Three-dimensionalization of ultrathin nanosheets in a two-dimensional nano-reactor: macroporous CuO microstructures with enhanced cycling performance†
Chuan-Yin
Jin
a,
Ming
Hu
a,
Xun-Liang
Cheng
a,
Fan-Xing
Bu
a,
Li
Xu
a,
Qing-Hong
Zhang
b and
Ji-Sen
Jiang
*a
aDepartment of Physics, Center for Functional Nanomaterials and Devices, East China Normal University, Shanghai, 200241, P.R. China. E-mail: jsjiang@phy.ecnu.edu.cn
bEngineering Research Center of Advanced Glasses Manufacturing Technology, MOE, Donghua University, Shanghai 201620, P.R. China
First published on 4th November 2014
Abstract
Three-dimensional (3D) macroporous CuO structures composed of ultrathin nanosheets were successfully synthesized by employing a liquid–liquid interface as a two-dimensional (2D) nano-reactor. The macroporous structure helped CuO to retain the exposed surface during reactions, thus significantly enhancing the long term cycling performance both in photocatalysis and lithium ion batteries.
Since the discovery of graphene,1,2 two-dimensional ultrathin nanosheets/flakes have attracted great interest owing to the excellent size-/shape dependent properties of nanosheets.3 Because of the large specific surface area and exposed surfaces,4 ultrathin nanosheets can interact with active species very well, or allow the insertion of external ions efficiently.4a,5 Such superiorities make the nanosheets to be great candidates in applications such as hetero-catalysis and energy storage.6
Copper oxide (CuO) has been widely utilized in various applications such as photocatalysis,7 adsorption,8 gas sensing9 and in lithium ion batteries.10 In recent years, CuO nanosheets have been synthesized, and showed enhanced performance in photocatalysis11 and electrochemical application.12 However, two main problems exist currently. Firstly, the CuO nanosheets are generally thicker than 20 nm, indicating that the nanosheets contain too many layers. Thick nanosheets have longer diffusion pathway perpendicular to the facet, and smaller accessible surface area, thus weaken the performance of CuO nanosheets in catalysis or energy storage. Secondly, agglomeration of nanosheets is hard to be avoided. The agglomeration causes the nanosheets to stack up seriously, reduces the chance of external species to interact with nanosheets, leading to low catalytic activity, or poor cycling capability.
To solve these problems, it is important to find a way to synthesize ultra-thin CuO nanosheets and organize them into a three-dimensional (3D) structure.13 Three-dimensionalization was considered to be an efficient way to improve the performance of nanosheets in applications. For example, Worsley et al.13a synthesized sp2-cross-linked three-dimensional graphene monoliths with high surface area, which exhibited extraordinarily high surface area and large pore volume, while maintaining the high conductivity observed in the resorcinol formaldehyde-derived graphene aerogels. Chen et al.13b reported a direct synthesis of 3D foam-like graphene macrostructures, which showed high electrical conductivity. In these cases, the nanosheets were well-supported, and a large fraction of the surface of nanosheets was exposed, thus good physicochemical properties of the material could be well maintained. Yin et al. synthesized 3D nanosheet-based flocculus-like hierarchical CuO nanostructures in solution, the thicknesses of the nanosheets were approximately 88.7 nm and 240 nm.14
Herein, we utilized a confined 2D space, the interface between immiscible liquids, as a nano-reactor. The nano-reactor could work as a template to confine the thickness of nanosheets. Moreover, we deduced that the obtained nanosheets could self-assemble in a 3D way in such confined space. We expected that 3D ultra-thin CuO nanosheets could show superior long-term activity over either commercial CuO or non-organized nanosheets in hetero-catalysis as well as electrochemical energy storage.
In a typical procedure, a liquid interface reactor was established by placing water above hydrophobic liquid, dichloromethane, as shown in Scheme 1. The height of the reactor is estimated to be about 1 nm.15 As shown in Scheme 1a, cupric acetylacetonate was dissolved in the dichloromethane phase, while sodium hydroxide was dissolved in the water phase. Then, the reaction between cupric ions and hydroxide ions was forced to be within the liquid–liquid interface which also meant that the generation of CuO was confined in this limited space. Digital photos taken from the reaction system before and after reaction were shown in Scheme 1b. The black area in the right side of Scheme 1b illustrated the formation of CuO inside the liquid–liquid interface. As the thicknesses of the interfaces can be modulated by using different types of solvents, or by changing the temperature of the reaction system,16 such an approach may open up a general way to prepare 3D structures composed of ultrathin nanosheets with different thicknesses.
 | ||
| Scheme 1 (a) Scheme of the 2D nano-reactor established at the interface of immiscible liquids; (b) photo of the reaction system before and after reaction. | ||
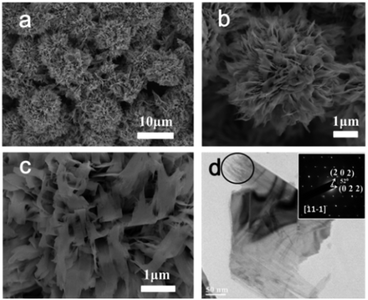
Fig. S1 (ESI†) shows the XRD pattern of the as-prepared sample. All the peaks can be assigned as monoclinic CuO (JCPDS file no. 48-1548, space group C2/c). No additional peak was detected, suggesting the purity of the as-prepared CuO sample. Fig. 1a is the SEM image of the as-prepared CuO product, from which numerous 3D flower-like macroporous spheres were clearly observed. These structures are of a mean size of around 7−8 μm. The low-magnified FESEM image (Fig. S2, ESI†) clearly reveals a high yield of the 3D flower-like macroporous spheres. Fig. 1b shows that the 3D structures are build up by nanosheets. The nanosheets are organized together, and support each other with almost all the surfaces exposed. The mean thickness of these nanosheet building blocks is of 6.42 ± 1.05 nm (Fig. S3, ESI†) according to the SEM image, which are much thinner than reported previously17 (Table S1, ESI†). The SEM image shown in Fig. 1c indicates that an individual microstructure is relatively robust after the sample preparation procedure. The pore size of the structure is between 100 and 400 nm. Fig. 1d is the TEM image of a non-organized nanosheet from the broken structures. The corner of the nanosheet contains some whiskers (inside the circle in Fig. 1d), suggesting that the nanosheets may be assembled by nanowires. The corresponding SAED pattern (Fig. 3d inset) of the nanosheet is constituted of periodic spots, indicating that the nanosheet is single crystalline. The projection can be indexed to the [11−1] zone axis, indicating that the exposed surface is (11−1).
Nitrogen sorption analysis was performed to investigate the specific surface area and porosity of such 3D macroporous CuO structures (Fig. S4, ESI†). Brunauer–Emmett–Teller (BET) surface area of the 3D macroporous CuO structures was calculated to be 14 m2 g−1 based on the desorption curve. The hysteresis loop from P/P0 = 0.6 to P/P0 = 1 suggests the existence of macroporous, which is in consistence with the shape of 3D CuO nanosheets observed by SEM.
Time-dependent experiment illustrates that the generation of 3D macroporous CuO structures is a multiple-step process, from Cu(OH)2 nanorods to 3D CuO structures. Fig. S5 and S6 (ESI†) present the XRD profiles and SEM images of the samples taken at different reaction periods, respectively. Cu(OH)2 nanorods were generated at 15 min, while a minor amount of CuO co-existed. After two hours, the nanorods aggregated together, and the main composition was Cu(OH)2 still. The morphology of the sample changed significantly when the reaction time was 6 h.
The 3D macroporous CuO structures became the main product, while more CuO was generated. When the time reached 10 h, Cu(OH)2 dismissed, while CuO was the only product. The 3D macroporous CuO structures formed from 6 h was retained after 12 h as shown in Fig. S6c and d, ESI.†
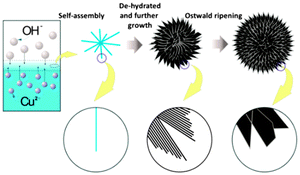
On the basis of the above multiple-step process, a possible mechanism was proposed as shown in Fig. 2. From the very beginning of the reaction, hydroxide ions in the water phase reacted with Cu(acac)2 in the oil phase at the liquid/liquid interface via ion diffusion. Cu(OH)2 was first formed after precipitation of Cu2+ and OH−. The shape of Cu(OH)2 was a typical nanorod which was common in previous reports owing to the principal crystal structure of Cu(OH)2.18 The limited space probably forced the nanorods to aggregate together to reduce the surface energy of nanorods. Subsequently, the 3D nanorods further fused together in an interesting way. The nanorods attached each other parallelly, and formed nanosheets. The self-assembly of nanorods to generate nanosheets could be supported by the TEM image (Fig. 1d). At the edge of nanosheets, whisker like shape could be observed. The driving force is probably the high surface energy of nanorods as well as the narrow reaction space in which concentrated species are located. The nanosheets retained the position and formed 3D nanosheets. In the meantime, Cu(OH)2 de-hydrated to generate CuO.
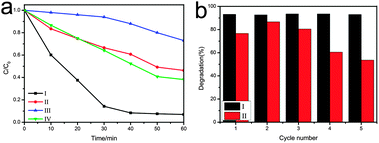
It has been reported that CuO can serve as an effective photocatalyst for the degradation of organic pollutants under visible light irradiation.7c,d,19 Herein, to qualitatively show the superiority of 3D macroporous CuO structures over non-organized nanosheets, commercial CuO or 3D structures composed of thicker sheets (Fig. S10, ESI†), we investigated photocatalytic decomposition of rhodamine B (RhB) under visible light irradiation. Fig. 3a shows the time-dependent degradation curves of RhB in the presence of CuO catalysts after five times cycling. The degradation of RhB catalyzed by 3D macroporous CuO structures, non-organized nanosheets and 3D structures composed of thicker sheets was faster than commercial CuO, suggesting that the nanosheets have higher catalytic activity. Better performance of 3D macroporous CuO structures than 3D structures composed of thicker sheets was observed, this could be attributed to the bigger surface area brought by the ultrathin nanosheets. Moreover, 3D macroporous CuO structures present an even higher efficiency than non-organized nanosheets during catalysis, which may come from the long term mesoscale structural stability of 3D macroporous CuO structures. Fig. 3b further confirms the long term catalytic activity of 3D nanosheets. The 3D macroporous CuO structures performed similar high activity during five times cycling test because the structures were maintained very well during cycling (Fig. S7, ESI†). However, the activity of non-organized nanosheets decayed significantly during five times tests. The SEM image of the samples taken after five times catalysis illustrated that non-organized nanosheets stack on each other (Fig. S8, ESI†). The non-organized nanosheets were well dispersed before catalysis (Fig. S9, ESI†). The reduced accessible surface of nanosheets is probably the reason that non-organized nanosheets performed worse than 3D nanosheets.
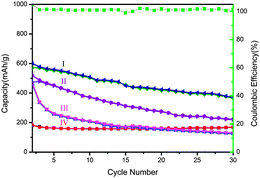
Not only in photocatalysis, the 3D macroporous CuO structures showed superior performance over 3D structures composed of thicker sheets, non-organized nanosheets but also as commercial CuO as the anode of lithium ion batteries. The cycling performance and coulombic efficiency of the 3D macroporous CuO structures at a rate of 0.1 C are shown in Fig. 4. 3D macroporous CuO structures can sustain 65% (390 mA h g−1) capacity of the 2nd cycle (600 mA h g−1) at a rate of 0.1 C after 30 cycles. The coulombic efficiency of the structures can sustain at about 100%. The relatively high reversible capacity could be attributed to its macroporous structure. The macroporous structure allows the nanosheets to interact with the electrolyte sufficiently, leading to a high discharging capacity. 3D structures composed of thicker sheets show lower capacity during cycling. The lower capacity of 3D CuO structures constituting of thick sheets could be attributed to the longer diffusion pathway perpendicular to the facet and smaller surface area. The non-organized nanosheets show even worse performance. The capacity reduced from 467 mA h g−1 to 130 mA h g−1, 72% percentage of capacity was lost. As the non-organized nanosheets would stack on each other easily, this could limit the chance of contact between the electrolyte and the surface of material, further decreasing the diffusion rate of ions in the battery. Commercial CuO shows lowest capacity because it processes no 3D structure or nanosheets when compared with the other samples. The stable cycling of commercial CuO probably comes from a better crystallinity. The XRD profiles of commercial CuO (Fig. S11, ESI†) suggest that the crystallinity of commercial CuO is better than our 3D CuO because higher and narrower diffraction peaks with smaller full width at half maximum (FWHM) values were observed at ((11−1) and (111)) of commercial CuO.
In summary, we have proposed an approach to fabricate 3D macroporous CuO structures composed of ultrathin nanosheets by utilization of an interface between immiscible liquids. The confined reaction space facilitated the generation of ultrathin nanosheets, and made them assemble into a 3D macroporous structure. Such a structure helped the nanosheets to keep exposed without stacking on each other during chemical reaction, and offered CuO superior long term activity both in photocatalysis and lithium ion batteries. Such an approach may open up a general way to prepare 3D structures composed of ultrathin nanosheets, and provide superior catalyst or energy storage materials with long term activity.
This work was supported by the National Natural Science Foundation of China (Grant No. 21173084) and the Large Instruments Open Foundation of East China Normal University.
Notes and references
- (a) K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed; (b) K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197 CrossRef CAS PubMed.
- (a) F. Zhang, C. Hou, Q. Zhang, H. Wang and Y. Li, Mater. Chem. Phys., 2012, 135, 826 CrossRef CAS PubMed; (b) R. Wang, C. Xu, J. Sun, L. Gao and C. Lin, J. Mater. Chem. A, 2013, 1, 1794 RSC; (c) M. Du, C. Xu, J. Sun and L. Gao, Electrochim. Acta, 2012, 80, 302 CrossRef CAS PubMed; (d) J. Chang, H. Xu, J. Sun and L. Gao, J. Mater. Chem., 2012, 22, 11146 RSC; (e) C. Xu, J. Sun and L. Gao, Nanoscale, 2012, 4, 5425 RSC; (f) S. Ding, J. S. Chen, D. Luan, F. Y. Boey, S. Madhavi and X. W. Lou, Chem. Commun., 2011, 47, 5780 RSC; (g) S. Ding, D. Luan, F. Y. Boey, J. S. Chen and X. W. Lou, Chem. Commun., 2011, 47, 7155 RSC; (h) X. Wang, Y. Zhang, C. Zhi, X. Wang, D. Tang, Y. Xu, Q. Weng, X. Jiang, M. Mitome, D. Golberg and Y. Bando, Nat. Commun., 2013, 4, 2905 Search PubMed; (i) Y. Zhang, K. Fugane, T. Mori, L. Niu and J. Ye, J. Mater. Chem., 2012, 22, 6575 RSC; (j) Y. Zhang, T. Mori, L. Niu and J. Ye, Energy Environ. Sci., 2011, 4, 4517 RSC.
- (a) D. Ma, G. Shi, H. Wang, Q. Zhang and Y. Li, Nanoscale, 2013, 5, 4808 RSC; (b) D. Ma, H. Wang, Q. Zhang and Y. Li, J. Mater. Chem., 2012, 22, 16633 RSC; (c) Q. Mu, Q. Zhang, H. Wang and Y. Li, J. Mater. Chem., 2012, 22, 16851 RSC; (d) D. Liu, X. Wang, X. Wang, W. Tian, J. Liu, C. Zhi, D. He, Y. Bando and D. Golberg, J. Mater. Chem. A, 2013, 1, 1952 RSC; (e) A. Pakdel, X. Wang, C. Zhi, Y. Bando, K. Watanabe, T. Sekiguchi, T. Nakayama and D. Golberg, J. Mater. Chem., 2012, 22, 4818 RSC; (f) X. Wang, C. Zhi, L. Li, H. Zeng, C. Li, M. Mitome, D. Golberg and Y. Bando, Adv. Mater., 2011, 23, 4072 CrossRef CAS PubMed; (g) C. Z. Yuan, L. Yang, L. R. Hou, L. F. Shen, X. G. Zhang and X. W. Lou, Energy Environ. Sci., 2012, 5, 7883 RSC; (h) S. H. Yang, X. F. Song, P. Zhang and L. Gao, ACS Appl. Mater. Interfaces, 2013, 5, 3317 CrossRef CAS PubMed; (i) Y. C. Jiang, S. D. Zhang, Q. Ji, J. Zhang, Z. P. Zhang and Z. Y. Wang, J. Mater. Chem. A, 2014, 2, 4574 RSC; (j) M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246 CrossRef CAS PubMed; (k) M. Hu, S. Ishihara and Y. Yamauchi, Angew. Chem., Int. Ed., 2013, 52, 1235 CrossRef CAS PubMed.
- (a) J. S. Chen, Y. L. Tan, C. M. Li, Y. L. Cheah, D. Luan, S. Madhavi, F. Y. C. Boey, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 6124 CrossRef CAS PubMed; (b) C. Hu, X. Zhang, W. T. Li, Y. Yan, G. C. Xi, H. F. Yang, J. F. Li and H. Bai, J. Mater. Chem. A, 2014, 2, 2040 RSC.
- (a) H. J. Zhang, Q. Q. He, F. J. Wei, Y. J. Tan, Y. Jiang, G. H. Zheng, G. J. Ding and Z. Jiao, Mater. Lett., 2014, 120, 200 CrossRef CAS PubMed; (b) R. J. Wei, J. C. Hu, T. F. Zhou, X. L. Zhou, J. X. Liu and J. L. Li, Acta Mater., 2014, 66, 163 CrossRef CAS PubMed; (c) Z. Sun, T. Liao, Y. Dou, S. M. Hwang, M. S. Park, L. Jiang, J. H. Kim and S. X. Dou, Nat. Commun., 2014, 5, 3813 Search PubMed; (d) M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263 CrossRef PubMed.
- (a) J. W. Sha, N. Q. Zhao, E. Z. Liu, C. S. Shi, C. N. He and J. J. Li, Carbon, 2014, 68, 352 CrossRef CAS PubMed; (b) Y. Zhao, K. Yao, Q. Cai, Z. J. Shi, M. Q. Sheng, H. Y. Lin and M. W. Shao, CrystEngComm, 2014, 16, 270 RSC; (c) F.-Y. Su, C. You, Y.-B. He, W. Lv, W. Cui, F. Jin, B. Li, Q.-H. Yang and F. Kang, J. Mater. Chem., 2010, 20, 9644 RSC; (d) S. Ding, J. S. Chen, D. Luan, F. Y. C. Boey, S. Madhavi and X. W. Lou, Chem. Commun., 2011, 47, 5780 RSC; (e) D. Rangappa, K. D. Murukanahally, T. Tomai, A. Unemoto and I. Honma, Nano Lett., 2012, 12, 1146 CrossRef CAS PubMed.
- (a) L. Huang, F. Peng, H. Yu and H. Wang, Solid State Sci., 2009, 11, 129 CrossRef CAS PubMed; (b) N. Mukherjee, B. Show, S. K. Maji, U. Madhu, S. K. Bhar, B. C. Mitra, G. G. Khan and A. Mondal, Mater. Lett., 2011, 65, 3248 CrossRef CAS PubMed; (c) J. Liu, J. Jin, Z. Deng, S. Z. Huang, Z. Y. Hu, L. Wang, C. Wang, L. H. Chen, Y. Li, G. Van Tendeloo and B. L. Su, J. Colloid Interface Sci., 2012, 384, 1 CrossRef CAS PubMed; (d) Y. Fan, R. Liu, W. Du, Q. Lu, H. Pang and F. Gao, J. Mater. Chem., 2012, 22, 12609 RSC.
- P. Tian, X. Y. Han, G. L. Ning, H. X. Fang, J. W. Ye, W. T. Gong and Y. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 12411 CAS.
- (a) X. Gou, G. Wang, J. Yang, J. Park and D. Wexler, J. Mater. Chem., 2008, 18, 965 RSC; (b) C. X. Wang, W. Zeng, H. Zhang, Y. Q. Li, W. G. Chen and Z. C. Wang, J. Mater. Sci.: Mater. Electron., 2014, 25, 2041 CrossRef CAS; (c) R. K. Bedi and I. Singh, ACS Appl. Mater. Interfaces, 2010, 2, 1361 CrossRef CAS PubMed.
- (a) J. Y. Xiang, J. P. Tu, L. Zhang, Y. Zhou, X. L. Wang and S. J. Shi, J. Power Sources, 2010, 195, 313 CrossRef CAS PubMed; (b) X. P. Gao, J. L. Bao, G. L. Pan, H. Y. Zhu, P. X. Huang, F. Wu and D. Y. Song, J. Phys. Chem. B, 2004, 108, 5547 CrossRef CAS; (c) J. C. Park, J. Kim, H. Kwon and H. Song, Adv. Mater., 2009, 21, 803 CrossRef CAS; (d) J. Wang, Y. Liu, S. Wang, X. Guo and Y. Liu, J. Mater. Chem. A, 2014, 2, 1224 RSC; (e) S. Yuan, X. L. Huang, D. L. Ma, H. G. Wang, F. Z. Meng and X. B. Zhang, Adv. Mater., 2014, 26, 2273 CrossRef CAS PubMed.
- L. J. Wang, Q. Zhou, Y. J. Liang, H. L. Shi, G. L. Zhang, B. S. Wang, W. W. Zhang, B. Lei and W. Z. Wang, Appl. Surf. Sci., 2013, 271, 136 CrossRef CAS PubMed.
- (a) Y. Liu, W. Wang, L. Gu, Y. Wang, Y. Ying, Y. Mao, L. Sun and X. Peng, ACS Appl. Mater. Interfaces, 2013, 5, 9850 CrossRef CAS PubMed; (b) X. Chen, N. Zhang and K. Sun, J. Mater. Chem., 2012, 22, 13637 RSC.
- (a) M. A. Worsley, T. Y. Olson, J. R. I. Lee, T. M. Willey, M. H. Nielsen, S. K. Roberts, P. J. Pauzauskie, J. Biener, J. H. Satcher and T. F. Baumann, J. Phys. Chem. Lett., 2011, 2, 921 CrossRef CAS; (b) Z. Chen, W. Ren, L. Gao, B. Liu, S. Pei and H.-M. Cheng, Nat. Mater., 2011, 10, 424 CrossRef CAS PubMed; (c) G. L. Wang, J. C. Huang, S. L. Chen, Y. Y. Gao and D. X. Cao, J. Power Sources, 2011, 196, 5756 CrossRef CAS PubMed; (d) L. Liu, Y. Li, S. Yuan, M. Ge, M. Ren, C. Sun and Z. Zhou, J. Phys. Chem. C, 2010, 114, 251 CrossRef CAS.
- Y. K. Yin, Y. L. Xu, Y. Li, F. Y. Ren, S. J. Li, G. Jin, M. J. Li and X. Y. Cui, Chem. Res. Chin. Univ., 2013, 29, 379 CrossRef CAS.
- D. Fu, J. Lu, J. Liu and Y. Li, J. Chem. Ind. Eng., 2002, 53, 7 Search PubMed.
- K.G. Weil, J. S. Rowlinson and B. Widom: Molecular Theory of Capillarity, Clarendon Press, Oxford 1982, 1984, p. 327.
- (a) J. Zhu and X. Qian, J. Solid State Chem., 2010, 183, 1632 CrossRef CAS PubMed; (b) Z. H. Ibupoto, K. Khun, V. Beni, X. Liu and M. Willander, Sensors, 2013, 13, 7926 CrossRef CAS PubMed; (c) Y. Xu, D. Chen, X. Jiao and K. Xue, Mater. Res. Bull., 2007, 42, 1723 CrossRef CAS PubMed; (d) L. Zheng and X. Liu, Mater. Lett., 2007, 61, 2222 CrossRef CAS PubMed; (e) J. G. Zhao, S. J. Liu, S. H. Yang and S. G. Yang, Appl. Surf. Sci., 2011, 257, 9678 CrossRef CAS PubMed; (f) M. Faisal, S. B. Khan, M. M. Rahman, A. Jamal and A. Umar, Mater. Lett., 2011, 65, 1400 CrossRef CAS PubMed; (g) F. Li, X. Liu, Q. Zhang, T. Kong and H. Jin, Cryst. Res. Technol., 2012, 47, 1140 CrossRef CAS.
- (a) X. G. Wen, W. X. Zhang and S. H. Yang, Langmuir, 2003, 19, 5898 CrossRef CAS; (b) C. H. Lu, L. M. Qi, J. H. Yang, D. Y. Zhang, N. Z. Wu and J. M. Ma, J. Phys. Chem. B, 2004, 108, 17825 CrossRef CAS.
- S. P. Meshram, P. V. Adhyapak, U. P. Mulik and D. P. Amalnerkar, Chem. Eng. J., 2012, 204–206, 158 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: XRD, FESEM pictures of the 3D macroporous CuO structures. FESEM images of the products obtained at different reaction times. TEM pictures of non-organized CuO nanosheets. The nitrogen adsorption–desorption isotherms of the 3D macroporous CuO structures. See DOI: 10.1039/c4cc05982d |
| This journal is © The Royal Society of Chemistry 2015 |