Trace copper ion detection by the suppressed decolorization of chromotrope 2R complex†
Lili
Fu
,
Yuan
Xiong
,
Shu
Chen
and
Yunfei
Long
*
Key Laboratory of Theoretical Organic Chemistry and Function Molecule, Ministry of Education, School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan, 411201, PR China. E-mail: l_yunfei927@163.com
First published on 31st October 2014
Abstract
Chromotrope 2R (CR) is a monoazo dye, which can be easily degraded under ultraviolet C (UVC) light irradiation. However, the degradation extent of CR is suppressed after it is chelated with Cu2+ ions to form a coordination complex (Cu2+–CR). This phenomenon was developed as a novel method for the quantitative detection of Cu2+ ions, which is based on determining the change in absorbance (ΔA, the absorbance of Cu2+–CR complex subtracted by that of CR after UVC light irradiation) by UV-visible absorption spectrum. Under the optimal detection conditions, ΔA at 509 nm highly depends on the concentration of Cu2+ ions in the range from 5.0 × 10−9 to 1.0 × 10−6 M as expressed by the following equation: ΔA = 0.3066 + 0.03605![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c with the correlation coefficient of r = 0.9912. The limit of detection (ld) is 3.4 nM as calculated by the formula 3σ = 0.3066 + 0.03605
c with the correlation coefficient of r = 0.9912. The limit of detection (ld) is 3.4 nM as calculated by the formula 3σ = 0.3066 + 0.03605![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ld. This method provides affordable and selective detection of Cu2+ ions and was used to detect Cu2+ ions in a human hair sample.
ld. This method provides affordable and selective detection of Cu2+ ions and was used to detect Cu2+ ions in a human hair sample.
1. Introduction
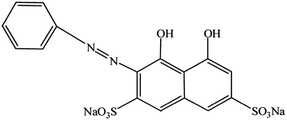
Copper ion (Cu2+) is one of the essential transition metal ions in the human body, and its deficiency or excess may cause health problems.1 The deficiency of Cu2+ may lead to hematological manifestations2 and diverse neurological problems.3 Moreover, excess amount of Cu2+ may cause gastrointestinal disturbance and neurotoxicity, which is commonly known as Parkinson's disease and Alzheimer's disease, through the deposition of Cu2+ in the lenticular nucleus of the brain and liver.4,5 Therefore, the analysis and detection of Cu2+ in environmental and/or biological samples is of great importance. Various sensors and techniques have been developed for the determination of Cu2+,6–9 such as organic fluorophores10 or chromogenic sensors,11 a DNAzyme-based method,12 the colorimetric detection,13 absorbance spectrophotometry,14 atomic absorption spectroscopy (AAS),15 and inductively coupled plasma mass spectroscopy (ICP-MS).16 However, a sensitive and selective method for Cu2+ detection is still needed.Chromotrope 2R (ref. 17) (abbreviated as CR, the structure is shown in Scheme 1) is a type of azo dye.18,19 CR and/or its homologs have been used to detect nitrate,20 formaldehyde,21 methanol,22 titanium,23 and unsymmetrical dimethylhydrazine.24 Moreover, they were used as chelating agents for metal ions, particularly Cu2+ ions.25–27 For example, chromotropic acid-intercalated Zn–Al layered double hydroxides have been used to remove Cu2+ ions,28 and polyurethane foam/2-(6′-ethyl-2′-benzothiazolylazo) chromotropic acid has been used to preconcentrate Cu2+ ions in water samples.29
Herein, we found that UVC light induced decolorization of CR could be suppressed after coordinating with Cu2+ ions. Furthermore, the decolorization extent of CR depends on the concentration of Cu2+ ions. Thus, a novel method for the detection of Cu2+ ions was established using UV-visible spectroscopy. The content of Cu2+ ions in a human hair sample was detected successfully, indicating its potential application in other environmental and biological samples.
2. Experiment
2.1 Instrumentation
UV-visible absorption spectra were recorded using a Lambda-35 UV-visible spectrophotometer (Perkin Elmer Instruments Inc., USA) and a quartz cell (1 × 1 cm2). Mass spectroscopy (MS) were recorded using an ACQUITY UPLC/Xevo Q-TOF (Waters, USA) instrument. A UVC lamp of 110 W (wavelength range 200–275 nm, Shanghai Yanguang Electronic Technology Co. Ltd., China) was used as the UVC light source. A QL-901 Vortex (Qilinbeier Instrument Manufacturing Co. Ltd., Haimen, China) was used to mix the reaction solution.2.2 Reagents
CR was purchased from Shanghai Chemical Co., and used as received without further purification. The concentration of the CR stock solution is 4.0 × 10−4 M. A Cu2+ stock solution with a concentration of 1.0 × 10−3 M was freshly prepared by dissolving pure Cu(NO3)2·3H2O (purchased from Tianjin Municipality Kemi'ou Chemical Reagent Co. Ltd.) with redistilled water, and then diluted to appropriate concentrations with water. The Britton–Robinson (BR) buffer solution was used to adjust the pH value of the reaction system from 1.81 to 8.69. All the chemicals were analytical reagents and redistilled water was used.2.3 Irradiation procedure
Irradiation experiments were carried out in a 4.0 mL centrifuge tube with the CR solution (0.1 mL, initial concentration 4.0 × 10−4 M). Then, appropriate concentrations of Cu2+ ions or sample solutions (3.5 mL) were added to the centrifuge tube and shaken for 30 s. Subsequently, the BR buffer solution (400 μL, pH = 4.78) was added, and the mixtures were illuminated under UVC irradiation for 50 min. Finally, the absorbance values of these samples were measured using a UV-visible spectrophotometer.2.4 Pretreatment of real samples
The hair sample from a barber shop was pretreated by following a procedure reported previously.30,31 The hair sample was first cut into pieces with a length <1.0 cm using stainless steel scissors, and then it was immersed in a detergent solution with a concentration of 1.0% for 0.5 h. Subsequently, the sample was washed four times with water and dried for 3 h in an oven at 105 °C. Then, 1.0 g of the hair sample and 2.0 mL of a mixture of HNO3/HClO4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) were added into a quartz beaker, covered with a watch glass, and allowed to stand overnight. The next day, the hair sample was heated until it dissolved completely. Finally, the sample was diluted to 50.0 mL with water and used as the stock solution.
1 v/v) were added into a quartz beaker, covered with a watch glass, and allowed to stand overnight. The next day, the hair sample was heated until it dissolved completely. Finally, the sample was diluted to 50.0 mL with water and used as the stock solution.
3. Results and discussion
3.1 Degradation of CR with and without Cu2+
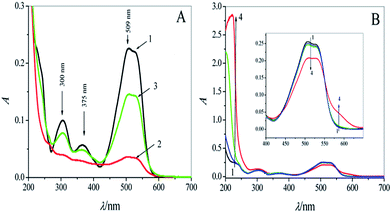
The UV-visible absorption spectra of CR (1.0 × 10−5 M) before and after UVC light irradiation are shown in Fig. 1A (curves 1 and 2). The absorbance (A) of the three absorption peaks at 300, 375, and 509 nm clearly decreased after irradiation for 50 min. The reduction in A was 84.1% at 509 nm; however, in the presence of a trace amount of Cu2+ ions (1.0 × 10−6 M), the photodegradation was significantly suppressed, and A decreased by only 35.4% at 509 nm (curve 3 in Fig. 1A), which can be ascribed to the formation of a coordination complex between CR and Cu2+ ions (CR–Cu2+).To identify the complexation of CR with Cu2+ ions, the UV-visible absorption and MS spectra of CR–Cu2+ in the presence of different concentrations of Cu2+ ions (from 0 to 1.0 × 10−3 M) were investigated. As shown in Fig. 1B, the absorbance of CR (curve 1, Fig. 1B) decreased slightly over the wavelength range of 450–550 nm in the presence of Cu2+ ions (curves 2–4, Fig. 1B). Two new absorption bands appeared in the wavelength ranges of 200–300 nm and 550–630 nm. Simultaneously, the color of the solution deepened with Cu2+ addition (Fig. S1 in the ESI†). These results can be attributed to the complexation between Cu2+ ions and the amine groups in CR. The result of MS showed that the molecular weight of the CR–Cu2+ mixture is 958.9 (Fig. S2A and B in the ESI†), indicating that the coordination ratio between CR and Cu2+ was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the possible structure of the complex is shown in Fig. S3.†
1, and the possible structure of the complex is shown in Fig. S3.†
To further understand the mechanism of the photodegradation of CR, we carried out the experiments by deoxygenating the solution. The UV-visible absorption spectra are shown in Fig. S4 in the ESI,† indicating that the degradation extent decreased significantly without dissolved oxygen (curve 1 of Fig. S4†) compared to that in the presence of dissolved oxygen (curve 2 of Fig. S4†). The MS spectra showed that the intensity of the peak at m/z of 468.9 for the CR molecule significantly decreased under UVC light irradiation in the presence of dissolved oxygen (Fig. S2C in the ESI†). Thus, we deduced that the photodegradation of CR involves oxidation by oxygen under UVC light irradiation.
3.2 Optimization of reaction conditions
Many studies showed that CR is sensitive to UV light. First, we confirmed that natural light has a negligible effect on CR at room temperature (Fig. S5 in the ESI†). We also investigated the effect of different powers of UVC light on the degradation of CR. It was found that CR could not be degraded within 50 min under low-power (30 W, 45 W) light irradiation. High-power (110 W) UVC light irradiation was efficient for this study.To study the effect of the concentration of CR on the detection sensitivity, we measured the ΔA values (difference between the absorbance of the CR solution in the presence of Cu2+ and in the absence of Cu2+) under different concentrations of CR solutions (Fig. S6 in the ESI†). The results indicate that the ΔA values are 0.142 for 1.0 × 10−4 M CR, 0.148 for 5.0 × 10−5 M CR, and 0.11 for 1.0 × 10−5 M CR. However, the signal to noise (S/N) ratio, which was calculated using the equation S/N = (A − AO)/AO, was 0.0796 for 1.0 × 10−4 M CR, 0.2016 for 5.0 × 10−5 M CR, and 3.056 for 1.0 × 10−5 M CR, i.e., the value of S/N is highest when the concentration of CR is 1.0 × 10−5 M.
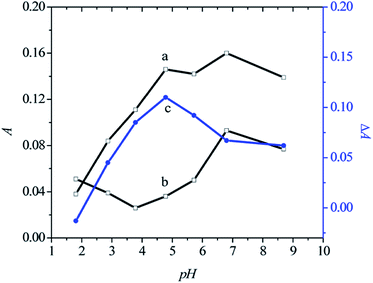
The pH of the solution is another key factor, affecting the sensing sensitivity. Fig. 2 shows the absorbance values at the maximum absorption peak of CR in the presence (curve a) and absence (curve b) of Cu2+ ions (1.0 × 10−6 M) at different pH values in the range from 1.81 to 8.69. Curve c in Fig. 2 shows the change of ΔA with different pH values. For pH ≤ 4.78, the ΔA values increased (curve c, Fig. 2) with increase in pH value, whereas for pH > 4.78, the ΔA values decreased with increase in pH value (curve c in Fig. 2). This indicates that weakly acidic media (pH = 4.78) are suitable for sensing Cu2+ions.
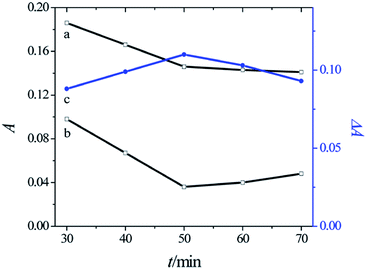
Fig. 3 shows the absorbance at the maximum absorption peak of CR in the presence (curve a) and absence (curve b) of Cu2+ ions (1.0 × 10−6 M) with different irradiation times in the range from 30 to 90 min at the optimum pH value of 4.78. Curve c in Fig. 3 shows that when the reaction time was 50 min, ΔA reached the maximum value. Therefore, the optimum irradiation time was 50 min for the determination of Cu2+ ions.
3.3 Selectivity of Cu2+ ion detection
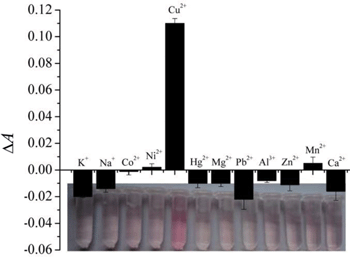
The selectivity of CR for the detection of Cu2+ ions over other ions is shown in Fig. 4. In addition to Cu2+ (1.0 × 10−6 M), the effects of other 11 types of cations, including K+, Na+, Co2+, Ni2+, Hg2+, Mg2+, Pb2+, Al3+, Zn2+, Mn2+, and Ca2+ ions, at the concentration of 5.0 × 10−6 M, on the UV-visible absorbance of CR, were investigated under UVC irradiation for 50 min. The results indicate that Mn2+ and Ni2+ ions slightly suppressed the CR degradation, and the other ions slightly accelerated the CR degradation. The signals responded by Cu2+ ions are the most pronounced among other cations. Therefore, the sensing system shows high selectivity for Cu2+ ion detection.3.4 Cu2+ ion detection in a human hair sample
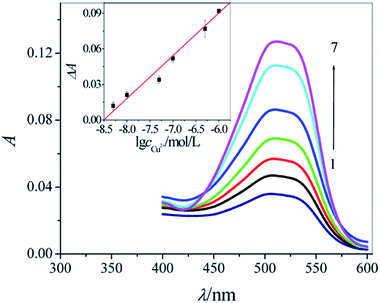
Fig. 5 shows that different concentrations of Cu2+ ions resulted in different degradation degrees of CR. Under the optimum conditions, this approach provides a linear response to Cu2+ ions in spiked samples at concentrations in the range from 5.0 × 10−9 to 1.0 × 10−6 M (ΔA = 0.3066 + 0.03605![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c, r = 0.9912, n = 6, the inset of Fig. 5). As the Table S1 in the ESI† shows, this method has comparable sensitivity with the fluorescence method32–34 and has relatively higher sensitivity than the absorption method.35,36 To evaluate the potential application of this method for detecting Cu2+ ions in a real sample, we tested its absorbance response for Cu2+ ions in a human hair sample. The content of Cu2+ ions in the human hair sample (n = 3) detected using this new approach was 3.72 μg g−1 (Table S2 in the ESI†), and the detection result agreed well with the Cu2+ content of healthy human hair37 with an excellent relative standard deviation (RSD, 3.62%). These results confirmed the reliability of this CR sensing method for the detection of Cu2+ ions in real samples.
c, r = 0.9912, n = 6, the inset of Fig. 5). As the Table S1 in the ESI† shows, this method has comparable sensitivity with the fluorescence method32–34 and has relatively higher sensitivity than the absorption method.35,36 To evaluate the potential application of this method for detecting Cu2+ ions in a real sample, we tested its absorbance response for Cu2+ ions in a human hair sample. The content of Cu2+ ions in the human hair sample (n = 3) detected using this new approach was 3.72 μg g−1 (Table S2 in the ESI†), and the detection result agreed well with the Cu2+ content of healthy human hair37 with an excellent relative standard deviation (RSD, 3.62%). These results confirmed the reliability of this CR sensing method for the detection of Cu2+ ions in real samples.
4. Conclusions
In this study, we found that CR could be degraded easily under UVC light irradiation. Cu2+ ions reacted with CR to form a stable complex, suppressing the CR degradation under UVC irradiation. After the optimization of the experimental conditions, a UV-visible absorption spectrum based sensing system was developed for the detection of Cu2+ ions. The results show that CR is an excellent UV-visible absorption spectrum probe for Cu2+ ion detection. The sensing system shows many advantages, such as good selectivity and high sensitivity, with the lowest detection concentration of 5.0 × 10−9 M. It was used for the detection of the content of Cu2+ ions in a real sample (human hair) with acceptable results.Acknowledgements
This work has been supported by the National Natural Science Foundation of China (No. 21275047 to YL and No. 21445008 to SC) and the Doctor Science Foundation of Hunan University of Science and Technology.References
- A. R. P. G. Georgopoulos, M. J. Yonone-Lioy, R. E. Opiekun and P. J. Lioy, J. Toxicol. Environ. Health, Part B, 2001, 4, 341–394 Search PubMed.
- T. R. Halfdanarson, N. Kumar, C.-Y. Li, R. L. Phyliky and W. J. Hogan, Eur. J. Haematol., 2008, 80, 523–531 CrossRef CAS PubMed.
- S. Jaiser and G. Winston, J. Neurol., 2010, 257, 869–881 CrossRef CAS PubMed.
- K. J. Barnham and A. I. Bush, Curr. Opin. Chem. Biol., 2008, 12, 222–228 CrossRef CAS PubMed.
- R. R. Crichton, D. Dexter and R. J. Ward, Coord. Chem. Rev., 2008, 252, 1189–1199 CrossRef CAS PubMed.
- Y. Zhou, S. Wang, K. Zhang and X. Jiang, Angew. Chem., 2008, 120, 7564–7566 CrossRef.
- P. Yang, Y. Zhao, Y. Lu, Q.-Z. Xu, X.-W. Xu, L. Dong and S.-H. Yu, Nano Lett., 2011, 5, 2147–2154 CAS.
- T. Lou, L. Chen, Z. Chen, Y. Wang, L. Chen and J. Li, ACS Appl. Mater. Interfaces, 2011, 3, 4215–4220 CAS.
- G. G. Huang and J. Yang, Anal. Chem., 2003, 75, 2262–2269 CrossRef CAS.
- R. F. H. Viguier and A. N. Hulme, J. Am. Chem. Soc., 2006, 128, 11370–11371 CrossRef CAS PubMed.
- S. Banthia and A. Samanta, New J. Chem., 2005, 29, 1007–1010 RSC.
- C. Ge, Q. Luo, D. Wang, S. Zhao, X. Liang, L. Yu, X. Xing and L. Zeng, Anal. Chem., 2014, 86, 6387–6392 CrossRef CAS PubMed.
- J.-M. Liu, H.-F. Wang and X.-P. Yan, Analyst, 2011, 136, 3904–3910 RSC.
- M. R. Callahan, J. B. Rose and R. H. Byrne, Talanta, 2002, 58, 891–898 CrossRef CAS.
- M.-S. Chan and S.-D. Huang, Talanta, 2000, 51, 373–380 CrossRef CAS.
- J. Wu and E. A. Boyle, Anal. Chem., 1997, 69, 2464–2470 CrossRef CAS PubMed.
- L. C. Almeida, S. Garcia-Segura, C. Arias, N. Bocchi and E. Brillas, Chemosphere, 2012, 89, 751–758 CrossRef CAS PubMed.
- C. G. Silva, W. Wang and J. L. Faria, J. Photochem. Photobiol., A, 2006, 181, 314–324 CrossRef CAS PubMed.
- N. M. Mahmoodi, M. Arami, N. Y. Limaee and N. S. Tabrizi, Chem. Eng. J., 2005, 112, 191–196 CrossRef CAS PubMed.
- J. J. Batten, Anal. Chem., 1964, 36, 939–940 CrossRef CAS.
- E. L. R. Krug and W. E. Hirt, Anal. Chem., 1977, 49, 1865–1867 CrossRef CAS.
- R. N. Boos, Anal. Chem., 1948, 20, 964–965 CrossRef CAS.
- W. W. Brandt and A. E. Preiser, Anal. Chem., 1953, 25, 567–571 CrossRef CAS.
- N. V. Sutton, Anal. Chem., 1964, 36, 2120–2121 CrossRef CAS.
- E. Bardez, V. Alain, É. Destandau, A. Fedorov and J. M. G. Martinho, J. Phys. Chem. A, 2001, 105, 10613–10620 CrossRef CAS.
- S. B. Dabhade and S. P. Sangal, Microchem. J., 1969, 14, 190–198 CrossRef CAS.
- G. A. L'Heureux and A. E. Martell, J. Inorg. Nucl. Chem., 1966, 28, 481–491 CrossRef.
- Y. Chen and Y.-F. Song, Ind. Eng. Chem. Res., 2013, 52, 4436–4442 CrossRef CAS.
- A. Moghimi and N. Tajodini, Asian J. Chem., 2010, 22, 3325–3334 CAS.
- W. W. Harrison, J. P. Yurachek and C. A. Benson, Clin. Chim. Acta, 1969, 23, 83–91 CrossRef CAS.
- J. P. Yurachek, G. G. Clemena and W. W. Harrison, Anal. Chem., 1969, 41, 1666–1668 CrossRef CAS.
- N. Shao, J. Y. Jin, H. Wang, Y. Zhang, R. H. Yang and W. H. Chan, Anal. Chem., 2008, 80, 3466–3475 CrossRef CAS PubMed.
- Y. Dong, R. Wang, G. Li, C. Chen, Y. Chi and G. Chen, Anal. Chem., 2012, 84, 6220–6224 CrossRef CAS PubMed.
- Y.-T. Su, G.-Y. Lan, W.-Y. Chen and H.-T. Chang, Anal. Chem., 2010, 82, 8566–8572 CrossRef CAS PubMed.
- Z.-Q. Guo, W.-Q. Chen and X.-M. Duan, Org. Lett., 2010, 12, 2202–2205 CrossRef CAS PubMed.
- B.-C. Yin, B.-C. Ye, W. Tan, H. Wang and C.-C. Xie, J. Am. Chem. Soc., 2009, 131, 14624–14625 CrossRef CAS PubMed.
- H. S. Ferreira, W. N. L. dos Santos, R. P. Fiuza, J. A. Nóbrega and S. L. C. Ferreira, Microchem. J., 2007, 87, 128–131 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ay02414a |
| This journal is © The Royal Society of Chemistry 2015 |