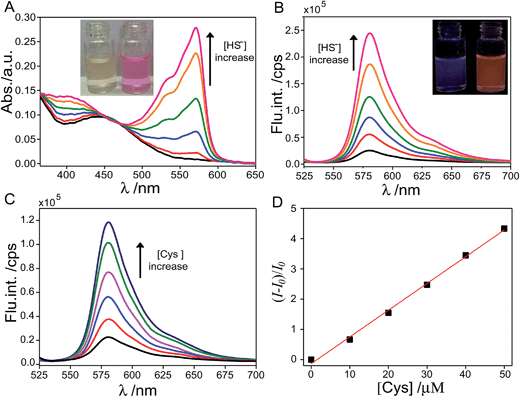
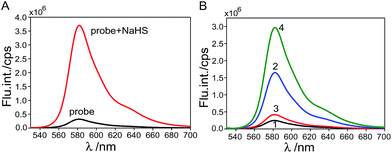
The target probe was synthesized via a facile nucleophilic substitution reaction of hexafluorobenzene with resorufin in the presence of NaOH with a relatively high yield, approximately 68%. The aqueous solution of the target probe (4 μM) is nearly colorless, as shown in Fig. 1A. To evaluate the response of the as-prepared probe to thiol, UV-vis absorption and fluorescence measurements of the probe in 0.2 M MOPS buffer were performed in the absence and presence of thiol and its inorganic counterpart, NaHS salt (Fig. 1). It was found that the aqueous sample of the probe displays weak absorption in the ultraviolet region. Upon addition of NaHS, significant absorption increase in the region of 475–600 nm with a maximum peak at ∼571 nm was clearly observed. As a result, the aqueous sample turned from nearly colorless to a vivid pink color (Fig. 1A). The fluorescence emission features of the probe sample also exhibited obvious change upon addition of NaHS. Specifically, emission intensity of the sample at 583 nm dramatically augmented 13-fold upon addition of 3 equiv. of NaHS and the sample presented a bright orange fluorescence color in contrast to the weak fluorescence of the sample prior to addition of NaHS (Fig. 1B).To evaluate the ability of the as-prepared probe for biothiol sensing, fluorescence emission features of the probe were investigated in the presence of cysteine (Cys) with the result illustrated in Fig. 1C. It can be seen that the emission intensity at 583 nm gradually increased with increasing the amount of Cys. Fig. 1D displays a plot of (I − I0)/I0 against the concentration of Cys ranging from 0 to 50 μM and the corresponding linear fit (R2 = 0.998) to the experimental data. I0 is the emission intensity at 583 nm of the probe in the absence of Cys and I is the counterpart intensity in the presence of different concentrations of Cys. From titrations, a detection limit of ∼0.52 μM of the as-prepared probe for Cys sensing was determined based on the 3-sigma method, suggesting the possibility of quantitative detection of biothiols using the as-prepared probe.
Aqueous solution of resorufin sodium salt displays an intense absorption band in the region of 475–600 nm and therefore presents a vivid pink color. It is noted that the resorufin anion possesses typical intramolecular push–pull character and therefore electron delocalization over the molecular skeleton that is responsible for the intense absorption in the visible region. Owing to the strong electron-withdrawing property of the tetrafluorobenzene (TFB) moiety, derivatization of the 7-OH of resorufin with TFB is expected to sequestrate the negative charge of the phenolate anion (Ph-O−) and consequently weakens the push–pull character.24,34–38 As a result, electron delocalization of the resorufin moiety decreases and the weak absorption and fluorescence features in the visible region of the probe were observed. The addition of thiols and HS− triggers the nucleophilic aromatic substitution reaction of the probe and eventually leads to cleavage of the ether bond of the probe and the release of the resorufin moiety. Consequently, the push–pull character of the resorufin moiety was recovered and the retrieved intense absorption features in the visible region and clear fluorescence enhancement were observed.
As demonstrated in Fig. 1, both Cys and HS− were capable of retrieving the fluorescence of the resorufin moiety by triggering the cleavage of the ether bond of the probe. However, the fluorescence emission features of the probe exhibited distinct dynamic changes upon addition of Cys and HS−, respectively. As shown in Fig. 2A, fluorescence intensity of the probe exhibited a ∼10-fold enhancement 200 s after the addition of excess amount of Cys and approached a maximum of ∼24-fold increase after 20 min. In sharp contrast, fluorescence intensity of the probe exhibited an enhancement of more than 10-fold in 5 s after addition of excess amount of HS− and reached a plateau after 160 s with 28-fold fluorescence enhancement. Such discrepancy in the response of the probe to HS− and Cys suggested the difference in the reaction rate of the probe with different thiol compounds. Specifically, release of the fluorescent resorufin moiety from the probe is based on thiol-mediated rupture of the C (TFB)–O bond and the nucleophilic aromatic substitution reaction of the probe, in which the Cys component was expected to suffer from a stronger steric hindrance effect owing to its large size as compared to that of HS−.
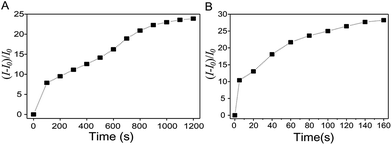 | ||
| Fig. 2 Evolution of the emission intensity (at 583 nm) of the probe (10 μM) in the presence of 100 equiv. of Cys (A) and NaHS (B) in 0.2 M MOPS buffer of pH 7.0. | ||
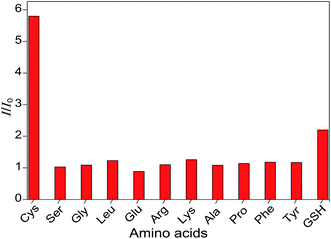
To evaluate the sensing selectivity of the as-prepared probes for Cys, the probes were tested against various other typical essential amino acids such as Ser, Gly, Leu, Glu, Arg, Lys, Ala, Pro, Phe, and Tyr as well as the thiol-containing GSH. The response time of the probe to Cys and various other amino acids was 5 min. As illustrated in Fig. 3, upon addition of these reference amino acids or GSH with identical concentrations, the fluorescence emission intensities of the probes nearly kept unchanged in the cases of reference amino acids or minimally affected in the case of GSH as compared to the original probe sample. In sharp contrast, the addition of Cys with identical concentrations induced ∼5-fold fluorescence enhancement. These results suggest a high selectivity of the as-prepared probes for thiols over other amino acids and indicate the diagnostic potential of the probes for thiol sensing in biological samples.
Owing to its superior characteristics such as high extinction coefficient, high fluorescence quantum yield, good water-solubility, good photostability, and biocompatibility, resorufin has been used greatly for different detection schemes such as proteases,40,41 ions42,43 and reactive oxygen species (ROS).44,45 Another salient feature of resorufin is its redox activity, namely it potential in acting as a redox indicator.46 Specifically, the fluorescent resorufin can be reversibly reduced to another colorless and nonfluorescent derivative of resorufin, dihydroresorufin, and the latter can be reoxidized to resorufin by dissolved oxygen in alkaline aqueous milieu. Owing to its redox activity, resorufin has been used in anaerobic microbiology to indicate contamination with oxygen,47 in glucose oxidase-catalyzed oxidation of glucose as an electron acceptor,48 and in tracing dissolved oxygen.33 Thus, thiol-triggered cleavage of the ether bond of the probe in the present work not only imparted fluorescence signaling for thiol sensing but also released active redox indicators for subsequent redox mapping in a reversible manner.
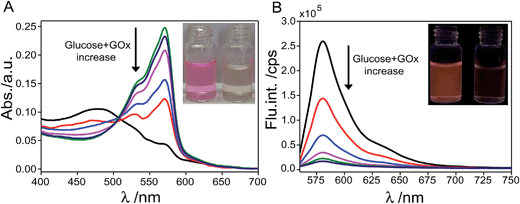
To evaluate the redox sensing ability of the probe, small aliquots of aqueous GOx and glucose solution were added to the aqueous sample of the probe (4 μM) after thiol treatment and the UV-vis absorption and fluorescence emission spectra of the sample were then recorded. As shown in Fig. 4A, the characteristic absorption with the maximum peak at ∼571 nm gradually decreased upon increasing the amount of glucose and GOx added to the sample and the vivid pink color of the sample gradually faded and regressed to colorless. Accompanying the obvious change in absorption features, fluorescence emission of the probe sample underwent remarkable regression upon addition of glucose and GOx, as shown in Fig. 4B. Specifically, only ∼5% of the initial emission intensity of the aqueous probe sample remained after incubation with 25 equiv. of glucose and the fluorescence of the sample became extremely faint from bright orange color before reduction treatment. Such remarkable changes in absorption and fluorescence emission features upon reduction treatment originate from the difference in the electronic configuration between resorufin and dihydroresorufin. Specifically, reduction treatment on resorufin essentially deprived it of its push–pull character and led to the formation of dihydroresorufin with limited electron delocalization features. As a result, significantly decreased absorption and fluorescence features in the visible region of the probe were observed.
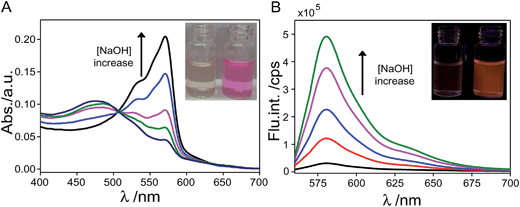
Fig. 5 displays the absorption and fluorescence emission features of the aqueous probe sample after sequential addition of thiols and reduction treatment upon following addition of aqueous NaOH solution under aerobic conditions. It was found that the colorless solution immediately changed to pink upon addition of a small amount of NaOH under aerobic conditions and a solution with nearly completely restored vivid pink color was obtained when 2 equiv. of NaOH was added to the sample. As shown in Fig. 5A, upon increasing the amount of NaOH added to the probe sample, the characteristic absorption band centered at ∼571 nm gradually increased and meanwhile the fluorescence emission of the probe sample centered at ∼583 nm significantly augmented. Specifically, addition of 2 equiv. of NaOH to the sample led to ∼16-fold fluorescence enhancement and the aqueous sample eventually restored to the bright orange fluorescence color from the faint fluorescence before re-oxidization treatment. Contrary to the reduction treatment, oxidization of dihydroresorufin by dissolved oxygen in alkaline milieu led to the recovery of resorufin and essentially restored the push–pull character of the probe.
As illustrated in Fig. 1 and 4, the probe sample underwent marked augment in fluorescence emission upon addition of thiol species, and the following addition of redox species, i.e. GOx and glucose aqueous solution, conversely led to a remarkable decrease in fluorescence emission. For a real target sample with the co-existence of thiol components and redox species, it is generally a concern whether the existence of one component perturbs the sensing of another. Fig. 6A displays the emission features of the probe prior to and after the addition of NaHS. It can be seen that the fluorescence intensity of the probe exhibited an ∼11-fold enhancement upon the addition of excess amount of NaHS. Fig. 6B displays the emission features of the probe sample in the absence of thiol components and redox species and of the sample in the co-existence of thiol components and redox species. It can be seen that the fluorescence emission of the sample immediately displayed a ∼7-fold enhancement in the emission intensity at 583 nm as compared to the emission spectrum of the sample before the addition of thiol components and redox species (line 2). Upon the following 10 min storage of the sample at 37 °C, fluorescence emission of the sample with remarkable regression was clearly observed (line 3). This means that the probe sample is capable of sensing thiol components and redox species in a sequential way even in their co-existence. Moreover, fluorescence emission of the probe sample significantly augmented upon the following addition of NaOH solution under aerobic conditions (line 4), consistent with the change observed from the aforementioned sequential sensing study results.
Fig. 7 displays the recoverability of fluorescence emission of the probe after thiol treatment upon alternating reduction and re-oxidization. It can be seen that the fluorescence intensity decreased significantly upon addition of glucose and GOx. Upon the subsequent addition of aqueous NaOH solution under aerobic conditions, such reduction-induced fluorescence decrease could be completely offset and the fluorescence intensity reverted to the level prior to the addition of glucose and GOx. The following reduction–oxidization cycle under sequential addition of glucose, GOx and NaOH also demonstrated recoverability of the fluorescence emission features of the probe. For several cycles, at least, such reduction-induced fluorescence decrease and then oxidization-induced fluorescence restoration were fully reversible, as shown in Fig. 7, suggesting the excellent photostability and the potential of such probes for multiple-cycle redox mapping applications.
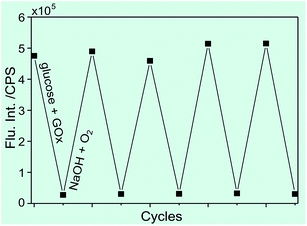 | ||
| Fig. 7 Fluorescence change of the aqueous probe sample after thiol treatment upon cyclic reduction–oxidation treatment. | ||
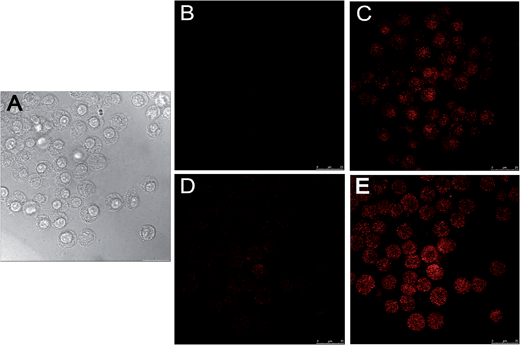
The applicability of the as-prepared probes for thiol sensing and redox homeostasis evaluation in live cells was also confirmed. Macrophage cell lines were used as the model because of their ability to efficiently endocytose cellular debris, pathogens, exogenous particles and molecules. To exclude the influence of interfering cellular fluorescence originating from the reaction of endogenous thiol compounds including Cys and GSH in cells with the probe, the cells were pretreated by the thiol blocking reagent N-ethyl maleimide (NEM), a trapping reagent of thiol species prior to the incubation of cells with the probe.24 Specifically, the macrophage cells were incubated with NEM (1 mM) for 30 min and then incubated with the probe (20 μM) for 15 min before the acquisition of DIC and fluorescence images of the cells (Fig. 8A and B). Then macrophages were pretreated with NaHS (20 μM) for 15 min and then the fluorescence images of the cells were acquired with confocal laser scanning microscopy (Fig. 8C). It can be clearly seen that the cells with internalized probes in the absence of thiols exhibited negligible background fluorescence. In sharp contrast, the cells after incubation with NaHS exhibited strong fluorescence that clearly brightened their cellular contours (Fig. 8C). Such a dramatic disparity in fluorescence brightness between Fig. 8B and C clearly demonstrated the usefulness of the as-prepared probe for intracellular thiol sensing. To evaluate the intracellular redox sensing ability of the probe, the cells after NaHS treatment were subsequently incubated with glucose (200 μM) and GOx (80 μg mL−1) for 10 min and then the fluorescence images of the cells were acquired. As shown in Fig. 8D, markedly decreased fluorescence brightness of the cells after reduction treatment was observed, consistent with the in vitro experiment results illustrated in Fig. 4. It is noted that as compared to the cells shown in Fig. 8B, a small amount of cells shown in Fig. 8C exhibited faint fluorescence and their cellular contours were dimly visible. Such faint fluorescence might be attributable to those residual resorufin moieties that did not react with the reductant in cells. Following the reduction treatment and image acquisition, 1 μL of aqueous NaOH solution (10 mM) was added to the medium in a cell culture dish with inlet oxygen and the incubation was kept for 10 min before fluorescence image acquisition. As illustrated in Fig. 8E, fluorescence brightness of the cells after the treatment of NaOH under aerobic conditions was completely restored to the level prior to the reduction treatment (Fig. 8C). Beyond doubt, such excellent fluorescence recoverability of the probe in intracellular milieu upon the redox reaction cycle suggests the usefulness of the as-prepared probes for cell redox homeostasis mapping.
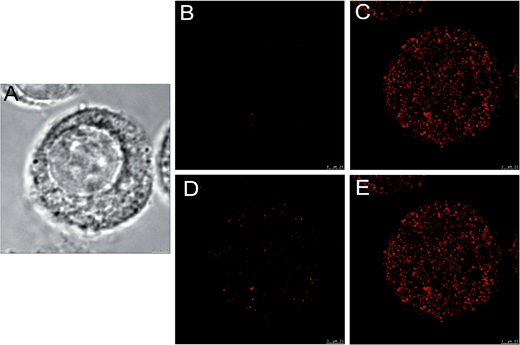
Fig. 9 displays the high magnification DIC and confocal fluorescence images of a macrophage monoplast that had been pretreated with NEM, subsequently incubated with probes for thiol sensing and redox homeostasis mapping. The fluorescence images acquired after NaHS treatment (Fig. 9C) and oxidization (Fig. 9E) clearly indicate internalization of the probes by the macrophage. It can be seen that the spotted fluorescence lights up the cytoplasm, cell organelles, and the nuclear membrane area, indicating a good appetite of the cell for the probes. Additionally, these images definitely demonstrate the sequential thiol sensing and redox homeostasis mapping abilities of the probes in cells, which is consistent with the cell imaging results shown in Fig. 8. It also deserves mentioning that the probes did not appear to exhibit appreciable cytotoxicity under the incubation time and loading concentration in the cell imaging experiments in the present work.
4 Conclusion
To conclude, we have developed a new type of dual-functional fluorescent probe whose fluorescence emission features are sensitive to thiol compounds and redox homeostasis. Specifically, thiol compounds may induce the transfer of the resorufin-based nonfluorescent probe to the highly fluorescent resorufin moiety; the released resorufin not only enables fluorescence signaling specific for thiol compounds but functions as a redox indicator with sensitive colorimetric and fluorescence emission change upon redox variation. A detection limit of 0.52 μM of the as-prepared probes for Cys was determined and the preliminary fluorescence imaging experiments have revealed the biocompatibility of the as-prepared probes and validated their practicability for thiol sensing and redox homeostasis mapping in living cells. This is the first paradigm where a single probe can be used for both intracellular thiol sensing and redox homeostasis mapping and is therefore potentially useful in bioresearch and disease diagnosis in the future.Acknowledgements
T. Ma, H. Ding and H. J. Xu contributed equally to this work. Financial support from the National Natural Science Foundation of China (grant no. 21173262 and 21373218) and the “Hundred-Talent Program” of CAS to ZT is acknowledged.References
- B. Han, J. Yuan and E. Wang, Anal. Chem., 2009, 81, 5569–5573 CrossRef CAS PubMed.
- S. Shahrokhian, Anal. Chem., 2001, 73, 5972–5978 CrossRef CAS.
- Y. Zhang, Y. Li and X.-P. Yan, Anal. Chem., 2009, 81, 5001–5007 CrossRef CAS PubMed.
- H. Refsum, P. M. Ueland, O. Nygård and S. E. Vollset, Annu. Rev. Med., 1998, 49, 31–62 CrossRef CAS PubMed.
- S. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D'Agostino, P. W. F. Wilson and P. A. Wolf, N. Engl. J. Med., 2002, 346, 476–483 CrossRef CAS PubMed.
- Y. Gao, Y. Li, X. Zou, H. Huang and X. Su, Anal. Chim. Acta, 2012, 731, 68–74 CrossRef CAS PubMed.
- R. Hong, G. Han, J. M. Fernández, B.-j. Kim, N. S. Forbes and V. M. Rotello, J. Am. Chem. Soc., 2006, 128, 1078–1079 CrossRef CAS PubMed.
- Z. A. Wood, E. Schröder, J. Robin Harris and L. B. Poole, Trends Biochem. Sci., 2003, 28, 32–40 CrossRef CAS.
- H. J. Forman and M. Torres, Mol. Aspects Med., 2001, 22, 189–216 CrossRef CAS.
- H. J. Forman and M. Torres, Am. J. Respir. Crit. Care Med., 2002, 166, S4–S8 CrossRef PubMed.
- Y. Liu and D. D. Gutterman, Clin. Exp. Pharmacol. Physiol., 2002, 29, 305–311 CrossRef CAS.
- W. Dröge, Free Radicals in the Physiological Control of Cell Function, 2002 Search PubMed.
- M. Rojkind, J. A. Domínguez-Rosales, N. Nieto and P. Greenwel, CMLS, Cell. Mol. Life Sci., 2002, 59, 1872–1891 CrossRef CAS.
- C. H. Foyer and G. Noctor, Plant Cell, 2005, 17, 1866–1875 CrossRef CAS PubMed.
- B. Morgan, D. Ezeriņa, T. N. E. Amoako, J. Riemer, M. Seedorf and T. P. Dick, Nat. Chem. Biol., 2013, 9, 119–125 CrossRef CAS PubMed.
- C. Chen, Y. Liu, R. Liu, T. Ikenoue, K.-L. Guan, Y. Liu and P. Zheng, J. Exp. Med., 2008, 205, 2397–2408 CrossRef CAS PubMed.
- M. M. Juntilla, V. D. Patil, M. Calamito, R. P. Joshi, M. J. Birnbaum and G. A. Koretzky, AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species, 2010 Search PubMed.
- S. Chuikov, B. P. Levi, M. L. Smith and S. J. Morrison, Nat. Cell Biol., 2010, 12, 999–1006 CrossRef CAS PubMed.
- K. Wang, T. Zhang, Q. Dong, E. C. Nice, C. Huang and Y. Wei, Cell Death Dis., 2013, 4, e537 CrossRef CAS PubMed.
- M. J. Percy, P. W. Furlow, G. S. Lucas, X. Li, T. R. J. Lappin, M. F. McMullin and F. S. Lee, N. Engl. J. Med., 2008, 358, 162–168 CrossRef CAS PubMed.
- Y. Mizutani, H. Nakanishi, K. Yamamoto, Y. N. Li, H. Matsubara, K. Mikami, K. Okihara, A. Kawauchi, B. Bonavida and T. Miki, J. Clin. Oncol., 2005, 23, 448–454 CrossRef CAS PubMed.
- M. Gutscher, A.-L. Pauleau, L. Marty, T. Brach, G. H. Wabnitz, Y. Samstag, A. J. Meyer and T. P. Dick, Nat. Methods, 2008, 5, 553–559 CrossRef CAS PubMed.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC.
- K. Cui, Z. Chen, Z. Wang, G. Zhang and D. Zhang, Analyst, 2011, 136, 191–195 RSC.
- Z. Guo, S. Nam, S. Park and J. Yoon, Chem. Sci., 2012, 3, 2760–2765 RSC.
- Q.-Q. Wu, Z.-F. Xiao, X.-J. Du and Q.-H. Song, Chem. – Asian J., 2013, 8, 2564–2568 CrossRef CAS PubMed.
- H. Østergaard, A. Henriksen, F. G. Hansen and J. R. Winther, Shedding light on disulfide bond formation: engineering a redox switch in green fluorescent protein, 2001 Search PubMed.
- G. T. Hanson, R. Aggeler, D. Oglesbee, M. Cannon, R. A. Capaldi, R. Y. Tsien and S. J. Remington, J. Biol. Chem., 2004, 279, 13044–13053 CrossRef CAS PubMed.
- C. T. Dooley, T. M. Dore, G. T. Hanson, W. C. Jackson, S. J. Remington and R. Y. Tsien, J. Biol. Chem., 2004, 279, 22284–22293 CrossRef CAS PubMed.
- V. V. Belousov, A. F. Fradkov, K. A. Lukyanov, D. B. Staroverov, K. S. Shakhbazov, A. V. Terskikh and S. Lukyanov, Nat. Methods, 2006, 3, 281–286 CrossRef CAS PubMed.
- W. Zhang, P. Li, F. Yang, X. Hu, C. Sun, W. Zhang, D. Chen and B. Tang, J. Am. Chem. Soc., 2013, 135, 14956–14959 CrossRef CAS PubMed.
- K. Xu, M. Qiang, W. Gao, R. Su, N. Li, Y. Gao, Y. Xie, F. Kong and B. Tang, Chem. Sci., 2013, 4, 1079–1086 RSC.
- H. Golnabi and M. Razani, Sens. Actuators, B, 2007, 122, 109–117 CrossRef CAS PubMed.
- H. Maeda, H. Matsuno, M. Ushida, K. Katayama, K. Saeki and N. Itoh, Angew. Chem., Int. Ed., 2005, 44, 2922–2925 CrossRef CAS PubMed.
- W. Jiang, Q. Fu, H. Fan, J. Ho and W. Wang, Angew. Chem., Int. Ed., 2007, 46, 8445–8448 CrossRef CAS PubMed.
- J. Bouffard, Y. Kim, T. M. Swager, R. Weissleder and S. A. Hilderbrand, Org. Lett., 2007, 10, 37–40 CrossRef PubMed.
- W. Zhang, R. Zhang, J. Zhang, Z. Ye, D. Jin and J. Yuan, Anal. Chim. Acta, 2012, 740, 80–87 CrossRef CAS PubMed.
- R. Jain and L. A. Cohen, Tetrahedron, 1996, 52, 5363–5370 CrossRef CAS.
- Y. Hitomi, T. Takeyasu, T. Funabiki and M. Kodera, Anal. Chem., 2011, 83, 9213–9216 CrossRef CAS PubMed.
- Z. Li, X. Li, X. Gao, Y. Zhang, W. Shi and H. Ma, Anal. Chem., 2013, 85, 3926–3932 CrossRef CAS PubMed.
- L. D. Lavis, T.-Y. Chao and R. T. Raines, Chem. Sci., 2011, 2, 521–530 RSC.
- M. G. Choi, J. Hwang, S. Eor and S.-K. Chang, Org. Lett., 2010, 12, 5624–5627 CrossRef CAS PubMed.
- X. Ma, J. Wang, Q. Shan, Z. Tan, G. Wei, D. Wei and Y. Du, Org. Lett., 2012, 14, 820–823 CrossRef CAS PubMed.
- E. W. Miller, O. Tulyathan, E. Y. Isacoff and C. J. Chang, Nat. Chem. Biol., 2007, 3, 263–267 CrossRef CAS PubMed.
- Y. Zhang, W. Shi, X. Li and H. Ma, Sci. Rep., 2013, 3, 2830 Search PubMed.
- P. Tratnyek, T. Reilkoff, A. Lemon, M. Scherer, B. Balko, L. Feik and B. Henegar, Chem. Educ., 2001, 6, 172–179 CrossRef CAS.
- P. A. Rublee, Am. Biol. Teach., 1984, 46, 63 CrossRef.
- H. Maeda, S. Matsu-Ura, T. Senba, S. Yamasaki, H. Takai, Y. Yamauchi and H. Ohmori, Chem. Pharm. Bull., 2000, 48, 897–902 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |