Determination of Cd, Co, Cr, Cu, Ni and Pb in cosmetic samples using a simple method for sample preparation
Érica Ferreira
Batista
,
Amanda dos Santos
Augusto
and
Edenir Rodrigues
Pereira-Filho
*
Federal University of São Carlos, Chemistry Department, Group of Applied Instrumental Analysis, P.O. Box 676, 13565-905 São Carlos, São Paulo State, Brazil. E-mail: erpf@ufscar.br
First published on 11th November 2014
Abstract
In this study, a method has been used to prepare and evaluate the content of some metals in samples of eye shadow. The samples were manufactured in different countries (Brazil, China and USA). The sample preparation was performed using dilute nitric acid and a hot block. The Pb content was measured using graphite furnace atomic absorption spectrometry (GFAAS), and the quantification of Cd, Co, Cr, Cu and Ni was performed using inductively coupled plasma optical emission spectrometry (ICP OES). Chemometric tools were used for multivariate optimisation and to perform an exploratory analysis according to the metal concentration levels. Some samples presented metal concentrations above the values indicated by the FDA (USA) and ANVISA (Brazil).
1 Introduction
Cosmetics are one of the most important products for the world economy. The cosmetic world market in 2012, for example, generated around US$40 billion.1 The first archaeological evidence for the use of cosmetics was found in ancient Egypt, where men, women and children used green and black paints as makeup.2 According to the Brazilian Health Surveillance Agency (ANVISA) and the U.S. Food and Drug Administration (FDA), cosmetics are products made of natural or synthetic substances and used for the purpose of cleaning, perfuming or changing the appearance of parts of the human body.3,4 Cosmetics are divided into several categories, such as makeup for children and adults, and include eye shadow, corrective facials, lipstick, blush and others. Most of these products have some metals in their pigmentation formulation that are used to provide a wide variety of colors.5 These metals are partially soluble in water and sweat and could be absorbed through the skin, causing an allergic reaction.6The U.S. and Brazilian legislative branches do not specify limits for the metal concentrations in makeup; however, the FDA and ANVISA have established a threshold limit for the metal content in raw materials. According to the FDA regulations, the metal concentration limits depend on each additive and its color.7 According to the Brazilian regulations, the limits of some metals in organic artificial colourants are 3 mg kg−1 for As, 20 mg kg−1 for Pb and 100 mg kg−1 for other elements.8
Canada has a guide on heavy metals found as impurities in cosmetic products. The country follows the European legislation, however, by testing its own samples, cosmetic limits for some metals were established in the final product. The maximum tolerable limits for Pb, As, Cd and Hg are 10, 3, 3 and 5 mg kg−1, respectively.9
Metals such as Ni, Co and Cr as mentioned before are considered major causes of allergies, but this risk can be reduced if the level of these metals remains below 5 μg g−1.10
The sample preparation, in the scope of the analytical sequence, is the process most likely to introduce errors; it is time consuming and involves high costs.11 Usually, during the sample preparation, concentrated HNO3 and HF are used to completely digest the cosmetic samples (e.g., lipstick and eye shadow).6,12,13 In this context, sample preparation using dilute HNO3 is an interesting alternative and environmentally friendly. This sample preparation is easier, safer and in accordance with green chemistry due to the low consumption of reagents and the consequent reduction of laboratory residues.14 Furthermore there is a reduction of the blank signal and dilution is avoided before determination.15 Although the use of diluted nitric acid was extremely evaluative for different types of samples, there are no studies employing diluted acids for metal extraction in eye shadows.16,17
The efficiency of using diluted acids is a result of the temperature gradient inside the reaction vessel during the initial steps of sample digestion and due to the presence of a rich oxygen atmosphere inside the closed vessel. The nitric acid is regenerated by nitrogen oxide species with hydrogen peroxide as shown below:18
| 2NO + 3H2O2 → 2HNO3 + 2H2O |
In some cases strong treatments (concentrated acids) may not be feasible; following this thought some authors have proposed to employ a partial digestion of cosmetics.10,19 Some parameters can be studied to optimize the process of sample preparation such as acid concentration, sample mass, heating time and the use of other reagents employed for digestion of the samples.20
One of the strategies to optimise the process of sample preparation is multivariate analysis. This approach enables the collection of information about the effects of each evaluated variable and their interactions to find the best possible or most favourable conditions.21 One of the chemometric tools used for system optimisation is factorial design. Using this approach, a reduced number of experiments are performed, and the information is maximised.21 When factorial design and multielement analysis are combined, several responses can be obtained. In this case, the desirability function becomes an alternative method to normalise these responses and combine them in a single value named the overall desirability (D) and then achieve better working conditions for all responses evaluated.21 Thus, the goal of this study was the development of a simple method of cosmetic sample preparation and determination of Cd, Co, Cr, Cu and Ni using ICP OES and Pb using GFAAS in eye shadow. Several chemometric tools were used for multivariate optimisation and to perform an exploratory analysis by categorising the samples according to the metal concentration levels.
2 Experimental
2.1 Reagents
All reagents were of analytical grade or higher purity. Deionised water (18.2 ΩM cm−1 resistivity) produced using a Milli-Q® Plus Total Water System (Millipore Corp., Bedford, MA, USA) was used to prepare all solutions. Prior to use, all glassware and polypropylene flasks were washed with soap, soaked in 10% v/v HNO3 for 24 h, rinsed with deionised water and dried to ensure that no contamination occurred. For sample mineralisation, a mixture of H2O2 (30% w/w) (Synth, Diadema, SP, Brazil), HNO3 (2 mol L−1) (Synth) and Triton X-100 (5% v/m) (Sigma Aldrich, St Louis, MO, USA) was used. The HNO3 was previously purified using sub-boiling distillation Distillacid™ BSB-939-IR (Berghof, Eningen, Germany). To compare the method of sample preparation we used a mixture of H2O2 (30% w/w), HF (40% v/v) (House of Chemistry, Diadema, SP, Brazil) and boric acid solution (4% w/v) (Mallinckrodt, Kentucky, USA).The multi-element standard solutions were prepared daily from 1000 mg L−1 Cd, Co, Cr, Cu, Ni and Pb stock solutions (Qhemis, Jundiaí, SP, Brazil) via dilution in 0.67 mol L−1 HNO3. This acid solution was also used as the blank.
A mixture of magnesium nitrate solution (0.03% w/v) and ammonium dihydrogen phosphate (0.5% w/v) solution was used as a chemical modifier in Pb determination using GFAAS.
2.2 Instrumentation
A hot block (Tecnal, Brazil), built to fit up to 30 units of PFA (perfluoroalkoxy, Savillex, Minnetonka, USA) in closed bottles of 50 mL was used for sample preparation. The HNO3 concentration, sample mass, and heating time were studied using a 23 full factorial design. An ICP OES (iCAP 6000, Thermo Scientific, Waltham, MA, USA) instrument was used for Cd, Co, Cr, Cu and Ni determination. This instrument allows sequential analytical signal collection using both axial and radial viewings. The ICP OES parameters were studied using a fractional factorial design 29−5. Argon 99.996% (White Martins-Praxair, Sertãozinho, SP, Brazil) was used in all ICP OES measurements.GFAAS (iCE 3000 Series, Thermo Scientific, Waltham, MA, USA) was used for Pb determination. The heating program (Table 1) for Pb determination was used under the recommended conditions provided by the manufacturer. For each measurement the autosampler of the GFAAS collected 15 μL of the sample or reference solution along with 5 μL of the modifier solution (a mixture of magnesium nitrate and ammonium dihydrogen phosphate), so this mixture was introduced into the graphite tube. Preliminary tests in ICP OES demonstrate high values of recuperation indicating possible spectral interferences arising due to the sample complexity. Graphite AAS and the mixture of chemical modifiers such as magnesium nitrate and ammonium dihydrogen phosphate were applied to overcome these problems. All measurements of integrated absorbance were made in triplicate.
| Step | Temp (°C) | Time (s) | Ramp (°C s−1) | Gas flow (L min−1) |
|---|---|---|---|---|
| 1 | 100 | 30 | 10 | 0.2 |
| 2 Pyrolysis | 800 | 20 | 150 | 0.2 |
| 3 Atomisation | 1200 | 3 | 0 | Off |
| 4 | 2500 | 3 | 0 | 0.2 |
The samples were also digested using a microwave oven (Speedwave four, Berghof) furnished with 12 digestion vessels (TFM™-PTFE) with an internal volume of 100 mL (DAP-100+). An analytical balance (model AY 220, max. 220 g, 0.1 mg resolution, Shimadzu, Kyoto, Japan) was used for sample preparation.
2.3 Sample preparation
Samples of powdered eye shadows (made in Brazil, China and USA) were purchased in a local market and analysed. Two sample groups were selected: cosmetics for adults and children. The prices of these samples ranged from US$ 3.00 to US$ 20.00.The samples were accurately weighed and were mineralised in a block digester. In the mineralization, 100 mg of the eye shadow sample was weighed in PFA tubes, followed by the addition of 5 mL of HNO3 (2 mol L−1), 2 mL of H2O2 (30% w/w) and 1 mL of Triton X-100 (5% w/v). The tubes were closed, the mixture was heated at 100 °C for 180 min and the solutions were quantitatively transferred to polypropylene flasks and diluted with water to 15 mL. The mineralization was made in triplicate with and without the addition of a standard to verify the accuracy of the analytical method and to detect possible losses of analytes during sample preparation. The final concentrations added were 40, 40, 700, 700, 200 and 80 μg L−1 for Cd, Co, Cr, Cu, Ni and Pb, respectively.
A sample of black color was used for microwave digestion with HF. Two hundred mg of each eye shadow sample was weighed into a Teflon (DAP 100) vessel and 6 mL of concentrated HNO3, 2 mL of H2O2 (30%) and 2 mL of concentrated HF were added to it.6 After cooling, the samples were transferred to 50 mL volumetric flasks, 24 mL of H3BO3 (4%) was added and the flasks were filled up to the required volume with deionized water. The microwave oven heating program performed was composed of two steps: (1) 5 minutes ramp (800 W), 5 minutes holding time (800 W); (2) 5 minutes ramp (1800 W), 30 minutes holding time (1800 W). The maximum temperature and pressure for steps 1 and 2 were 180 °C and 70 bar and 210 °C and 70 bar, respectively.
3 Results and discussion
3.1 Optimisation of the sample preparation
The sample preparation was optimised with the help of a full factorial design (23 = 8 experiments). The variables studied were HNO3 concentration (2 or 7 mol L−1), sample mass (150 or 250 mg) and heating time (1 or 3 hours). Sample mass and HNO3 variables were studied in order to identify the best compromise condition for low dilution factor and acidity. As the system (digester block) has no pressure control, we tried to achieve a suitable sample preparation by controlling the heating time. In this case, this variable was also studied. The objective was to identify the best condition for sample mineralisation. In all of the experiments, 2 mL of 30% w/w H2O2 and 1 mL of Triton X-100 (5% w/v) were added to the eye shadow.Triton X-100 was used as a surfactant because it was observed that the eye shadows remained on the solution surfaces due to the small particle size and a high value of surface water tension, and the surfactant helps with homogenization.22
The analyses were performed in axial and radial modes and a total of 12 responses were obtained for each experiment.
As several responses were analysed simultaneously, an important aspect is how to combine these data. In this case, the desirability function23 was used, and the signals were normalised between 0 (the lowest signals) and 1 (the highest signals). Eqn (1) shows how this transformation was performed:
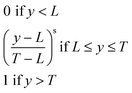 | (1) |
After the calculation of the individual desirability values (di), it is possible to combine the results and obtain the global desirability (D), calculated using the geometric mean (eqn (2)):
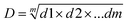 | (2) |
The global desirability (D) values can be viewed in Table 2. As can be observed, the best working conditions (values near 1) for axial mode (D1) were obtained in experiment 5 (0.95), while for radial mode (D2), the best result was obtained in experiment 8 (0.96). However, the goal was to identify a commitment condition for both modes. Thus, a new calculation of the geometric mean was made using the global desirability values of each mode 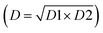 .
.
| Experiments | Desirability values | ||
|---|---|---|---|
| Axial (D1) | Radial (D2) | Axial and radial (D) | |
| 1 | 0.62 | 0.64 | 0.63 |
| 2 | 0.44 | 0.47 | 0.46 |
| 3 | 0.88 | 0.83 | 0.85 |
| 4 | 0.71 | 0.77 | 0.74 |
| 5 | 0.95 | 0.89 | 0.92 |
| 6 | 0.85 | 0.88 | 0.86 |
| 7 | 0.93 | 0.93 | 0.93 |
| 8 | 0.90 | 0.96 | 0.93 |
After performing the new global desirability calculation, it was observed that the experiments 5, 7 and 8 showed the best conditions with very similar desirability values (ranging from 0.92 to 0.93). The acid concentration used in the experiments 7 and 8 was 7 mol L−1, while in experiment 5 it was 2 mol L−1. Thus, the acid concentration was a decisive parameter, and the experimental condition presented in experiment 5 (2 mol L-1) was selected as the ideal. In addition the advantages of the use of dilute acid, which require lower dilution for analysis by ICP OES, have been reported. Table 3 shows the final conditions of the sample preparation.
| Parameters | Conditions |
|---|---|
| Heating time | 3 hours |
| Temperature | 100 °C |
| Volume of H2O2 (30% m/v) | 2 mL |
| Sample mass | 250 mg |
| Volume and concentration of HNO3 | 5 mL (2 mol L−1) |
| Volume of Triton X-100 | 1 mL (5% m/v) |
3.2 Analytical performance parameters
The ICP OES parameters were studied using a fractional factorial design (29−5), requiring 16 experiments to study 9 variables simultaneously. A 1 mg L−1 aqueous multi-element solution was used. Table 4 shows the variables and the working conditions.24 The determination of Pb was performed with GFAAS due to the interference of Fe in the most intense emission lines and the lack of adequate sensitivity in the analysis with ICP OES.| Variables | Conditions |
|---|---|
| V1: Integration time for low emission line (s) | 5 |
| V2: Integration time for high emission line (s) | 5 |
| V3: Sample introduction flow rate (mL min−1) | 4.2 |
| V4: Sample flow rate during the analyses (mL min−1) | 2.1 |
| V5: Pump stabilisation time (s) | 25 |
| V6: Radio frequency applied power (W) | 1200 |
| V7: Auxiliary gas flow rate (L min−1) | 0.25 |
| V8: Nebulisation gas flow rate (L min−1) | 0.83 |
| V9: Cooling gas flow rate (L min−1) | 16 |
After optimising the conditions of sample preparation and instrumental parameters of the ICP OES, the analytical performance parameters were evaluated. Table 5 shows the limits of detection and quantification, linearity, sensitivity and precision to axial and radial views in ICP OES and GFAAS (Pb determination).
| Parameters | Cd 228.802 nm | Co 228.616 nm | Cr 357.869 nm | Cu 224.700 nm | Ni 341.476 nm | Pba 283.3 nm |
|---|---|---|---|---|---|---|
| a GFAAS. b Radial view. | ||||||
| Calibration curve | y = 7.3x + 10.4 | y = 5.03x + 6.25 | y = 14.6x + 5.9 | y = 2.7x + 16.8 | y = 3.7x + 2.6 | y = 0.004x + 0.008 |
| y = 1.03x + 1.28b | y = 0.7x + 0.6b | y = 0.9x + 0.6b | y = 0.3x + 1.8b | y = 0.3x + 0.1b | ||
| Linearity (μg L−1) | 2.5–80 | 5–1500 | 5–1500 | 5–1500 | 5–1500 | 5–120 |
| Regression coefficient (r) | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| LOD (mg kg−1) | 0.03 | 0.06 | 0.06 | 0.08 | 0.3 | 0.02 |
| 0.2b | 0.2b | 0.8b | 0.8b | 0.9b | ||
| LOQ (mg kg−1) | 0.1 | 0.2 | 0.2 | 0.3 | 0.9 | 0.06 |
| 0.7b | 0.7b | 3b | 3b | 3b | ||
| Precision (%) n = 10 | 0.2 | 0.2 | 0.3 | 0.2 | 0.1 | 0.9 |
| 0.9b | 0.7b | 0.7b | 0.4b | 0.8b | ||
Particularly for the calculation of limits of detection in ICP OES, the concept of background equivalent concentration (BEC), defined as the concentration of the analyte that produces a signal equivalent to the emission intensity of the background at the spectral line measured, was used. The incorporation of BEC values in the calculation of LOD allows the evaluation of any change in operational conditions.25 The LOD and LOQ for the GFAAS were calculated from 10 independent blank samples measured once each in accordance with IUPAC recommendations.26
The precision was evaluated in terms of repeatability and the relative standard deviations (RSDs) of 10 measurements of a multielement solution were determined with a concentration of 40 μg L−1 for Cd, 50 μg L−1 for Pb and 250 μg L−1 for Co, Cr, Cu and Ni. The precision for each element is adequate according to the standards of the acceptability criterion set by INMETRO (Instituto Nacional de Metrologia) which establishes an acceptable relative standard deviation of up to 10% for concentrations above 100 ng g−1.27 The linearity was confirmed in the working range of each element, and the correlation coefficients found were equal to or exceeded 0.99.
The methods with analytical performance parameters are adequate to determine these elements in eye shadow samples and meet the limits required by legislations.7,9
Certified reference materials similar to eye shadows were not available; thus, the validity of the proposed method was verified by addition–recovery studies and by comparing the proposed method with the conventional acid digestion procedure performed with a microwave oven (closed vessel system) and HF.
The results, obtained from the proposed method and the microwave-assisted digestion procedure (Table 6), were compared using the paired t test. The sample used was the same employed in all optimisation studies. The results obtained by both procedures for all elements were in concordance at the 95% confidence level. The values of Cd were lower than the LOQ (see details in Table 5).
| Cd | Co | Cr | Cu | Ni | Pb | |
|---|---|---|---|---|---|---|
| Concentration by the proposed method (mg kg−1) | <LOQ | 3.8 ± 0.1 | 30.4 ± 0.3 | 39 ± 1 | 19.5 ± 0.6 | 10 ± 1 |
| Concentration by the comparative method (mg kg−1) | <LOQ | 3.7 ± 0.2 | 38 ± 3 | 42 ± 1 | 16.8 ± 0.9 | 8.3 ± 0.7 |
Recovery rates between 80% and 120% were obtained (Table 7); these findings are considered according to FDA7 (which requires recoveries between 80% and 120% for concentrations above 1 μg g−1). Thus, the results of the performance parameters studied confirmed quality assurance when using the proposed method for the determination of Cd, Co, Cr, Cu, Ni and Pb in eye shadow samples.
| Concentration (mg kg−1) | Recovery (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Origin country | Cd | Co | Cr | Cu | Ni | Pb* | Cd | Co | Cr | Cu | Ni | Pb* | |
| Children | 1-Purple | China | 0.13 ± 0.01 | 0.29 ± 0.02 | 1.07 ± 0.01 | <LOQ | <LOQ | 1.34 ± 0.08 | 94 | 81 | 85 | — | — | 115 |
| 2-Green | China | <LOQ | 0.32 ± 0.02 | 0.78 ± 0.06 | 0.74 ± 0.01 | <LOQ | 0.94 ± 0.05 | — | 83 | 85 | 90 | — | 119 | |
| 3-Blue | China | <LOQ | 0.34 ± 0.04 | 1.39 ± 0.09 | <LOQ | <LOQ | 0.99 ± 0.04 | — | 85 | 83 | 67 | — | 114 | |
| 4-Blue | China | <LOQ | 0.37 ± 0.02 | 1.06 ± 0.01 | 0.9 ± 0.3 | <LOQ | 1.00 ± 0.03 | — | 83 | 91 | 85 | — | 115 | |
| 5-Red | China | <LOQ | <LOQ | 2.5 ± 0.2 | 1.1 ± 0.1 | <LOQ | 7.7 ± 0.2 | — | — | 90 | 86 | — | 100 | |
| 6-Orange | China | 4.9 ± 0.2 | <LOQ | 3.5 ± 0.1 | 1.1 ± 0.2 | <LOQ | 44 ± 2 | 89 | — | 89 | 83 | — | 120 | |
| 7-Pink | China | <LOQ | 0.29 ± 0.01 | 0.65 ± 0.03 | 0.4 ± 0.2 | <LOQ | 0.46 ± 0.04 | — | 80 | 88 | 83 | — | 86 | |
| 8-Yellow | China | <LOQ | 0.47 ± 0.02 | 1.8 ± 0.1 | 0.9 ± 0.3 | <LOQ | 0.50 ± 0.06 | — | 100 | 98 | 103 | — | 103 | |
| 9-Blue | China | 6.0 ± 0.1 | <LOQ | 0.89 ± 0.03 | 48.2 ± 0.9 | <LOQ | 34 ± 4 | 89 | — | 101 | 105 | — | 105 | |
| 10-Pink | China | 4.6 ± 0.1 | <LOQ | 1.05 ± 0.05 | 0.48 ± 0.03 | <LOQ | 26 ± 2 | 87 | — | 102 | 90 | — | 97 | |
| 11-Black | China | <LOQ | 3.88 ± 0.09 | 44 ± 3 | 16.7 ± 0.6 | 23.1 ± 0.5 | 1.36 ± 0.08 | — | 92 | 91 | 90 | 103 | 81 | |
| 12-Blue | China | <LOQ | 2.4 ± 0.4 | 2.3 ± 0.3 | 4.6 ± 0.4 | <LOQ | 1.54 ± 0.06 | — | 80 | 94 | 98 | — | 94 | |
| 13-Pink | China | <LOQ | 1.47 ± 0.04 | 1.75 ± 0.06 | 3.2 ± 0.5 | <LOQ | 1.59 ± 0.07 | — | 91 | 92 | 98 | — | 95 | |
| 14-Gold | China | <LOQ | 1.22 ± 0.05 | 11.5 ± 0.7 | 15.3 ± 0.8 | 3.4 ± 0.1 | 2.25 ± 0.05 | — | 86 | 92 | 87 | 95 | 95 | |
| Adults | 15-Black | China | <LOQ | 4.9 ± 0.2 | 62.7 ± 0.9 | 21.1 ± 0.7 | 10.4 ± 0.3 | 1.05 ± 0.09 | — | 90 | 97 | 102 | 98 | 86 |
| 16-Black | China | <LOQ | 1.8 ± 0.2 | 2.4 ± 0.2 | <LOQ | 2.8 ± 0.9 | 2.0 ± 0.3 | — | 91 | 91 | 116 | 101 | 93 | |
| 17-Black | Brazil | <LOQ | 3.8 ± 0.5 | 28 ± 4 | 36 ± 1 | 19.5 ± 0.5 | 0.09 ± 0.01 | — | 89 | 91 | 94 | 92 | 91 | |
| 18-Black | Brazil | <LOQ | 3.5 ± 0.7 | 29 ± 1 | 37 ± 2 | 19.6 ± 0.8 | 0.58 ± 0.05 | — | 93 | 96 | 96 | 100 | 117 | |
| 19-Black | USA | <LOQ | 3.45 ± 0.09 | 12.8 ± 0.4 | 39.1 ± 0.7 | 4.9 ± 0.2 | <LOQ | — | 80 | 89 | 81 | 95 | — | |
| 20-Black | USA | <LOQ | 3.4 ± 0.1 | 91 ± 1 | 26.5 ± 0.6 | 16.0 ± 0.4 | 0.78 ± 0.06 | — | 92 | 90 | 94 | 93 | 86 | |
| 21-Blue | China | 0.39 ± 0.01 | 1.02 ± 0.05 | 1.0 ± 0.1 | 61 ± 8 | <LOQ | 1.74 ± 0.07 | 103 | 103 | 101 | 101 | — | 96 | |
| 22-Blue | China | 0.41 ± 0.01 | 0.80 ± 0.07 | 0.5 ± 0.2 | <LOQ | <LOQ | 1.22 ± 0.04 | 106 | 110 | 96 | — | — | 82 | |
| 23-Blue | Brazil | 0.9 ± 0.1 | <LOQ | <LOQ | <LOQ | <LOQ | 1.8 ± 0.2 | 93 | — | — | — | — | 85 | |
| 24-Blue | Brazil | <LOQ | 1.85 ± 0.07 | 31 ± 1 | 10.0 ± 0.4 | 3.2 ± 0.3 | 1.09 ± 0.09 | — | 91 | 93 | 104 | 92 | 94 | |
| 25-Blue | USA | <LOQ | 2.2 ± 0.09 | 21.8 ± 0.9 | 9.7 ± 0.4 | 10.0 ± 0.4 | 1.51 ± 0.08 | — | 87 | 88 | 82 | 87 | 84 | |
| 26-Orange | China | <LOQ | 0.8 ± 0.1 | 17.2 ± 0.7 | 9.2 ± 0.5 | 3.3 ± 0.3 | 1.84 ± 0.07 | — | 100 | 95 | 98 | 96 | 90 | |
| 27-Orange | China | 0.36 ± 0.02 | 0.58 ± 0.03 | 1.73 ± 0.09 | 0.90 ± 0.08 | 0.73 ± 0.06 | 3.3 ± 0.2 | 83 | 103 | 98 | 108 | 99 | 93 | |
| 28-Orange | Brazil | 0.41 ± 0.02 | 0.37 ± 0.04 | 3.0 ± 0.2 | 7.5 ± 0.5 | 2.9 ± 0.2 | 0.59 ± 0.06 | 80 | 80 | 85 | 98 | 80 | 86 | |
| 29-Orange | Brazil | <LOQ | 0.75 ± 0.01 | 2.4 ± 0.1 | <LOQ | 1.23 ± 0.07 | 0.10 ± 0.02 | — | 84 | 87 | — | 88 | 89 | |
| 30-Orange | USA | <LOQ | 0.88 ± 0.04 | 7.4 ± 0.1 | <LOQ | 16.9 ± 0.3 | 1.3 ± 0.2 | — | 91 | 89 | — | 89 | 108 | |
| 31-Pink | China | <LOQ | 0.36 ± 0.02 | 1.7 ± 0.1 | 1.14 ± 0.06 | 1.8 ± 0.5 | 3.21 ± 0.09 | — | 83 | 94 | 99 | 98 | 104 | |
| 32-Pink | China | <LOQ | 0.10 ± 0.02 | 0.81 ± 0.07 | <LOQ | <LOQ | 3.40 ± 0.09 | — | 87 | 89 | — | — | 90 | |
| 33-Pink | Brazil | <LOQ | 0.15 ± 0.03 | 3.0 ± 0.6 | 21 ± 3 | <LOQ | 0.33 ± 0.09 | — | 97 | 82 | 107 | — | 95 | |
| 34-Pink | Brazil | <LOQ | <LOQ | 2.1 ± 0.2 | <LOQ | <LOQ | 2.1 ± 0.2 | — | — | 94 | — | — | 86 | |
| 35-Pink | USA | <LOQ | 0.44 ± 0.02 | 1.00 ± 0.07 | <LOQ | <LOQ | 1.4 ± 0.1 | — | 86 | 89 | — | — | 107 | |
3.3 Determination of Cd, Co, Cr, Cu, Ni and Pb in the eye shadow samples
Table 7 shows the concentration values for the analytes in the samples. Among the 14 child eye shadow samples, 5 had concentrations above those permitted by the legislation. Samples 6 and 9 had higher concentrations than allowed for Cd (ca. 5 mg kg−1) and Pb (ca. 30 mg kg−1). Sample number 10 presented concentrations near the allowed ones for Cd and Pb. Sample 11 showed concentration above advisable levels (5 mg kg−1) for Cr (44 mg kg−1) and Ni (23.1 mg kg−1) and in sample 14, the concentration exceeded the recommended concentration for Cr (11.5 mg kg−1). For adult eye shadow samples, 9 among 21 samples presented concentrations above the tolerable value for Cr (samples 15, 17, 18, 19, 20, 24, 25, 26 and 30) and 6 samples for Ni (samples 15, 17, 18, 20, 25 and 30).7,9To analyse the behaviour of the samples in relation to the levels of the elements, PCA (Principal Component Analysis) was performed on a 104 × 11 data matrix that contained samples with three authentic replicates in the rows (except for the sample 16, which contains two authentic replicates) and the analytes in the columns, which were determined in the axial and radial modes (except for Pb). The data were auto-scaled and Pirouette version 4.5 was used in the calculation (Infometrix, Bothell, USA).
A model with 4 principal components (PCs) was selected to evaluate the behaviour of the samples and explained 96% of the total variance. The results are shown in Fig. 1 as graphical representations of the scores (related to samples, Fig. 1a) and loadings (related to the analyte, Fig. 1b).
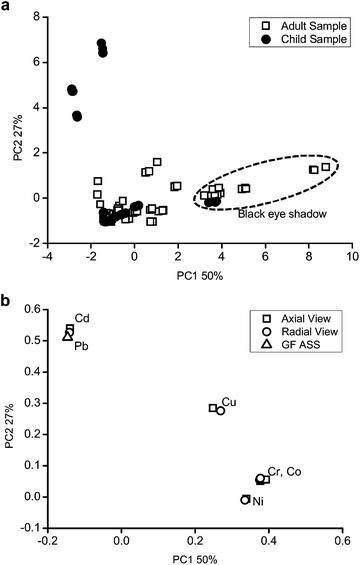 | ||
| Fig. 1 Graphical representation of the (a) scores and (b) loadings of PC1 × PC2 in relation to element concentrations presented in Table 7. | ||
The authentic replicates and data obtained from the radial and axial views are very close, showing the low standard deviation in the measurements, but it was not possible to see the separation between the child and adult samples in the figure.
If the scores (Fig. 1a) are analysed with the graph of loadings (Fig. 1b), it is possible to see that some child samples have high values of Cd and Pb. These samples have values which are above those recommended by the legislation.
It is also possible to observe the separation of the samples with respect to the black color. The black color samples (see dotted line ellipses) have higher concentrations of Co, Cr, Cu and Ni.
4 Conclusions
A simple and fast analytical method for the determination of Cd, Co, Cr, Cu, Ni and Pb in eye shadow samples, using diluted HNO3 (2 mol L−1) for the sample preparation, has been proposed. It was possible to optimise the mineralisation procedure for samples of eye shadow using the experimental design and the results were compared with a microwave-assisted digestion procedure. Despite what is proposed in the literature, that this type of sample is digested with concentrated acids, it was possible to obtain good results using an acid concentration of 2 mol L−1.10,13Under the optimised conditions of sample preparation and instrumental parameters of the ICP OES and GFAAS, it was possible to determine the Cd, Co, Cr, Cu, Ni and Pb concentrations in the eye shadow samples from different brands, consumers, colors and countries and, by employing chemometric tools, observing separation of the samples according to the concentration levels of the elements.
The analyte concentrations found in some samples were above the values recommended for both adult and child samples. These results confirm the importance of quality control in the production of cosmetics as well as the applicability of the proposed method.
Acknowledgements
This work was supported by the São Paulo Research Foundation (FAPESP) (grants 2014/04251-0, 2012/10680-6, 2012/01769-3 and 2012/50827-6), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, process 474357/2012-0), and CAPES-Embrapa and Thermo Scientific – Analítica for the instruments.References
- Industrial Development Agency of Brazilian, III Notebook trends 2014/2015, http://www.abdi.com.br/Estudo/III%20Caderno%20de%20Tend%C3%AAncias%202014-2015.pdf, (accessed May 2014).
- A. D. Hardy, R. I. Walton, R. Vaishnav, K. A. Myers, M. R. Power and D. Pirrie, in Physical Techniques in the Study of Art, Archaeology and Cultural Heritage, ed. D. Bradley and D. Creagh, Elsevier, Amsterdam, 1st edn, 2006, vol. 1, ch. 5, pp. 173–202 Search PubMed.
- Brazilian Health Surveillance Agency, http://portal.anvisa.gov.br/wps/wcm/connect/dfa9b6804aee482bb7a1bfa337abae9d/Resolu%C3%A7%C3%A3o+RDC+n%C2%BA+211%2C+de+14+de+julho+de+2005.pdf?MOD=AJPERES, (accessed June 2014).
- U.S. Food and Drug Administration, http://www.fda.gov/Cosmetics/GuidanceComplianceRegulatoryInformation/ucm074201.htm, (accessed June 2014).
- E. L. Sainio, R. Jolanki, E. Hakala and L. Kanerva, Contact Dermatitis, 2000, 42, 5 CAS.
- C. Contado and A. Pagnoni, Sci. Total Environ., 2012, 432, 173 CrossRef CAS PubMed.
- U.S. Food and Drug Administration, http://www.fda.gov/ForIndustry/ColorAdditives/ColorAdditivesinSpecificProducts/InCosmetics/ucm130125.htm, (accessed June 2014).
- Brazilian Health Surveillance Agency, http://www.anvisa.gov.br/cosmeticos/guia/html/79_2000.pdf ( accessed May 2014).
- Health Canada. Guidance on heavy metal impurities in cosmetics, http://www.hc-sc.gc.ca/cps-spc/pubs/indust/heavy_metals-metaux_lourds/index-eng.php, (accessed May 2014).
- C. Capelli, D. Foppiano, G. Venturelli, E. Carlini, E. Magi and C. Ianni, Anal. Lett., 2014, 47, 1201 CrossRef CAS.
- M. A. Z. Arruda and R. E. Santelli, Quim. Nova, 1997, 20, 638 CAS.
- P. Piccinini, M. Piecha and S. F. Torrent, J. Pharm. Biomed. Anal., 2013, 76, 225 CrossRef CAS PubMed.
- M. G. Volpe, M. Nazzaro, R. Coppola, F. Rapuano and R. P. Aquino, Microchem. J., 2012, 101, 65 CrossRef CAS PubMed.
- American Chemical Society, http://www.acs.org/content/acs/en/greenchemistry/what-is-green-chemistry/principles/12-principles-of-green-chemistry.html, (accessed April 2014).
- J. A. Nobrega, L. C. Trevizan, G. C. L. Araujo and A. R. A. Nogueira, Spectrochim. Acta, Part B, 2002, 57, 1855 CrossRef.
- C. A. Bizzi, J. S. Barin, E. E. Garcia, J. A. Nóbrega, V. L. Dressler and E. M. M. Flores, Spectrochim. Acta, Part B, 2011, 66, 394 CrossRef CAS PubMed.
- J. T. Castro, E. C. Santos, W. P. C. Santos, L. M. Costa, M. Korn, J. A. Nobrega and M. G. A. Korn, Talanta, 2009, 78, 1378 CrossRef CAS PubMed.
- N. J. Rossi and K. G. Unfried, Met. Finish., 1997, 16 CrossRef CAS.
- I. Lavilla, N. Cabaleiro, M. Costas, I. de la Calle and C. Bendicho, Talanta, 2009, 80, 109 CrossRef CAS PubMed.
- G. C. L. Araujo, M. H. Gonzalez, A. G. Ferreira, A. A. Nogueira and J. A. Nobrega, Spectrochim. Acta, Part B, 2002, 57, 2121 CrossRef.
- B. Barros Neto, I. S. Scarminio and R. E. Bruns, in Como Fazer Experimentos: Pesquisa e Desenvolvimento na Ciência e na Indústria, ed B. Barros Neto and Bookman, São Paulo, 2nd edn, 2010, ch. 1, pp. 1–7 Search PubMed.
- N. Maniasso, Quim. Nova, 2001, 24, 87 CrossRef CAS PubMed.
- G. Derringer and R. Suich, J. Qual. Tech., 1980, 12, 214 Search PubMed.
- A. S. Augusto, E. F. Batista and E. R. Pereira-Filho, BrJAC--Braz. J. Anal. Chem., 2014 Search PubMed , Accepted for publication.
- D. Schiavo, L. C. Trevizan, E. R. Pereira-Filho and J. A. Nóbrega, Spectrochim. Acta, Part B, 2009, 64, 544 CrossRef PubMed.
- IUPAC. International Union of Pure and Applied Chemistry, http://iupac.org/publications/analytical_compendium/, (accessed April 2014).
- INMETRO, Instituto Nacional de Metrologia, Normalização de Qualidade, Orientação sobre Validação de Métodos Analíticos, http://www.inmetro.gov.br/sidoq/arquivos/Cgcre/DOQ/DOQ-Cgcre-8_04.pdf, (accessed April 2014).
| This journal is © The Royal Society of Chemistry 2015 |
