Rapid detection of the mature form of cystic fibrosis transmembrane regulator by surface plasmon resonance
Pascal
Trouvé
*a,
Marie-Laure
Calvez
ab,
Stéphanie
Moisan
ac,
Sophie
Le Hir
ad,
Florentin
Huguet
ac,
Nathalie
Benz
ab,
Mathieu
Kerbiriou
ac and
Claude
Férec
*acde
aInserm, UMR1078, 46, rue Félix le Dantec, CS 51819, 29218 Brest Cedex 2, F-29218, France. E-mail: pascal.trouve@univ-brest.fr; claude.ferec@univ-brest.fr; Fax: +33 2 98 01 83 42; Tel: +33 2 98 22 36 79
bAssociation G Saleun, Brest, F-29218, France
cUniversité de Brest, Faculté de Médecine et des sciences de la santé, Brest, F-29200, France
dC.H.R.U. Brest, Hôpital Morvan, Laboratoire de Génétique Moléculaire, Brest, F-29200, France
eEtablissement Français du Sang-Bretagne, Brest, F-29200, France
First published on 5th November 2014
Abstract
Cystic fibrosis is the most common lethal autosomal recessive disease in the Caucasian population, and is due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The CFTR protein functions as a chloride channel. Electrophoretic analysis shows that normal CFTR exists in three different molecular weight forms of 127, 131 and 170 kDa (bands A, B and C, respectively) representing different glycosylation forms. Band A is the non-glycosylated form of CFTR, band B is the core-glycosylated CFTR and band C is the mature form of CFTR with complex glycosylation. The glycosylation state of CFTR is representative of its maturation and is an important marker of the protein processing and function. The most common mutation in CF is a missing phenylalanine at position 508 (F508del-CFTR). The misfolded F508del-CFTR protein, which exhibits an altered glycosylation, is observed as bands A and B only and does not traffic correctly to the plasma membrane. Laboratory experiments (SDS-PAGE, immunoblotting, glycosidase digestions, and mobility shift of deglycosylated CFTR) aimed to assess the expression of CFTR and to depict which band is observed have been developed. Nevertheless, these experimental procedures are time consuming and poorly specific. The aim of the present study was to provide an easy, rapid and reproducible methodology to assess whether the CFTR protein within a protein extract is expressed and matured. We show here that surface plasmon resonance (SPR) permits a direct detection of the mature form of CFTR in crude cell lysates, providing a new tool to characterize CFTR in cells without any labelling or pre-treatment before cell lysis. The study of the effects of correctors for F508del-CFTR is the main task of many laboratories. Therefore, the proposed method is likely a useful tool for a rapid detection of mature CFTR in complex samples. We also show here that our method permits the characterization of CFTR in patients' cell extracts in a minimum time.
1. Introduction
Cystic fibrosis (CF) is the most common lethal autosomal recessive disease in the Caucasian population and is due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene.1–3 CFTR, which is an ATP-binding cassette transporter, functions as a chloride (Cl−) channel4–6 and comprises two hydrophobic core regions, two nucleotide-binding domains (NBDs) with ATP-binding activity7 and a regulatory domain (R domain). Two N-linked glycosylation sites are present in the fourth extracellular loop of the normal CFTR protein (wt-CFTR2). The newly synthesized wt-CFTR is rapidly degraded in the ER, as a core-glycosylated folding intermediate and depending on the expression system only 20 to 50% of the protein attains maturation.8 Electrophoretic analysis shows that wt-CFTR exists in three different molecular weight forms of 127, 131 and 170 kDa referred to as bands A, B and C respectively.9 These three bands represent the different glycosylation forms of wt-CFTR. Band A is the non-glycosylated form of CFTR, band B is the core-glycosylated CFTR and band C is the mature form of CFTR with complex glycosylation.9 The glycosylation state of CFTR is representative of its maturation and is an important marker of the protein processing and function.9The most common mutation in CF is a missing phenylalanine at position 508 (F508del-CFTR), which occurs in the first nucleotide-binding domain of the CFTR protein. The misfolded F508del-CFTR protein does not traffic correctly to the plasma membrane and is retained in the endoplasmic reticulum (ER) and degraded by proteasome.10–17 This F508del-CFTR protein which is unable to traffic normally to the Golgi apparatus exhibits an altered glycosylation and is observed as bands A and B only.10,18 Besides F508del-CFTR, over 1940 mutations (http://www.genet.sickkids.on.ca/cftr/) have been identified in the CFTR gene and a classification of mutations by which different mechanisms induce CF has been proposed.20
The appearance of the complex type N-linked oligosaccharides of the wt-CFTR and thus its glycosylation state is of main importance to characterize the maturation state of the protein. It is also important to assess whether a given mutation has an impact on CFTR maturation and membrane localization. Therefore, laboratory experiments aimed to assess the expression of CFTR and to depict which band is observed have been developed, keeping in view that the C band corresponds to a protein which is exported to membranes and is likely active as a Cl− channel. Nevertheless, these experimental procedures are time consuming and poorly specific, needing SDS-PAGE and immunoblotting. To be fully sure of the glycosylation state of CFTR, distinction between the core- and the complex-glycosylated CFTR needs to be confirmed by glycosidase digestions by endoglycosidase H and peptide N-glycosidase F, the mobility shift of deglycosylated CFTR being observed by immunoblotting.12 Because the glycosylation state of CFTR is correlated with its membrane localization and function, the aim of the present study was to provide an easy, rapid and reproducible methodology to assess whether the CFTR protein within a protein extract is matured or not. We show here that surface plasmon resonance (SPR19) permits a direct detection of the mature form of CFTR in crude cell lysates, providing a new tool to characterize CFTR in cells without any labelling of pre-treatment before cell lysis.
2. Methods
2.1 Antibody and chemicals
CFTR proteins were probed using a monoclonal anti-CFTR antibody (24-1, R&D system). Wheat Germ Agglutinin (WGA, Triticum vulgaris) was from Calbiochem (France). Sensor chip CM5, Amine Coupling Kit (N-hydroxysuccinimide, (NHS)), N-ethyl-N-(3-diethylaminopropyl)carbodiimide hydrochloride (EDC), ethanolamine (1 M, pH 8.5) and HBS-EP buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.005% surfactant P20 at pH 7.4) were obtained from GE Healthcare Bio-Sciences AB.Purified wt-CFTR and F508del-CFTR proteins were a generous gift from Pr Robert Ford (University of Manchester, UK20).
All experiments were performed at the PurIProb core facility (Inserm, UMR1078, Brest).
2.2 Ant-CFTR antibody and WGA immobilization
Real-time detection of CFTR in cell lysates was performed using a Biacore system (Biacore 3000; GE Healthcare) and its Control Software version 3.2. All injections were performed at 25 °C in HBS-P 1× running buffer (GE Healthcare). The Biacore 3000 was set at 25 °C for all steps during the analytical process, and experimental data were collected at a medium rate. Biacore sensorgrams were analyzed using the BIAevaluation software. For each sample the indicated RU (Resonance Unit) value is the value of the active flow cell (FC, FC2 and FC4) minus the value of the reference FC (FC1 and FC3), 20 seconds after the beginning of the dissociation phase.Binding of the CFTR antibody was performed according to Biacore recommendations on a CM5 sensor chip which is a carboxymethylated dextran matrix covalently attached to a gold surface. The antibody was immobilized on a CM5 biosensor chip using an Amine Coupling Kit (GE Healthcare). The active surface was activated with equal volumes of NHS and EDC for 7 min with a 5 μl min−1 flow. The binding events at a sensor surface induce changes in SPR signals which are expressed in resonance units (RUs). One RU is given to be equivalent to one picogram of protein per square millimeter on the sensor surface. The surface was then blocked with 1 M ethanolamine hydrochloride (pH 8.5) injection for 7 min with a 5 μl min−1 flow. The reference channel was activated with equal volumes of NHS and EDC and immediately saturated with ethanolamine.
WGA was immobilized on FC2 and the anti-CFTR antibody was immobilized on FC4. FC1 and FC3 were their respective control FCs. All proteins were diluted in HBS-EP buffer and injected at a flow rate of 5 μl min−1. Injected volumes passed over the FCs were 40 μl and 10 μl according to Biacore 3000 functioning. Absolute amounts of injected proteins are indicated in figures, instead of concentrations, to give a more clear idea of the bound quantities of proteins. All injections were performed at least in duplicate and BSA was used as a negative control.
2.3 Cell cultures and protein extraction
Hela cells stably expressing either wt-CFTR or F508del-CFTR were obtained and characterized previously.21 wt-CFTR and F508del-CFTR expressing cells were collected and homogenized in 2 ml of RIPA buffer. Lysates were ultra-centrifuged (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm, 30 min, 4 °C) and pellets were resuspended in HBS-P 1× running buffer (GE Healthcare) and injected over the sensor chip.
500 rpm, 30 min, 4 °C) and pellets were resuspended in HBS-P 1× running buffer (GE Healthcare) and injected over the sensor chip.
In some experiments, proteins from nasal cells were used. Nasal cells were obtained after brushing of healthy controls (n = 3) and CF patients (n = 3) who were informed and from whom full consent was obtained. Cells were cultured as previously described.22 In brief, after nasal lavage with physiological saline buffer, a brushing was performed in both nostrils, along the tip of the inferior turbinate and the lateral nasal wall, by a gentle circular movement for 1 minute, using a sterile brush (sterile endocervical brush, Diam 5.5, Laboratoire Gyneas, Goussainville, France). Brushes were immersed in 1 ml of Ham's F12 containing Ultroser G 2% (Lifescience) and an antibiotic/antimycotic solution and transported to the laboratory. Epithelial cells were detached from the brushes by tweezing in a culture dish. Samples obtained from both nostrils were pooled and centrifuged for 5 minutes (300g) at room temperature. The cell pellets were resuspended in 1 ml of SAGM (SAGM Bullekit, Lonza). To improve cell adhesion, culture plates were coated with collagen from human placenta (Type VI, Sigma Aldrich). When the cells were confluent, they were harvested and lysed in NP40 buffer (50 mM Tris–HCl at pH 7.4, 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 1% Nonidet P-40 and 0.02% NaN3; NP40 Cell Lysis Buffer, Invitrogen).
2.4 Statistical analysis
Student's t-test was used and differences were considered significant when p < 0.05 (*) and p < 0.0001 (***).3. Results
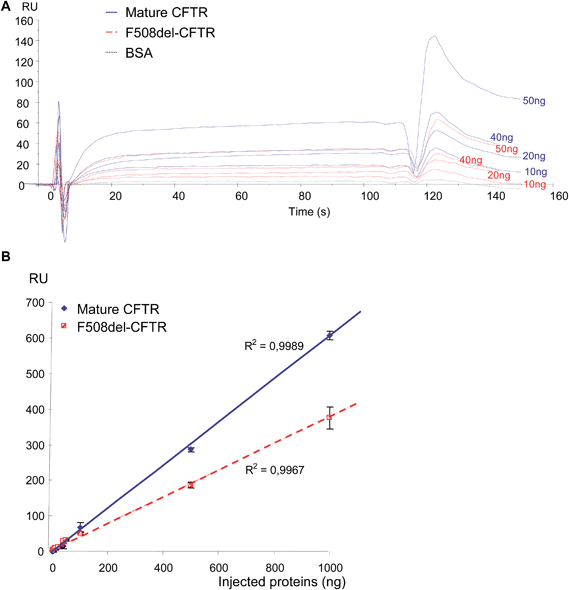
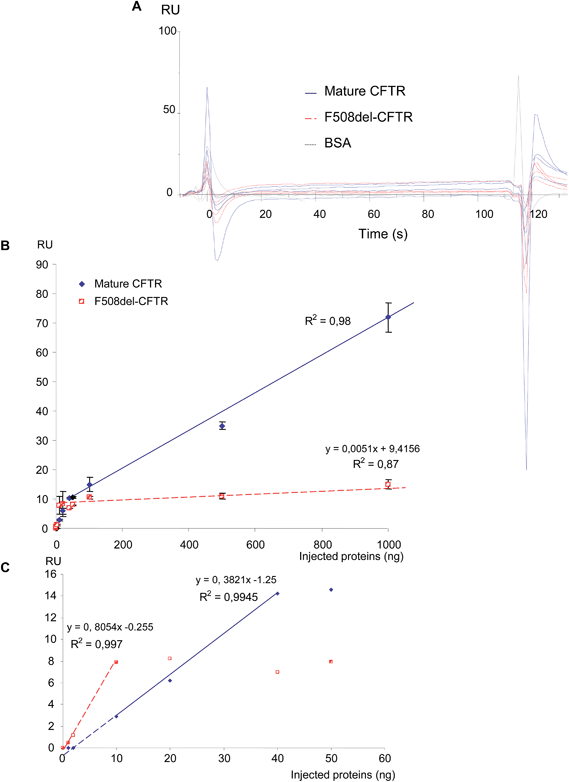
Before final immobilization, the immobilization pH scouting test was performed in order to determine the buffer to use for the antibody dilution. The suitable buffer for the antibody was determined to be 10 mM acetate pH 5.0 and the CFTR antibody was injected to achieve 9509 RU. First, increasing amounts of purified non-mutated CFTR and F508del-CFTR proteins (0, 1, 2, 10, 20, 40, 50, 100, 500 and 1000 ng) were injected on the anti-CFTR antibody and sensorgrams were recorded. As shown in Fig. 1A, non-mutated CFTR and F508del CFTR proteins were recognized by the immobilized anti-CFTR. Nevertheless, a difference was observed. When the same amount of the two proteins was injected, a higher RU number was observed during the equilibrium and dissociation phases for the non-mutated CFTR. RU values were collected 20 s after the beginning of the dissociation phase and were plotted against the amount of proteins. 20 s was chosen to represent the amount of bound protein on the antibody, after washing, to remove any non-specifically bound material contribution and to reach the equilibrium of the bound material. As shown in Fig. 1B, the responses were linear for both proteins. Nevertheless, the response was significantly lower for the F508del-CFTR protein, indicating that the affinity is likely lower for the mutated protein. Indeed, the maximum response for 1000 ng of proteins was 606 ± 12 RU and 375 ± 31 RU for non-mutated CFTR and F508del-CFTR, respectively. This difference is likely not due to the mutation which corresponds to the deletion of a single amino acid.The pH scouting test indicated that WGA had to be diluted in 10 mM acetate buffer, pH 4.5. Binding of WGA was performed as described above to achieve 5329 RU. The same quantities of non-mutated CFTR and F508del-CFTR proteins were injected on WGA. As shown in Fig. 2A, they were recognized by the immobilized lectin but the responses were low and the simple analysis of the sensorgrams was poorly informative despite a likely higher response for the non-mutated CFTR. Surprisingly, a positive response was observed for the F508del-CFTR protein. RU values were then plotted against the amount of injected proteins. As shown in Fig. 2B, two phases were observed, depending on the amount of injected proteins. Above 50 ng (Fig. 2B) the response for both proteins was linear and the RU values were significantly higher for the non-mutated CFTR than for F508del-CFTR. Indeed, the maximal response for F508del-CFTR was only 15 ± 1.5 RU. It was also observed that the slope of the curves were very different. The slope of the curve obtained by the injection of the F508del-CFTR protein was 0.0051, indicating a very loose association of the protein with the lectin, as a function of the amount of injected protein. For low quantities of injected proteins (ranging from 0 to 50 ng), the curves were not linear. A specific fitting is therefore presented in Fig. 2C. From the equations of the curves it was estimated that a positive response was obtained above 3.27 and 0.3 ng for non-mutated CFTR and F508del-CFTR, respectively. This calculation was in accordance with the experimental result. From the curves shown in Fig. 2B and C it was concluded that for very low concentrations a response was observed for F508del-CFTR but that the maximum was reached sooner than non-mutated CFTR, with no further increase of the response above 10 ng. Apparent constants according to the fitting model 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (“Langmuir binding”) were calculated and the KD values were 1.3 × 10−11 M and <2 × 10−8 M for wt-CFTR and F508del-CFTR proteins, respectively, whereas KD values were different, the KD constants were close for both proteins (1.7 × 10−5 s−1 and 1 × 10−5 s−1 for non-mutated CFTR and F508del-CFTR proteins, respectively). The final conclusion of this experiment was that the binding of wt-CFTR on WGA was stronger and that the binding of the F508del-CFTR protein was close to background.
1 (“Langmuir binding”) were calculated and the KD values were 1.3 × 10−11 M and <2 × 10−8 M for wt-CFTR and F508del-CFTR proteins, respectively, whereas KD values were different, the KD constants were close for both proteins (1.7 × 10−5 s−1 and 1 × 10−5 s−1 for non-mutated CFTR and F508del-CFTR proteins, respectively). The final conclusion of this experiment was that the binding of wt-CFTR on WGA was stronger and that the binding of the F508del-CFTR protein was close to background.
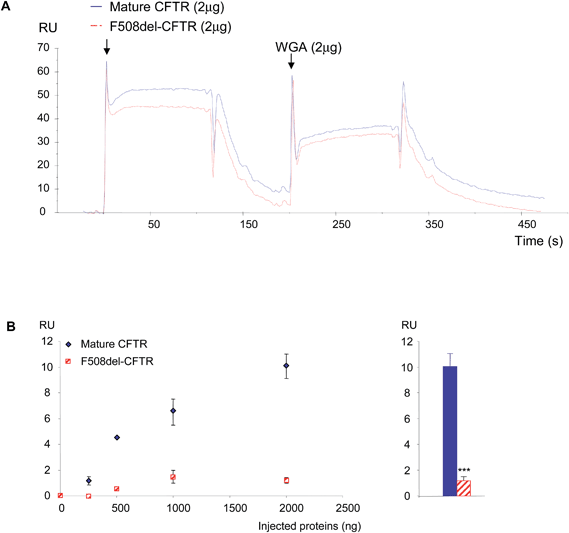
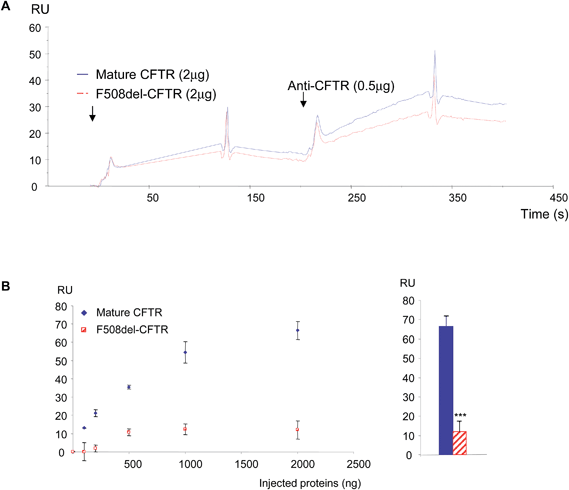
Because the binding of the purified proteins on the anti-CFTR antibody does not permit the discrimination of the non-mutated CFTR from the F508del-CFTR protein due to the presence of its epitope on both proteins and because their binding on WGA did not follow a binary mode, double injections were tested. First they were injected over the immobilized antibody. Before the base line was reached, WGA was injected. According to the results observed in Fig. 1, proteins associated with the antibody showed a higher response for the normal protein (Fig. 3A). The injection of WGA showed a higher response on the non-mutated CFTR than on F508del-CFTR (Fig. 3A). Increasing amounts of proteins (0, 100, 500, 1000 and 1500 ng) were injected and 2 μg of WGA were injected on the proteins which were immobilized on the antibody. Using the RU value before WGA injection as a base line, the responses were collected 20 s after the beginning of WGA dissociation. The RU values were plotted against the amount of injected purified proteins. As shown in Fig. 3B (left panel), the response due to WGA binding increased according to the amount of bound non-mutated CFTR on the antibody. The response due to WGA binding on F508del-CFTR remained low (<2 RU). A response was observed only above 100 ng of immobilized F508del-CFTR and remained stable above 1000 ng. The quantitation of the responses was further performed when 2 μg of purified proteins were injected on the immobilized antibody and when WGA was injected. As shown in Fig. 3B (right panel), the quantity of bound WGA was significantly higher on the non-mutated CFTR than on the F508del-CFTR. The opposite experiment was performed. The purified proteins were injected over WGA and the anti-CFTR antibody was injected. An example of the resulting sensorgram is shown in Fig. 4A. The binding of the non-mutated CFTR on WGA was stronger than the one of F508del-CFTR. Further injection of the antibody showed that indeed, WGA retains more non-mutated CFTR protein. This was confirmed by the injection of increasing quantities of protein over WGA (Fig. 4B, left panel). The maximum binding of WGA on the F508del-CFTR was reached (<10 RU) with 500 ng immobilized proteins onto the antibody whereas it was not so for the non-mutated CFTR. The quantitation of the responses was further performed when 2 μg of purified proteins were injected on the immobilized WGA and when the antibody was injected. As shown in Fig. 4B (right panel), the quantity of bound antibody was significantly higher on the non-mutated CFTR than on F508del-CFTR. This indicated that more non-mutated CFTR was bound on WGA than F508del-CFTR.
Further experiments were aimed to detect wt-CFTR and F508del-CFTR and to detect the mature form of the protein in crude protein extracts from cells.
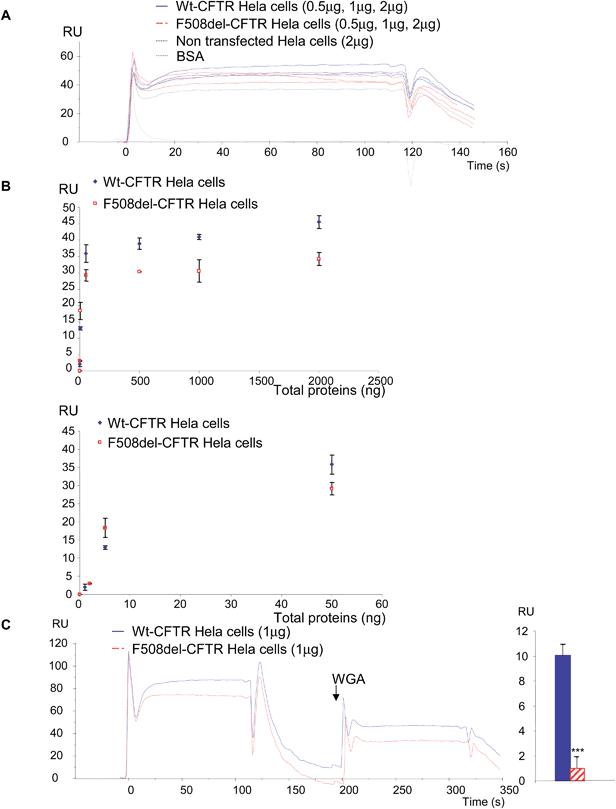
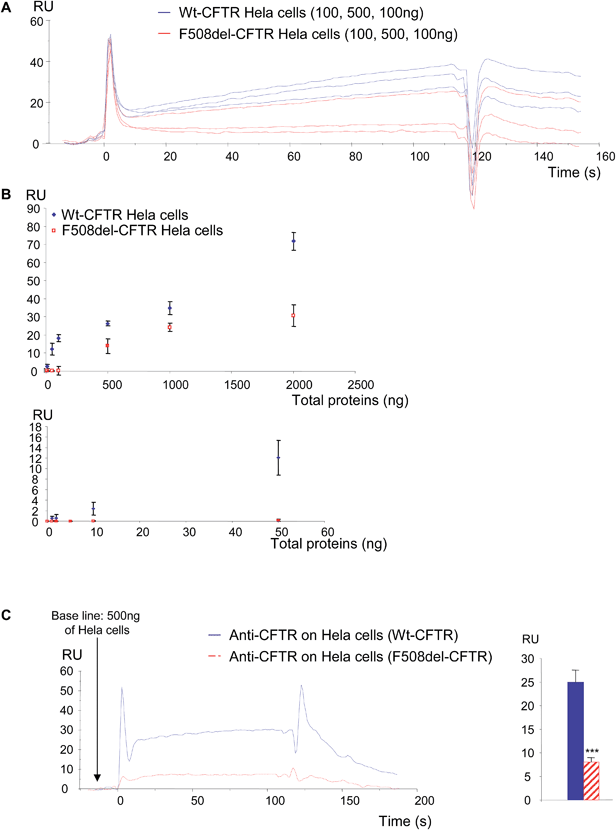
In a first set of experiments, stably transfected Hela cells expressing either wt-CFTR or F508del-CFTR were used. Total proteins from those cells were injected on FC4 on which the anti-CFTR antibody was immobilized. As shown in Fig. 5A, both protein extracts gave a positive signal showing that the wt-CFTR as well as the F508del-CFTR proteins were present in the cell lysates. Nevertheless, as it was observed with purified proteins (Fig. 1), a higher response was obtained with wt-CFTR expressing cells. Various amounts of protein extracts (0 to 2 μg) were injected on the immobilized antibody and RU values were plotted against the injected quantities (Fig. 5B). The upper panel of Fig. 5B shows the whole curves. Above 100 ng of proteins a plateau phase was observed. In order to see the behaviour of the injections below 100 ng a second curve was plotted (Fig. 5B, lower panel). It was observed that for low amounts (<50 ng) of injected proteins, there was no difference between the extracts of wt-CFTR and F508del-CFTR expressing cells. From these injections it was concluded that it is possible to detect the CFTR protein by the use of an immobilized antibody. Nevertheless, because the antibody recognizes wt-CFTR and F508del-CFTR, the observed difference between wt-CFTR and F508del-CFTR expressing cells was not sufficient to discriminate the mature protein. Therefore, 0.5 μg WGA was injected during the dissociation phase of the whole cell extracts (Fig. 5C, left). A higher signal was observed for the wt-CFTR expressing cells than for the F508del-CFTR expressing cells, corresponding to the glycosylated form of the protein (mature form). The response due to the injection of WGA was further quantified (Fig. 5C, right) to show that indeed, there was a difference between the proteins from the two cell lines and to show that the observed difference was significant. To avoid the bias due to a stronger binding of proteins from wt-CFTR expressing cells the base line was taken just before the injection of WGA. Transfected Hela cells were then injected over the immobilized WGA. As it is shown in the resulting sensorgrams (Fig. 6A), the difference between the proteins extracted from wt-CFTR expressing cells and F508del-CFTR expressing cells was important, with RU values for the latest cells close to zero. These values were plotted against the amount of injected proteins (Fig. 6B). The observation of the upper curve of Fig. 6B shows that below 100 ng of injected proteins, the RU values were very low for the proteins from F508del-CFTR expressing cells. Therefore, another curve was drawn for quantities ranging from 0 to 50 ng (Fig. 6B, lower panel). These curves show that the values of the protein extracts containing the mutated CFTR were close to 0. Because RU values of F508del-CFTR expressing cells were positive for concentrations above 50 ng and because CFTR is not the only glycosylated protein in cell lysates that could bind to WGA, the CFTR antibody was injected during the dissociation phase. An example of the resulting sensorgram is given in Fig. 6C (left). When the quantitation was performed, a medium amount of proteins was injected (500 ng). It was observed that the binding of the antibody on the protein extracts themselves injected over WGA was significantly stronger in proteins from the wt-CFTR expressing cells. For the quantitation, the base line was taken just before the injection of the antibody. From the experiments performed with Hela cells, we concluded that it is possible using an immobilized anti-CFTR antibody to detect the protein in whole cell lysates and that the use of a combination of antibody and WGA permits us to discriminate between fully glycosylated CFTR and F508del-CFTR.
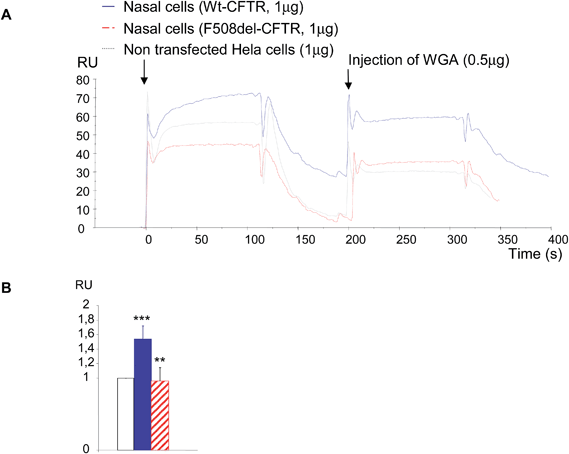
Because the amount of CFTR protein is higher in transfected cells than in cells from patients, it is challenging to detect the protein in primary cell lines. Therefore, some experiments were done with cells from CF patients (n = 3) and compared with controls (n = 3). Due to the difficulty to obtain nasal cells from patients, to culture them and to obtain a sufficient amount of proteins, only one sort of experiment was performed. 1 μg of total proteins was injected on the immobilized anti-CFTR antibody and 0.5 μg of WGA was injected during the dissociation phase (Fig. 7A). Proteins extracted from Hela cells transfected with an empty plasmid were used as a control. Each injection was performed twice. Once more, a stronger binding of CFTR on the antibody was observed for the proteins extracted from mutated CFTR expressing cells. Proteins extracted from Hela cells transfected with the empty plasmid gave a medium association but dissociation comparable to the one of CF cells. The base line was taken just before WGA injection to avoid the bias due to the binding on the antibody. When WGA was injected, a stronger binding on CF proteins was also observed. 20 seconds after the beginning of the dissociation phase RU values were collected and used in statistical analysis. The result is shown in Fig. 7B as a histogram in which the RU value of non-transfected Hela cells was given to be 1 unit. Comparison of this control with the binding of WGA on the control protein indicated that nasal cells significantly express more CFTR than Hela cells. The comparison of the results between proteins from control nasal cells and CF nasal cells showed that WGA was significantly less bound on CF proteins and that the level of bound WGA was identical to the one observed for empty Hela cells. We concluded that it is possible to detect CFTR in proteins from primary cells and that the use of WGA permits us to discriminate mature CFTR from F508del-CFTR.
4. Discussion and conclusion
The assessment of the expression of CFTR, as well as the assessment of its maturation is of main importance in the CF field of research, at the basic science level but also when the aim is to test correctors that could rescue F508del-CFTR from the ER. Knowing whether CFTR is expressed in a given cell line is not sufficient. Indeed, it is fundamental to know whether the fully glycosylated mature form is expressed due to the fact that this form solely acts as a normal Cl− channel. Experimental procedures are time consuming and poorly specific, needing SDS-PAGE and immunoblotting with antibodies that are not always fully specific. The glycosylation state of CFTR has to be tested by glycosidase digestions and immunoblotting.12 The aim of the present study was to provide an easy, rapid and reproducible methodology to assess whether the CFTR protein is expressed in a complex protein extract and whether it is mature or not. SPR is a very sensitive tool to detect and quantify protein interactions without any labelling of pre-treatment.19 Nevertheless, it is often used to study the interactions of purified proteins. We and others previously showed that the detection of a single protein with a low expression, in a complex sample such as serum, is feasible using SPR, and we show here that it can also be used to detect CFTR in a complex sample.23 Nevertheless, two detections in a single protein injection were necessary. The anti-CFTR antibody may be poorly specific, leading to false results or to over estimations. Furthermore, they do not permit us to distinguish the mature form of CFTR. To avoid this bias, we decided to use WGA that specifically recognizes the mature form of the protein.24 Using total proteins from cells, we had to face the fact that WGA recognizes many proteins. Therefore, the single use of an antibody or WGA was not sufficient to detect the mature form of CFTR. A sandwich method had to be used and we propose here that the immobilization of the anti-CFTR antibody permits us to assess the expression of the protein and that a subsequent injection of WGA permits us to distinguish its mature form. Furthermore, using a purified CFTR protein or a control protein extract permits the absolute or relative quantitation of CFTR in a complex sample. When compared to the classical Western blot, another advantage of SPR is the use of a very low amount of proteins, whereas 50 to 100 μg of total proteins are necessary for the detection of CFTR in cell lysates, here we show that while using SPR a few nanograms are sufficient. The proposed method is of interest for fundamental research aimed at rapidly finding correctors as well as for research aimed at showing the potential therapeutic use of a given compound in cells obtained from patients.In conclusion, we propose SPR as an alternative method to detect and give a relative quantitation of CFTR in a complex protein sample, in a short time, despite the low expression of the protein. Furthermore, we propose, for the first time, that this technology can also be used to discriminate the mature form of CFTR from its immature state providing a less time consuming and more simple method than Western-blottings and enzymatic digestions.
Abbreviations
| SPR | Surface plasmon resonance |
| RU | Resonance units |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
Acknowledgements
First, we want to thank the patients and the healthy donors who agreed to participate in the study. We also thank the associations Gaetan Saleun and Vaincre la Mucoviscidose for their financial support.References
- B. S. Kerem, J. M. Rommens, J. A. Buchana, D. Markiewicz, T. K. Cox, A. Chakravarti, M. Buchwald and L. C. Tsui, Identification of the cystic fibrosis gene: genetic analysis, Science, 1989, 245, 1073–1080 CAS.
- J. R. Riordan, J. M. Rommens, B. S. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, J. L. Chou, M. L. Drumm, M. C. Lannuzzi, F. S. Collins and L. C. Tsui, Identification of the cystic fibrosis gene: clonic and characterization of complementary DNA, Science, 1989, 245, 1066–1073 CAS.
- J. M. Rommens, M. C. Lannuzzi, B. S. Kerem, M. L. Drumm, G. Melmer, M. Dean, R. Rozmahel, J. L. Cole, D. Kennedy, N. Hidaka, M. Zsiga, M. Buchwald, J. R. Riordan, L. C. Tsui and F. S. Collins, Identification of the cystic fibrosis gene: chromosome walking and jumping, Science, 1989, 245, 1059–1065 CAS.
- M. J. Welsh, L. C. Tsui, T. F. Boat and A. L. Beaudet, in The Metabolic and Molecular Bases of Inherited Disease, ed. C. R. Scriver, A. L. Beaudet, W. S. Sly and D. Valle, McGraw-Hill, New York, 7th edn, 1995, pp. 3799–3876 Search PubMed.
- M. L. Drumm, H. A. Pope, W. H. Cliff, J. M. Rommens, S. A. Marvin, L. Tsui, F. S. Collins, R. A. Frizzel and J. M. Wilson, Correction of the cystic fibrosis defect in vitro by retrovirus-madiated gene transfer, Cell, 1990, 62, 1227–1233 CrossRef CAS.
- D. P. Rich, M. P. Anderson, R. J. Gregory, S. H. Cheng, S. Paul, D. M. Jefferson, J. D. McCann, K. W. Klinger, A. E. Smith and M. J. Welsh, Expression of the cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells, Nature, 1990, 347, 358–363 CrossRef CAS PubMed.
- T. Szellas and G. Nagel, Apparent affinity of CFTR for ATP is increased by continuous kinase activity, FEBS Lett., 2003, 535, 141–146 CrossRef CAS.
- C. L. Ward and R. R. Kopito, Intracellular turnover of Cystic Fibrosis Transmembrane Conductance Regulator, J. Biol. Chem., 1994, 269, 25710–25718 CAS.
- R. J. Gregory, S. H. Cheng, D. P. Rich, J. Marshall, S. Paul, K. Hehir, L. Ostedgarrd, M. J. Welsh and A. E. Smith, Expression and characterization of the Cystic Fibrosis Transmembrane Conductance Regulator, Nature, 1990, 347, 382–386 CrossRef CAS PubMed.
- (a) S. H. Cheng, R. J. Gregory, J. Marshall, S. Paul, D. W. Souza, G. A. White, C. R. O'Riordan and A. E. Smith, Defective intracellular transport and processing of CFTR is the molecular basis for most of cystic fibrosis, Cell, 1990, 63, 827–834 CrossRef CAS; (b) K. Du, M. Sharma and G. L. Lukacs, The ΔF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR, Nat. Struct. Mol. Biol., 2005, 12, 17–25 CrossRef CAS PubMed.
- G. M. Denning, L. S. Ostedgaard and M. J. Welsh, Abnormal localization of Cystic Fibrosis Transmembrane Conductance Regulator in primary cultures of cystic fibrosis airway epithelia, J. Cell Biol., 1992, 118, 551–559 CrossRef CAS.
- N. Kartner, O. Augustinas, T. J. Jensen, A. L. Naismith and J. R. Riordan, Mislocalization of ΔF508 CFTR in cystic fibrosis sweat glands, Nat. Genet., 1992, 1, 321–327 CrossRef CAS PubMed.
- G. L. Lukacs, A. Mohamed, N. Kartner, X. B. Chang, J. R. Riordan and S. Grinstein, Conformational maturation of CFTR but not its mutant counterpart (Delta F508) occurs in the endoplasmic reticulum and requires ATP, EMBO J., 1994, 13, 6076–6086 CAS.
- G. M. Denning, G. M. Denning, M. P. Anderson, J. F. Amara, J. Marshall, A. E. Smith and M. J. Welsh, Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive, Nature, 1992, 358, 761–764 CrossRef CAS PubMed.
- R. R. Kopito, Biosynthesis and degradation of CFTR, Physiol. Rev., 1999, 79, S167–S173 CAS.
- J. M. Younger, H. Y. Ren, L. Chen, C. Y. Fan, A. Fields, C. Patterson and D. M. Cyr, A foldable CFTR (Delta) F508 biogenic intermediate accumulates upon inhibition of the Hsc70-CHIP E3 ubiquitin ligase, J. Cell Biol., 2004, 167(6), 1075–1085 CrossRef CAS PubMed.
- A. Gilbert, M. Jadot, E. Leontieva, S. Wattiaux-De Coninck and R. Wattiaux, ΔF508 CFTR localizes in the endoplasmic reticulum-Golgi intermediate compartment in cystic fibrosis cells, Exp. Cell Res., 1998, 242, 144–152 CrossRef CAS PubMed.
- R. J. Gregory, D. P. Rich, S. H. Cheng, D. W. Souza, S. Paul, P. Manavalan, M. P. Anderson, M. J. Welsh and A. E. Smith, Maturation and function of Cystic Fibrosis Transmembrane Conductance Regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2, Mol. Cell. Biol., 1991, 11, 3886–3893 CAS.
- J. F. Place, R. M. Sutherland and C. Dähne, Opto-electronic immunosensors: a review of optical immunoassay at continuous surfaces, Biosensors, 1985, 1(4), 321–353 CrossRef CAS.
- N. Pollock, N. Cant, T. Rimington and R. C. Ford, Purification of the Cystic Fibrosis Transmembrane Conductance Regulator Protein Expressed in Saccharomyces cerevisiae, J. Visualized Exp., 2014, 10(87), e51447, DOI:10.3791/51447.
- L. Teng, M. Kerbiriou, M. Taiya, S. Le Hir, N. Benz, P. Trouvé and C. Férec, Proteomic Identification of Calumenin as a G551D - CFTR Associated Protein, PLoS One, 2012, 7(6), e40173 CAS.
- K. Mosler, C. Coraux, K. Fragaki, J. M. Zahm, O. Bajolet, K. Bessaci-Kabouya, E. Puchelle, M. Abély and P. Mauran, Feasibility of nasal epithelial brushing for the study of airway epithelial functions in CF infants, J. Cystic Fibrosis, 2008, 7(1), 44–53 CrossRef CAS PubMed.
- P. Trouvé, M. Kerbiriou, S. Le Hir, N. Benz and C. Férec, Surface Plasmon Resonance shows a gender difference in circulating annexin A5 in human, Talanta, 2012, 93, 219–223 CrossRef PubMed.
- C. R. O'Riordan, A. L. Lachapelle, J. Marshall, E. A. Higgins and S. H. Cheng, Characterization of the oligosaccharide structures associated with the cystic fibrosis transmembrane conductance regulator, Glycobiology, 2000, 10(11), 1225–1233 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |