A novel ionic liquid-based monolithic column and its application in the efficient separation of proteins and small molecules by high-performance liquid chromatography
Junxiao
Qin
ab,
Ligai
Bai
*abc,
Jiafei
Wang
ab,
Yamin
Ma
abc,
Haiyan
Liu
*abc,
Shuai
He
b,
Tengteng
Li
b and
Ying
An
b
aKey Laboratory of Pharmaceutical Quality Control of Hebei Province, China. E-mail: bailigai@163.com; lhy1610@126.com
bCollege of Pharmaceutical Sciences, Hebei University, China
cKey Laboratory of Medicinal Chemistry and Molecular Diagnosis, Ministry of Education, Hebei University, Baoding 071002, China
First published on 8th October 2014
Abstract
A novel skeleton porous polymer-based monolith chromatography column has successfully been prepared using an in situ free radical polymerization technique. A 50 mm × 4.6 mm i.d. stainless steel chromatographic column used dodecanol as porogen and ionic liquid (IL), 1-dodecene (C12) and trimethylol propane triacrylate (TMPTA) as monomers, and ethylene dimethacrylate as crosslinker. The effect of variables such as temperature and porogen solvent content on the porous structure was studied in detail. The polymer-based monolith obtained was characterized by scanning electron microscopy, infrared spectroscopy, mercury intrusion porosimetry, and nitrogen adsorption. The results indicated that the monolithic column had a porous structure, good mechanical stability, high permeability (6.77 × 10−14 m2), and a high specific surface area (155.62 m2 g−1). The liquid chromatographic performance of the monolith was evaluated in the separation of lysozyme from egg white and in the separation of a variety of mixtures of small molecules, such as amines and benzene analogues. The column showed good repeatability and reproducibility, and the column-to-column (n = 7) and batch-to-batch (n = 5) reproducibility was 2.85 and 3.15%, respectively.
1. Introduction
Polymeric monoliths were introduced about 20 years ago as materials facilitating rapid mass transport driven by convection though large pores in the monolith.1 As fourth-generation chromatographic sorbents, monolithic columns possess a unique structure and exhibit unusual characteristics, making them suitable as a continuous porous separation medium.2 During recent decades monolithic columns have developed rapidly due to their low back pressure, high permeability, rapid mass transfer, simple preparation and easy modification.3 As a result, monolithic columns have become an excellent tool in analytical techniques, not only in separation techniques, such as ion-exchange, hydrophobic interaction, size exclusion, and affinity chromatography, but also in sample preparation, including solid-phase and microextraction.4,5As a separation medium for high-performance liquid chromatography (HPLC), monolithic columns normally have a matrix which is either inorganic silica-based, or organic polymer-based derived from polystyrenes, polymethacrylate esters or polyacrylamides.6–8 Silica- and polymer-based matrices have different properties. Those based on silica allow rapid separation of small molecules, but the preparation process is complex and difficult to control,9 and a further disadvantage is that the Si–O linkage is prone to hydrolysis, giving a relatively narrow pH range (2–8) in use.
Organic polymer-based monoliths, on the other hand, can readily be post-modified and are stable over a wide pH range (1–14).10 In particular, polymeric materials provide an excellent stationary phase in HPLC for the rapid separation of large molecules, such as proteins, nucleic acids and peptides.11 Nevertheless, they have a number of disadvantages, such as poor reproducibility and resolution, and they have a non-uniform structure due to inadequate solubility of the monomers and porogens. To overcome these problems, we have therefore developed an alternative basis using ionic liquids (ILs) as a co-monomer.
Ionic liquids are a type of non-volatile organic molten salts which are non-molecular, and are stable and chemically inert. At temperatures below 100 °C they are liquid,12–14 and they have attracted wide interest due to their unique properties, including low volatility, tunable viscosity and good biocompatibility.15,16 ILs are of wide interest in a variety of applicational areas, not only in analytical chemistry but also more widely.17,18 Based on the properties of ILs,19 a new IL-based monolithic material has now been synthesized.
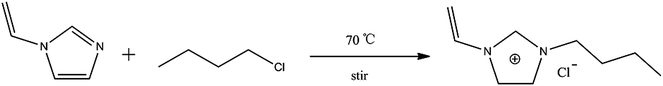
In the present study a novel HPLC monolithic column was prepared by in situ free-radical polymerization using 1-vinyl-3-butylimidazolium chloride as co-monomer. The effect of the variables influencing the porous structure have been studied in detail, and the optimized monoliths were assessed by separating lysine (Lys) from egg white and aromatic compounds.
2. Experimental
2.1 Materials
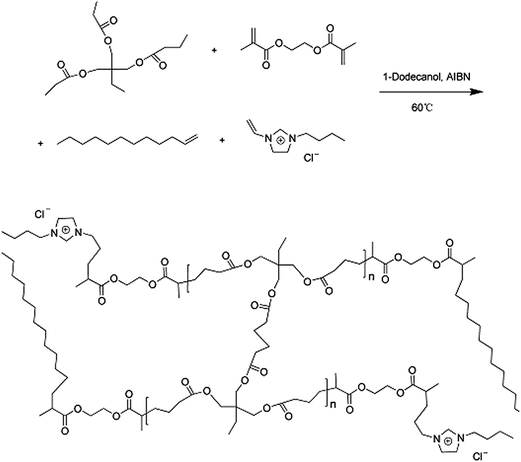
1-Dodecene (C12), 1-vinylimidazole and 1-chlorobutane were purchased from Shanghai Aladdin Co. Trimethylol propane triacrylate (TMPTA), ethylene dimethacrylate (EDMA) and azo-bis-isobutyronitrile (AIBN) were obtained from the Tianjin Chemistry Reagent Factory, and dodecanol, polyethylene glycol (PEG-200) and hexadecanol from the Shanghai Chemical Plant. The aromatic compounds studied were provided by the National Institute for the Control of Pharmaceutical and Biological Products of China (Beijing). HPLC-grade methanol and potassium bromide (KBr) were supplied by Kermel Chemical Reagent Co Ltd. (Tianjin), and the stainless steel columns (i.d. 50 × 4.6 mm) were purchased from Beijing Xinyu Instrument Co Ltd. Lys was obtained from Sigma Chemical Co. (St Louis, MO, USA). Triple-distilled water was used for all the experiments, and all liquids and solutions were filtered through a 0.45 μm membrane before use.2.2 Instrumental
An 1100 system from Agilent Technologies (USA) was used in the chromatographic studies. The HPLC system consisted of a quaternary pump with an online vacuum degasser, an autosampler with variable injection capacity from 0.1 to 100 μL, and a UV detector. All the sample solutions injected into the chromatographic system were filtered through a millipore membrane (0.45 μm) to remove particles and large aggregates. The morphology of the monolithic columns was studied using a Hitachi S-3400 scanning electron microscope (Hitachi High Technologies, Tokyo), and the FT-IR spectra were recorded on an FTIR-8400S IR spectrometer (Shimadzu, Kyoto, Japan) over the range 400–4000 cm−1.2.3 Preparation of the polymer-based monolithic columns
| Columna | IL (mL) | C12 (mL) | TMPTA (mL) | EDMA (mL) | Dodecanol (mL) | PEG-200 (mL) | Hexadecanol (mL) | Polymerization temperature (°C) | Mechanical/physical properties | Pressureb (bar) | Permeability (K, 10−14 m2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a All columns were initiated by incorporation of 0.01 g AIBN. b Pressure was obtained using methanol as the mobile phase at 1.0 mL min−1. | |||||||||||
| A | 0.1 | 0.2 | 0.4 | 0.8 | 2.0 | — | — | 60 | Proper hard | 4 | 6.77 |
| B | — | 0.2 | 0.4 | 0.8 | 2.0 | — | — | 60 | Hard | 9 | 3.01 |
| C | 0.1 | — | 0.4 | 0.8 | 2.0 | — | — | 60 | Granulous | 10 | 2.71 |
| D | 0.1 | 0.2 | 0.4 | 0.5 | 2.0 | — | — | 60 | Brittle | 3 | 9.03 |
| E | 0.1 | 0.2 | 0.4 | 1.1 | 2.0 | — | — | 60 | Hard | 13 | 2.08 |
| F | 0.1 | 0.2 | 0.4 | 0.8 | 1.6 | — | — | 60 | Quite hard | 15 | 1.81 |
| G | 0.1 | 0.2 | 0.4 | 0.8 | 2.1 | — | — | 60 | Soft | 3 | 6.92 |
| H | 0.1 | 0.2 | 0.4 | 0.8 | 2.3 | — | — | 60 | Soft | 2 | 7.38 |
| I | 0.1 | 0.2 | 0.4 | 0.8 | 2.4 | — | — | 60 | Quite soft | <2 | — |
| J | 0.1 | 0.2 | 0.4 | 0.8 | — | 2.0 | — | 60 | Immiscible | — | — |
| K | 0.1 | 0.2 | 0.4 | 0.8 | — | — | 2.0 | 60 | Quite hard | — | 0.91 |
| L | 0.1 | 0.2 | 0.4 | 0.8 | 2.0 | — | — | 40 | — | — | — |
| M | 0.1 | 0.2 | 0.4 | 0.8 | 2.0 | — | — | 50 | Quite soft | <1 | — |
| N | 0.1 | 0.2 | 0.4 | 0.8 | 2.0 | — | — | 70 | Quite hard | >20 | 0.65 |
2.4 Characterization methods
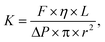 | (1) |
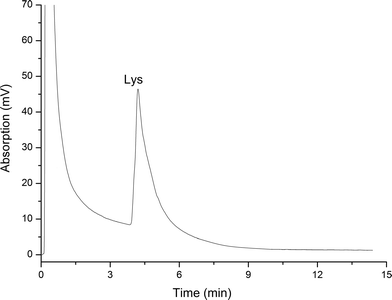
2.5 The separation of Lys and egg white
Lysine (abbreviated as Lys or K)1 is an α-amino acid with structure HO2C·CH(NH2)·(CH2)4NH2. It is an essential amino acid in humans.The IL-based monolithic column was used to separate Lys from egg white by ionic interaction, in the following manner. Chicken egg white was separated from fresh eggs and diluted to 50% (v/v) using phosphate buffer (50 mmol, pH 7.0). The diluted egg white was homogenized in an ice-bath and centrifuged at 4 °C and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min.
000 rpm for 10 min.
The following conditions were used for the chromatographic separation. The UV detector was used at 280 nm, the injection volume was 5.0 μL, the gradient was 0–3 min and an aqueous solution of 0.02 mol L−1 Na2HPO4 (adjusted to pH 12 with aqueous NaOH solution) was used as the mobile phase; from 3.01–10 min, water was used as the mobile phase.
3. Results and discussion
3.1 Optimization of preparation conditions
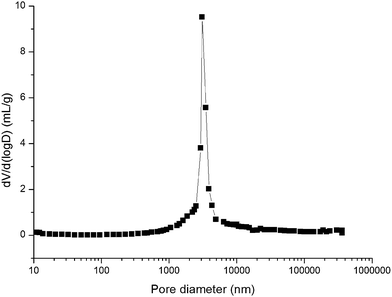
In order to investigate the influence of dodecanol content of the pre-polymerization solution on the preparation of the poly(IL-co-C12-co-TMPTA -co-EDMA) monolith, a range of dodecanol contents were considered, listed in Table 1. The results showed that with an increasing proportion of dodecanol, the column permeability increased, but the hardness and back pressure decreased (columns A, F–I). On the other hand, when the proportion of dodecanol was increased to 2.4 mL (column I), the mechanical properties of the column became unacceptably poor. When the amount of dodecanol was 2.0 mL (column A), the monolithic column exhibited good permeability, moderate hardness, and low back pressure.
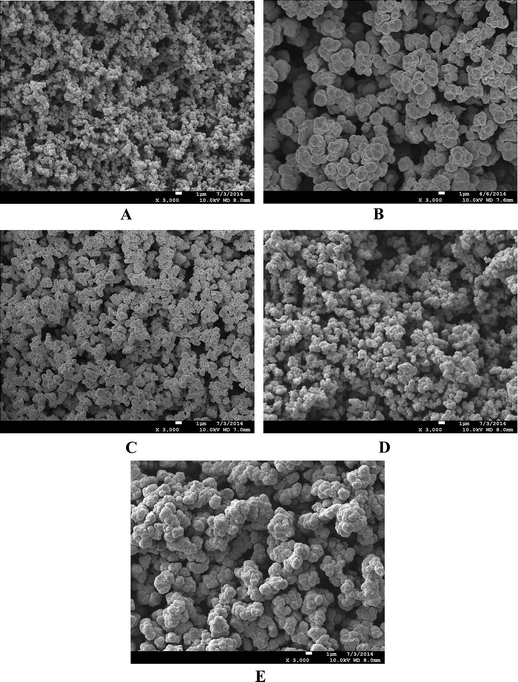
3.2 Characterization of the monoliths
The performance of the three monoliths, columns A, B and C, was confirmed by the liquid chromatographic separation of small molecules.
The peak band broadening from B and peak tailing from C indicate the effect of the composition of the monomer on the structure and the resulting chromatographic performance. After optimization and comparison, column A was adopted for the subsequent experiments.
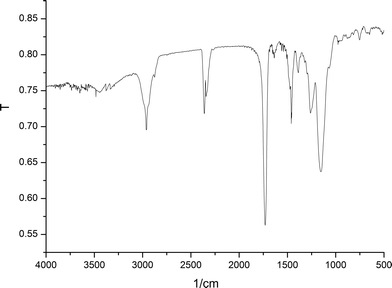
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds was seen at 1750 cm−1, and in addition the C–H bond stretching, vibration around the imidazole ring at 1169 cm−1, could be observed, confirming the presence of IL on the IL-based monolith.
O double bonds was seen at 1750 cm−1, and in addition the C–H bond stretching, vibration around the imidazole ring at 1169 cm−1, could be observed, confirming the presence of IL on the IL-based monolith.
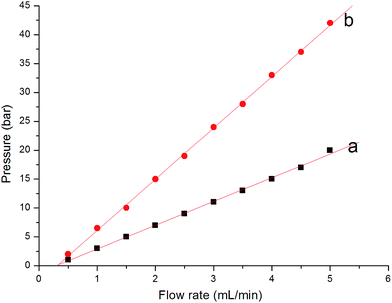
Fig. 3 shows the back pressure in Column A at different flow rates using methanol and water, respectively, as mobile phase. Although the flow rate was increased to 5 mL min−1 using water as the mobile phase, the maximum pressure was 43 bar. In addition, the good linear relationship (r2 > 0.999) between back pressure and flow rate confirmed its good mechanical stability.
3.3 Chromatographic behavior of the IL-based monolithic column
In addition, the Lys content was assayed by ultraviolet spectrometry and its purity was calculated after vacuum freeze-drying as 92.1%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 v/v, and a flow rate of 1.0 mL min−1, was selected as providing optimal chromatographic conditions.
25 v/v, and a flow rate of 1.0 mL min−1, was selected as providing optimal chromatographic conditions.
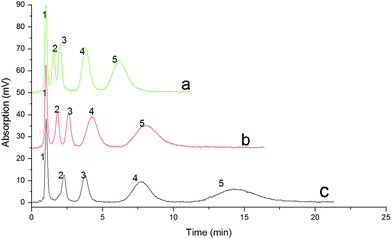
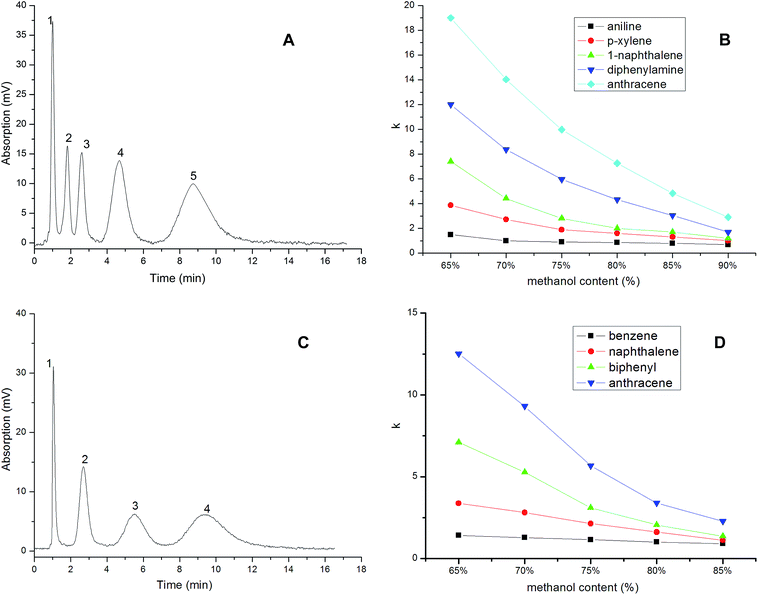
The chromatographic behavior of the IL-based monolithic column in the separation of aromatic compounds is shown in Fig. 7, in which the chromatogram (a) shows the baseline separation of four compounds with a methanol–water mobile phase (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, v/v). The analytes were eluted in the following order: aniline, p-xylene, naphthalene, diphenylamine and triphenylamine, which corresponded to the hydrophobicity of the five analytes from low to high. The result indicated a typical reversed-phase separation mode.
25, v/v). The analytes were eluted in the following order: aniline, p-xylene, naphthalene, diphenylamine and triphenylamine, which corresponded to the hydrophobicity of the five analytes from low to high. The result indicated a typical reversed-phase separation mode.
The retention factor (k) of each sample on the IL-based monolith was determined at different methanol contents in the mobile phase, and the results are shown in Fig. 7(b), confirming a typical reversed-phase liquid chromatographic mechanism. A typical separation of neutral aromatic compounds is shown in Fig. 7(c), with a baseline separation of four compounds with a mobile phase of methanol–water (68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32, v/v). The four compounds were eluted in accordance with their polarities from high to low in the order benzene, naphthalene, biphenyl and anthracene, and the column efficiencies for the four compounds were about 5940–9249 theoretical plates per meter. The retention factors (k) of the four analytes on the IL-based monolith are presented in Fig. 7(d), which also shows that the retention factor of each sample decreased following the increase in methanol content in the mobile phase, confirming the typical reversed-phase chromatographic mechanism of the separation.
32, v/v). The four compounds were eluted in accordance with their polarities from high to low in the order benzene, naphthalene, biphenyl and anthracene, and the column efficiencies for the four compounds were about 5940–9249 theoretical plates per meter. The retention factors (k) of the four analytes on the IL-based monolith are presented in Fig. 7(d), which also shows that the retention factor of each sample decreased following the increase in methanol content in the mobile phase, confirming the typical reversed-phase chromatographic mechanism of the separation.
3.4 Reproducibility
The reproducibility of the monolithic column was calculated from the percentage relative standard deviations of the retention factors of the test compounds on Column A. The average run-to-run reproducibility (n = 5) of benzene was 1.07%, while the average day-to-day reproducibility (n = 3) was 1.75%. This demonstrates the stability of the monolithic columns. In addition, the column-to-column and batch-to-batch reproducibility were also investigated using the same or different batches of polymerization mixture, respectively. The column-to-column (n = 7) and batch-to-batch (n = 3) reproducibility were 2.85 and 3.15%, respectively. This confirmed the intrinsic reproducibility and stability of the monolithic columns.4. Conclusions
In this study a porous uniform poly-IL-based monolithic column with high specific surface area was successfully prepared using IL as co-monomer by in situ free radical polymerization, and was successfully used to separate Lys from egg white. In addition the monolithic column exhibited good performance in reversed-phase liquid chromatographic separation. The monolithic column offers a potentially useful alternative for the efficient separation of proteins and small molecules.Acknowledgements
We are grateful for the financial support provided by the National Natural Science Foundation of China (21175031), the National Science Foundation of Hebei Province (B2012201052, B2013201082), and the National Science Foundation of Hebei University (2013-247).References
- G. Guiochon, J. Chromatogr. A, 2007, 1168, 101 CrossRef CAS PubMed.
- J. J. Ou, H. Lin, Z. B. Zhang, G. Huang, J. Dong and H. F. Zou, Electrophoresis, 2013, 34, 126 CrossRef CAS PubMed.
- H. F. Han, Q. Wang, X. Liu and S. X. Jiang, J. Chromatogr. A, 2012, 1246, 9 CrossRef CAS PubMed.
- W. J. Li, X. Zhou, S. S. Tong and Q. Jia, Talanta, 2013, 105, 386 CrossRef CAS PubMed.
- Z. H. Liu, Y. B. Peng, T. T. Wang, G. X. Yuan, Q. X. Zhang, J. L. Guo and Z. J. Zhang, J. Sep. Sci., 2013, 36, 262 CrossRef CAS PubMed.
- M. M. Zheng, G. D. Ruan and Y. Q. Feng, J. Chromatogr. A, 2009, 1216, 7739 CrossRef CAS PubMed.
- Y. J. Ueki, T. Umemura, J. X. Li, T. Odake and K. Tsunoda, Anal. Chem., 2009, 76, 7007 CrossRef PubMed.
- F. Maya and F. Svec, Polymer, 2014, 55, 340 CrossRef CAS PubMed.
- J. Chen, P. F. Zhang and J. Li, J. Chromatogr. A, 2011, 1218, 3699 CrossRef CAS PubMed.
- Y. Tian, R. Feng, L. P. Liao, H. L. Liu, H. Chen and Z. R. Zeng, Electrophoresis, 2008, 29, 3153 CrossRef CAS PubMed.
- J. Liu, F. J. Wang, H. Lin, J. Zhu, Y. Y. Bian, K. Cheng and H. F. Zou, Anal. Chem., 2013, 85, 2847 CrossRef CAS PubMed.
- H. M. Albishri and D. A. El-Hady, Talanta, 2014, 118, 129 CrossRef CAS PubMed.
- T. Zhu, W. T. Bi and K. H. Row, J. Appl. Polym. Sci., 2010, 118, 3425 CrossRef CAS.
- Y. Wang, Q. L. Deng, G. Z. Fang, M. F. Pan, Y. Yu and S. Wang, Anal. Chim. Acta, 2012, 712, 1 CrossRef CAS PubMed.
- C. C. Liu, Q. L. Deng, G. Z. Fang, H. L. Liu and J. H. Wu, Anal. Chim. Acta, 2013, 804, 313 CrossRef CAS PubMed.
- X. J. Huang, L. L. Chen, D. X. Yuan and S. S. Bi, J. Chromatogr. A, 2012, 1248, 67 CrossRef CAS PubMed.
- H. J. Zheng, Q. W. Liu and Q. Jia, J. Chromatogr. A, 2014, 1343, 47 CrossRef CAS PubMed.
- B. Singco, C. L. Lin, Y. J. Cheng, Y. H. Shih and H. Y. Huang, Anal. Chim. Acta, 2012, 746, 123 CrossRef CAS PubMed.
- Y. Y. Fan, S. H. Liu and Q. L. Xie, Talanta, 2014, 119, 291 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |