High pH sensing with water-soluble porpholactone derivatives and their incorporation into a Nafion® optode membrane†
Jill L.
Worlinsky
a,
Steven
Halepas
a,
Masoud
Ghandehari
b,
Gamal
Khalil
c and
Christian
Brückner
*a
aDepartment of Chemistry, University of Connecticut, Storrs, CT 06269-3060, USA. E-mail: c.bruckner@uconn.edu; Fax: +1 860-486-2981
bDepartment of Civil and Environmental Engineering, Polytechnic Institute of New York University, Six MetroTech Center, Brooklyn, NY 11201, USA
cDepartment of Aeronautics & Astronautics, University of Washington, Box 352250, Seattle, WA 98195-2250, USA
First published on 7th November 2014
Abstract
The known optical high pH sensing chromophores, free base and metal complexes (M = 2H, Zn(II), Pt(II)) of meso-tetrakis(pentafluorophenyl)porpholactone, and the as yet untested Ga(III) complex, were made freely water-soluble by derivatization at the aryl group with PEG chains. Their halochromic response profiles were determined and found to be surprisingly shifted toward greater base sensitivity when compared to the parent sensors in aqueous solution in the presence of a surfactant. Select PEG-derivatized chromophores were also incorporated into Nafion®-based membranes. The immobilized sensor was shown to be suitable for a moderately rapid (response time in minutes) sensing of high concentrations of hydroxides (pH 11 and above, up to 5 M NaOH concentrations). The lesser sensitivity of the indicators in the membrane is rationalized by the anionic nature of the membrane material. The membrane shows a perfectly reversible response and remains transparent and stable even under extended times of exposure to very caustic environments, and no leaching of the chromophore is observed. The membrane might find use in fiber optics-based optodes suitable for the monitoring of high hydroxide environments inside chemical reactors or fuel cells.
Introduction
The measurement of the pH value of aqueous solutions is of fundamental importance in the sciences, in engineering, and many environmental fields.1 Many methods were developed for the determination of pH values. The most popular are the use of glass pH electrodes but their performance deteriorates in the highly alkaline region; they are prone to an ‘alkaline error’ (also known as Na+ error), making them unsuitable for longer duration monitoring applications within a very high pH range.2,3Among other pH sensing techniques developed are optical methods that are based on pH-dependent changes of the absorbance or luminescence of an indicator molecule. Examples of high pH halochromic sensors are the well-known compound Titan Yellow 1 (pH range 12 to 13) or a porphyrin-based sensor, [tetrakis(4-hydroxyphenyl)porphyrinato]cobalt(II) 2, that is characterized by a particularly broad sensing regime due to multiple sites that can be deprotonated (pH 8 to 12).4
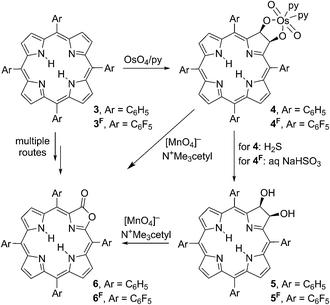
We recently demonstrated that the metal complexes of meso-tetraarylporpholactones, a class of porphyrinoids in which a β,β′-double bond of a porphyrin was replaced by a lactone moiety, are suitable as optical high pH sensors in aqueous solutions containing a surfactant (Cremophore®).5,6 Porpholactones 6/6F can be prepared from the corresponding tetraarylporphyrins 3/3F along a number of complementary routes.7–12 We developed an efficient and scalable two-step oxidation process (Scheme 1):11,12 first, the porphyrins 3/3F are converted to diol osmate esters 4/4F using osmium tetroxide. Second, the diol osmate esters 4/4F (or the corresponding diol chlorins 5/5F, generated by reduction of the osmate ester) are then oxidized using permanganate in an organic solvent-soluble form, producing selectively the porpholactones 6/6F in high yields.
We also investigated the modulation of the dynamic high pH sensing ranges of the porpholactone-based halochromic indicators by variation of the meso-aryl group (C6H5versus C6F5), the introduction of a β-nitro group, and the variation of the central metal (free bases, Zn(II), Cu(II), Ni(II), Pd(II), Ag(II), Pt(II)).6 Overall, the tight electronic coupling of the lactone functionality with the porphyrinic chromophore,12 the facile physical accessibility of the lactone moiety by analytes, and the intrinsic optical properties of porpholactones highlight their potential to be utilized as chemosensing platforms. In fact, we demonstrated the use of the free base porphothionolactone derivatives as fluorescent switch-on chemodosimeters for hypochlorite (OCl−).13 Most recently, we also presented the use of porpholactone free base and a range of metal complexes as cyanide sensors in purely aqueous solutions.14 The utilization of platinum porpholactone complexes in oxygen-sensing, ‘pressure-sensing’ paints is well known.15–17 Select porpholactone metal complexes were also shown to be more emissive compared to their porphyrin analogues.18
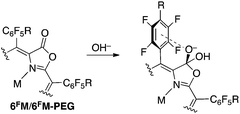
The sensing mechanism for the detection of OH− (or alkoxides, RO−, or CN−) relies on a nucleophilic attack of OH− on the lactone moiety, forming a chlorin-like species (Scheme 2).5,6 The orthoester anion functionality thus generated is believed to be stabilized by the neighboring pentafluorophenyl group. The central metal does not directly interact with the hydroxide, but the nucleophilicity of the lactone moiety is modulated by the central metal.5,14
A range of metal complexes of the porpholactones were described,18–22 including the recently reported Ga(III) complexes.14 Others, utilizing the well-described ability of the pentafluorophenyl groups to undergo a nucleophilic aromatic substitution at the p-aryl position, have shown that these meso-substituents can be used as a synthetic handle to generate p-arylsubstituted derivatives.18,23–29 We applied this reaction to generate water-soluble oxazole and porpholactone derivatives using a PEGylation strategy (Scheme 3).14,30
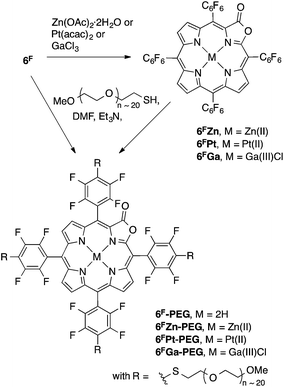 | ||
| Scheme 3 Formation of the metal complexes studied and the solubilization of the porpholactones by PEGylation. | ||
An indicator molecule that is chemically or physically immobilized on/in a solid substrate may generate an optical sensor material (optode).31 Optical sensors overcome limitations of conventional (electrochemical) methods of pH measurements by eliminating the need for a reference electrode and the mechanical and chemical limitations posed by conventional glass pH electrodes, they may be less susceptible to interferences by other ions, and possibly offer a decrease in cost, higher sensitivity, while offering the potential for facile remote sensing.32,33 The requirements for an ideal optode are well established and include a rapid enough response time for the demands of any particular application, full reversibility, and long-term stability.5,34,35
There are multiple reports on the immobilization of colorimetric molecules onto various films and membranes for the construction of pH optode materials.5,32–42 Among these reports, however, the construction of optical pH sensors for high pH (pH > 10) values are less common,5,34,35,39–41 and none are capable of sensing the differences between extremely high hydroxide concentrations, such as 1 M and 3 M aqueous NaOH, even though these very high concentrations are frequently being used in industry. Moreover, many of the sensors reported show a significant overlap between the spectra of the indicator acid and base sensor forms, complicating an optical pH detection, or they possess insufficient chemical stability at high pH values.
We will report here the evaluation of a number of recently reported PEGylated and fully water-soluble porpholactones as high pH sensors in purely aqueous solutions. We will show that they are surprisingly more sensitive to hydroxide than the corresponding non-water soluble derivatives that were solubilized with the help of a surfactant. Also more sensitive to hydroxide than the M(II) porpholactone complexes thus far investigated is the Ga(III) complex, hitherto not at all evaluated as a pH sensor. Lastly, we will report the incorporation of the PEGylated derivatives into optically transparent Nafion®-based optode membranes. We will demonstrate the halochromic response profiles and the chemical stability of these pH sensing optode materials.
Experimental section
Materials
All solvents and reagents (Aldrich, Acros) were used as received. For the materials used in the fabrication of the membranes, see below. Flash column silica gel (premium grade, 60 Å, 32–63 μm) was provided by Sorbent Technologies, Atlanta, GA. The PEGylated porpholactones 6F-PEG, 6FZn-PEG, 6FGa-PEG, and 6FPt-PEG were prepared as described previously.14Preparation of the sensor membrane
The Nafion® membranes were, using a procedure adopted with changes from the literature,43 prepared as Nafion®-PTFE-Nafion® sandwiches using Tetratex PTFE Film (0.7 mil) and using Nafion® solutions (Ion Power, Inc., 5% DuPont™ D521 Nafion® solution in H2O/iPrOH, EtOH) as described before for a 6FGa-PEG-based cyanide-sensing membrane.14 Quantities of sensor dyes and Nafion® solutions used here: dyes 6F (3 mg, 6.0 × 10−7 mol), 6FPt (7 mg, 1.4 × 10−7 mol), or 6FGa (4 mg, 7.9 × 10−7 mol) were dissolved in 13 g (∼14 mL) of the commercial Nafion® solution.Spectroscopic measurements
UV-vis spectra were recorded on a Cary 50 spectrophotometer (Varian Inc). The pH was recorded on an Accumet Basic AB15 pH meter (Fisher Scientific).Two 1.5 cm diameter stainless steel washers were braised parallel to each other to the ends of a pair of stainless steel curved tweezers. A ∼1.6 cm diameter piece of the sensor membrane was held taut between the washers and the tweezer was held clamped shut using a metal binder clip (bulldog clip). The washers holding the membrane were submersed in a 5 × 5 × 1 cm glass cuvette containing the desired aqueous solutions, with the 1 cm path length of the cuvette perpendicular to the washer and the hole in the washer carefully placed into the center of the beam of the UV-vis spectrometer. In case of pH titrations, a small home-built magnetic stirrer was placed underneath the cuvette, a small stir bar was added to the cuvette, and a pH meter was inserted. The titrant was added using an Eppendorf micropipette.
Results and discussion
Halochromic response of the PEGylated porpholactones in aqueous solution
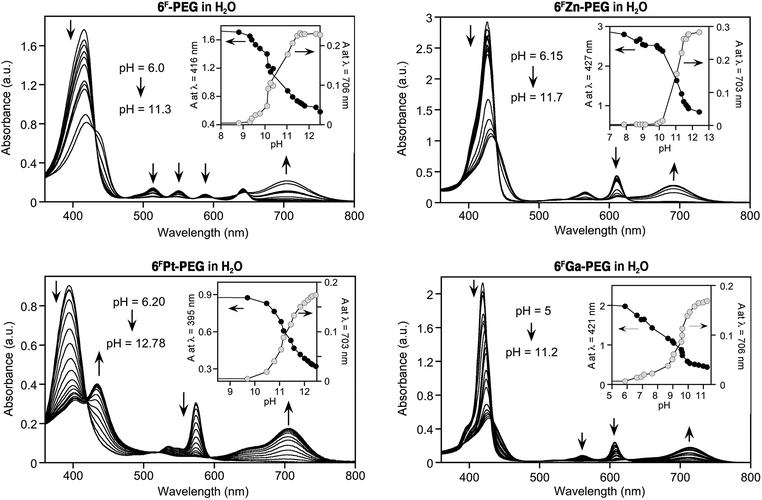
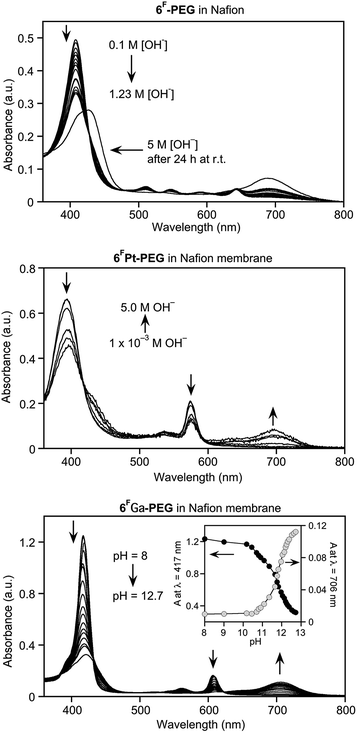
Fig. 1 shows the results of the titrations of the sensor dyes 6F-PEG, 6FZn-PEG, 6FGa-PEG, and 6FPt-PEG with base. Except for minor solvochromic shifts, their optical response is essentially identical to that of the non-PEGylated sensors in water in the presence of a surfactant, or in organic solvents.5,6 The halochromic response is also rapid, i.e., completed upon mixing of the solutions. Important for their potential use is the up to 280 nm wide spectral separation of the diagnostic peaks for the determination of the pH.Much to our surprise, however, the freely water-soluble sensors are significantly more sensitive to hydroxide. In other words, their pKOH values, i.e., the pH values at which they are converted to 50% into the base adduct form (cf.Scheme 2), possess much lower values. For example, while we determined a pKOH value of 12.6 for 6F in H2O in the presence of Cremophore EL®,6 this value is 10.5 for 6F-PEG in water in the absence of any surfactant.
The number of electron-withdrawing fluorine atoms present on the meso-aryl functionalities in 6F is higher (20 fluorine atoms) than in 6F-PEG (16 fluorine atoms). Hence, if the fluorine substituents affect the susceptibility of the lactone toward nucleophilic attack – and this was conclusively demonstrated by comparison of the pKOH values of the fluorinated and non-fluorinated porpholactones,6 then the opposite trend would have been expected. We thus conclude that the (unknown) micellar structures likely formed by the surfactant concentrated the dyes into vesicles (the solutions appeared clear) and thereby inhibited the formation or accumulation of charged hydroxide adducts. Inversely, free in solution, this spatial accumulation of charge does not take place, and lower hydroxide concentrations lead to the formation of the hydroxide adduct form of the sensor.
The pKOH values of the metal complexes 6FZn-PEG and 6FPt-PEG are 10.9 and 11.2, compared to 12.5 and 12.4 for 6FZn and 6FPt in the presence of surfactant, respectively.6 Again, the freely soluble systems are much more sensitive to base. The slightly higher pKOH values when compared to the value for free base 6FPEG contrast against the slightly higher base sensitivity of the metallated dyes in the surfactant-mediated solutions when compared to the corresponding free base system. We also found the metallated porpholactones to be more sensitive toward cyanide than the corresponding free base.14
The most hydroxide-sensitive dye, 6FGa-PEG (pKOH value of 9.3), was also the most cyanide-sensitive dye, and we rationalize this likewise with the presence of a strongly polarizing tricationic metal ion in the centre of the dye.14
Halochromic optode membrane
Nafion®-PTFE-Nafion® sandwiches were reported as membranes in fuel cells.43 The high chemical, thermal and mechanical stability of the materials involved, their transparency, thinness (few mil), hydrophilicity (Nafion® is a sulfonated tetrafluoroethylene based fluoropolymer-copolymer, see Fig. 2), and high ion conductivity also make Nafion® an attractive matrix material for our high pH-sensing porpholactone complexes. Nafion® membranes were previously reported as a carrier for optical chemosensors.38,42,44 The embedding of water-soluble (tetrasulfonated) tetraarylporphyrin into Nafion® membranes was also reported.42The Nafion® membranes are cast from H2O/DMF/EtOH Nafion® solutions, potentially allowing the easy introduction of water-soluble dyes into the matrix. Indeed, we found that free water-solubility of the porphyrins was needed for the homogenous distribution of the porphyrins throughout the otherwise clear membranes. Initial experiments using 6FPt led to the precipitation of the metalloporpholactone in the polymer matrix, resulting in a grossly inhomogeneous dye distribution. Thus, we embedded the PEGylated porpholactones 6F-PEG, 6FPt-PEG, and 6FGa-PEG into the membranes, resulting in thin (1–2 mil, 0.025–0.050 mm) and clear (purple-brown-after neutralization, pink, and green, respectively) sensor membranes containing about 10 μmol sensor per cm2 (Fig. 3).
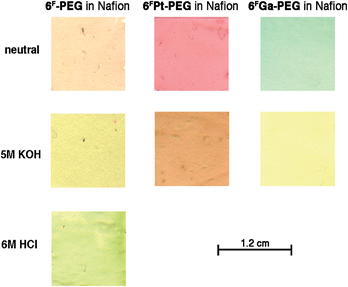 | ||
| Fig. 3 Color scans of 1.2 × 1.2 cm sections of the membranes incorporating the sensor dyes indicated under the conditions listed. | ||
The incorporation of 6FZn-PEG failed due to the high acidity of the Nafion® solution, demetalating the porpholactone, also resulting in the formation of a 6F-PEG-embedded membrane, in its diprotonated form. This matrix-induced protonation of porphyrins in Nafion® solutions was observed before.42 The neutral form of the free base porphyrin could be generated by washings of the membrane in neutral buffer.
The UV-vis spectra of the membranes show the presence of the porphyrinoids, with only very minor solvatochromic shifts and spectra broadening, compared to the spectra of the corresponding porpholactones in water. See, for instance, the metallochlorin-like UV-vis spectrum of 2Pt-PEG1000 in aqueous solution and embedded in a Nafion® membrane (Fig. 4). The similarity of the spectra of the porpholactones in aqueous solution and in the Nafion® membrane proves the presence of the unaltered chromophore in the film; it also indicates that the sensor is not significantly stacked or in an environment that otherwise changed its optical properties.
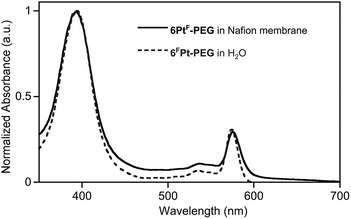 | ||
| Fig. 4 Normalized UV-vis spectra of 6FPt-PEG in aqueous solution, pH 7 buffer, and embedded into a Nafion® membrane, both at ambient T. | ||
Exposure of the purple-brown Nafion® film containing free base sensor 6F-PEG1000 to a high pH solution turns it yellow-brown (Fig. 3). The resulting high-base spectrum (Fig. 5) is qualitatively equivalent to that of the sensor in aqueous solution, notably the appearance of the diagnostic new band at 695 nm, except for two major quantitative differences. Firstly, the response of the sensor in the membrane is diffusion-limited and, thus, much slower than in solution state, and reached equilibrium after ∼20 min (see also below). Secondly, sensor 6F-PEG1000 in free solution is at pH 12.5 quantitatively converted to its base-form (Fig. 1). On the other hand, 6FPEG1000 embedded in the Nafion® membrane is much less sensitive to base. Even when exposed to a 1.2 M NaOH solution it had not fully converted to the base form after 20 min, as indicated by the presence of a strong residual Soret peak for the neutral form of the sensor. Only extreme base concentrations for extended periods of time (such as 5 M NaOH over 24 h) fully converted the dye to its base form.
A comparable situation is presented by the metalloporpholactones 6FPt-PEG1000 and 6FGa-PEG1000 embedded in Nafion®, except these sensors are much more sensitive compared to the free base sensor 6F-PEG1000 in Nafion® (Fig. 5). The Nafion® matrix is likely responsible for these effects. Even at the present thickness of only 0.025 mm (1–2 mil), the membrane matrix presents a diffusional barrier. More importantly, the anionic Nafion® polymer (Fig. 2) poses a significant electrostatic barrier for hydroxide anions. Thus, the use of a Nafion® polymer matrix for our optodes trades response time for chemical stability and ability to differentiate very high hydroxide concentrations, as demonstrated below.
The dynamic range of the platinum complex allows a differentiation of 10−2 M to 5 M NaOH solutions, after 20 min. As is the case in solution, the gallium complex is more sensitive yet, with a pKOH of 11.8. Thus, embedding this dye in the Nafion® matrix renders it about 320-fold less sensitive to base. This sensor is an excellent pH sensor in the regime between pH 10.5 and 13.
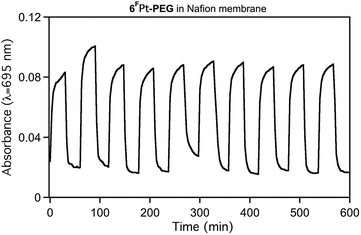
The membrane-embedded dyes are chemically stable even under harshly basic conditions. This can be demonstrated (Fig. 6). Alternating high pH and buffer solutions show that the halochromic response is reversible, and the maintenance of the signal strength shows the durability of the sensor membrane. Most, of not all, of the intensity fluctuations observed from one signal maximum to the next can be attributed to slight position changes of the soft and flexible membrane in the solution. Importantly, no degradation or leaching of the dye is observed. Furthermore, we note that, upon exposure to high hydroxide conditions, ∼80% of the signal height is achieved within 3 min, but the reverse reaction is much faster (∼30 s), supporting the electrostatic argument brought forward to explain the slow diffusion of hydroxide into the anionic Nafion® framework.
Free base porpholactones, like porphyrins, are susceptible to protonation, as seen by the expression of a diagnostic optical spectrum.5 Is the free base high-pH sensor 6F-PEG1000 also suitable as low-pH sensor? The question can, in principle, be answered in the affirmative. However, 6F-PEG1000 is not very basic. We previously estimated that, in organic solvents, the basicity of meso-tetraphenylporphyrin is 10-fold diminished by introduction of the meso-pentafluorophenyl groups, and another 10-fold diminished by introduction of the lactone moiety.5 As a consequence, 6F-PEG1000 requires conc. aqueous HCl to become fully protonated (Fig. 7).
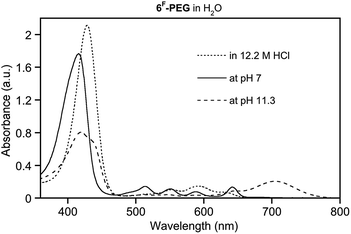 | ||
| Fig. 7 UV-vis spectra of 6F-PEG in water at acidic, neutral, and basic conditions. Dye at equal concentrations (∼10−6 M) in the solutions indicated, at ambient T, after 20 min. | ||
Conclusions
In conclusion, we presented here the evaluation of the response profile of a number of freely water-soluble porpholactone-based halochromic sensors. Particularly the known Pt(II)-based chromophore and the novel Ga(III)-based complex show favourable response profiles (strong signals, large peak separation of the neutral and base form signals) in the high pH regime (between pH 10 and 12.5 and 8 and 11, respectively).The water-soluble derivatives were also incorporated into a Nafion®-based membrane, generating a high pH optical sensing optode material, again with excellent on/off signal separations, high absorption coefficients, good chemical and physical stability, and reproducibility. In contrast to the free sensor in aqueous solution, the sensor embedded in the anionic Nafion® matrix responses to high pH solutions slower and with significantly lower sensitivity. Many applications in industry use highly concentrated hydroxide solutions. These optode materials may be utilized for the optical determination of the concentration of high concentration of hydroxide. Alas, the slow response time of the optodes presented here may prohibit their application in certain areas. We expect that further developments with respect to the Nafion® formulations used and the optode membrane thickness will result in faster response times.
Acknowledgements
We thank Philip Baker and Leonard Bonville, Center for Clean Energy Engineering C2E2), University of Connecticut, for technical assistance in the preparation of the Nafion® membranes. This material is based upon work supported by the US National Science Foundation under Grants CHE-0517782, CHE-1058846, and CMMI-0730826.Notes and references
- R. G. Bates, Determination of pH: Theory and Practice, John Wiley and Sons, New York, 1973 Search PubMed.
- N. Landqvist, Acta Chem. Scand., 1955, 9, 595–612 CrossRef CAS.
- B. Karlberg and G. Johansson, Talanta, 1969, 16, 1545–1551 CrossRef CAS PubMed.
- T. L. Blair, J. R. Allen, S. Daunert and L. G. Bachas, Anal. Chem., 1993, 65, 2155–2158 CrossRef CAS.
- G. E. Khalil, P. Daddario, K. S. F. Lau, S. Imtiaz, M. King, M. Gouterman, A. Sidelev, N. Puran, M. Ghandehari and C. Brückner, Analyst, 2010, 135, 2125–2131 RSC.
- J. L. Worlinsky, G. Zarate, M. Zeller, M. Ghandehari, G. Khalil and C. Brückner, J. Porphyrins Phthalocyanines, 2013, 17, 836–849 CrossRef CAS.
- M. J. Crossley and L. G. King, J. Chem. Soc., Chem. Commun., 1984, 920–922 RSC.
- M. Gouterman, R. J. Hall, G. E. Khalil, P. C. Martin, E. G. Shankland and R. L. Cerny, J. Am. Chem. Soc., 1989, 111, 3702–3707 CrossRef CAS.
- T. Köpke, M. Pink and J. M. Zaleski, Chem. Commun., 2006, 4940–4942 RSC.
- Y. Yu, H. Lv, X. Ke, B. Yang and J.-L. Zhang, Adv. Synth. Catal., 2012, 354, 3509–3516 CrossRef CAS.
- J. R. McCarthy, H. A. Jenkins and C. Brückner, Org. Lett., 2003, 5, 19–22 CrossRef CAS PubMed.
- C. Brückner, J. Ogikubo, J. R. McCarthy, J. Akhigbe, M. A. Hyland, P. Daddario, J. L. Worlinsky, M. Zeller, J. T. Engle, C. J. Ziegler, M. J. Ranaghan, M. N. Sandberg and R. R. Birge, J. Org. Chem., 2012, 77, 6480–6494 CrossRef PubMed.
- Y. Yu, B. Czepukojc, C. Jacob, Y. Jiang, M. Zeller, C. Brückner and J.-L. Zhang, Org. Biomol. Chem., 2013, 11, 4613–4621 CAS.
- J. L. Worlinsky, S. Halepas and C. Brückner, Org. Biomol. Chem., 2014, 12, 3991–4001 CAS.
- B. Zelelow, G. E. Khalil, G. Phelan, B. Carlson, M. Gouterman, J. B. Callis and L. R. Dalton, Sens. Actuators, B, 2003, 96, 304–314 CrossRef CAS.
- M. Gouterman, J. Callis, L. Dalton, G. Khalil, Y. Mebarki, K. R. Cooper and M. Grenier, Meas. Sci. Technol., 2004, 15, 1986–1994 CrossRef CAS.
- G. E. Khalil, C. Costin, J. Crafton, G. Jones, S. Grenoble, M. Gouterman, J. B. Callis and L. R. Dalton, Sens. Actuators, B, 2004, 97, 13–21 CrossRef CAS.
- X. S. Ke, B. Y. Yang, X. Cheng, S. L. F. Chan and J. L. Zhang, Chem.–Eur. J., 2014, 20, 4324–4333 CrossRef CAS PubMed.
- K. Jayaraj, A. Gold, R. N. Austin, L. M. Ball, J. Terner, D. Mandon, R. Weiss, J. Fischer, A. DeCian, E. Bill, M. Müther, V. Schünemann and A. X. Trautwein, Inorg. Chem., 1997, 36, 4555–4566 CrossRef CAS PubMed.
- G. Khalil, M. Gouterman, S. Ching, C. Costin, L. Coyle, S. Gouin, E. Green, M. Sadilek, R. Wan, J. Yearyean and B. Zelelow, J. Porphyrins Phthalocyanines, 2002, 6, 135–145 CrossRef CAS.
- A. Cetin and C. J. Ziegler, Dalton Trans., 2005, 25–26 RSC.
- L. Liang, H. Lv, Y. Yu, P. Wang and J.-L. Zhang, Dalton Trans., 2012, 41, 1457–1460 RSC.
- K. M. Kadish, B. C. Han, M. M. Franzen and C. Araullo-McAdams, J. Am. Chem. Soc., 1990, 112, 8364–8368 CrossRef CAS.
- P. Battioni, O. Brigaud, H. Desvaux, D. Mansuy and T. G. Traylor, Tetrahedron Lett., 1991, 32, 2893–2896 CrossRef CAS.
- S. J. Shaw, C. Edwards and R. W. Boyle, Tetrahedron Lett., 1999, 40, 7585–7586 CrossRef CAS.
- S. J. Shaw, K. J. Elgie, C. Edwards and R. W. Boyle, Tetrahedron Lett., 1999, 40, 1595–1596 CrossRef CAS.
- D. Samaroo, M. Vinodu, X. Chen and C. M. Drain, J. Comb. Chem., 2007, 9, 998–1011 CrossRef CAS PubMed.
- T. Bříza, R. Kaplánek, M. Havlík, B. Dolenský, Z. Kejík, P. Martásek and V. Král, Supramol. Chem., 2008, 20, 267–242 CrossRef.
- J. Tüxen, S. Eibenberger, S. Gerlich, M. Arndt and M. Mayor, Eur. J. Org. Chem., 2011, 4823–4833 Search PubMed.
- J. Ogikubo, J. L. Worlinsky, Y.-J. Fu and C. Brückner, Tetrahedron Lett., 2013, 54, 1707–1710 CrossRef CAS.
- For a current overview, see, Fiber Optic Chemical and Biological Sensors thematic issue of: Curr. Anal. Chem., 2008, 4, 271–402 Search PubMed.
- S. Igarashi, K. Kuwae and T. Yotsuyanagi, Anal. Sci., 1994, 10, 821–822 CrossRef CAS.
- C. Y. Li, X. B. Zhang, Z. X. Han, B. Åkermark, L. Sun, G. L. Shen and R. Q. Yu, Analyst, 2006, 131, 388–393 RSC.
- T. Werner and O. S. Wolfbeis, Fresenius' J. Anal. Chem., 1993, 346, 564–568 CrossRef CAS.
- A. Safavi and M. Sadeghi, Spectrochim. Acta, Part A, 2007, 66, 575–577 CrossRef PubMed.
- A. Gulino, P. Mineo, S. Bazzano, D. Vitalini and I. Fragala, Chem. Mater., 2005, 17, 4043–4045 CrossRef CAS.
- C.-G. Niu, X.-Q. Gui, G.-M. Zeng and X.-Z. Yuan, Analyst, 2005, 130, 1551–1556 RSC.
- V. Misra, H. Mishra, H. C. Joshi and T. C. Pant, Sens. Actuators, B, 2002, 82, 133–141 CrossRef CAS.
- A. Safavi and H. Abdollahi, Anal. Chim. Acta, 1998, 367, 167–173 CrossRef CAS.
- A. A. Ensafi and A. Kazemzadeh, Microchem. J., 1999, 63, 381–388 CrossRef CAS.
- T. L. Blair, J. R. Allen, S. Daunert and L. G. Bachas, Anal. Chem., 1993, 65, 2155–2158 CrossRef CAS.
- M. A. Castriciano, A. Carbone, A. Sacca, M. G. Donato, N. Micali, A. Romeo, G. De Luca and L. M. Scolaro, J. Mater. Chem., 2010, 20, 2882–2886 RSC.
- V. Ramani, H. R. Kunz and J. M. Fenton, J. Power Sources, 2005, 152, 182–188 CrossRef CAS.
- K. Sendhil, C. Vijayan and M. P. Kothiyal, Opt. Mater., 2005, 27, 1606–1609 CrossRef CAS.
Footnote |
| † Part 15 of the Oxazolochlorins series: Oxazolochlorins. 14. M. Zeller, S. Banerjee and C. Brückner, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2014, 70, 707–711. |
| This journal is © The Royal Society of Chemistry 2015 |