Dithiafulvenyl-grafted phenylene ethynylene polymers as selective and reversible dispersants for single-walled carbon nanotubes†
Karimulla
Mulla
a,
Shuai
Liang
b,
Haseena
Shaik
a,
Eyad A.
Younes
a,
Alex
Adronov
b and
Yuming
Zhao
*a
aDepartment of Chemistry, Memorial University, St. John's, NL, Canada A1B 3X7. E-mail: yuming@mun.ca; Fax: +1 709 864 8747; Tel: +1 709 864 3702
bDepartment of Chemistry, McMaster University, Hamilton, ON, Canada L8S 4M1
First published on 5th November 2014
Abstract
Phenylene ethynylene-based π-conjugated polymers grafted with dithiafulvenyl groups on their side chains were found to be efficient in dispersing single-walled carbon nanotubes in a selective and controllable way.
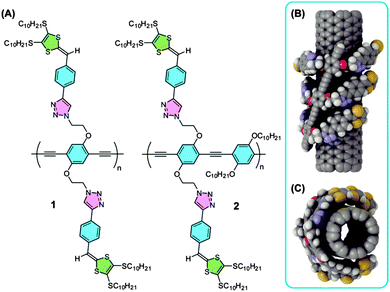
The widespread application of single-walled carbon nanotubes (SWNTs) in materials science and biological technology1,2 has greatly promoted the research on new methods to produce structurally homogeneous SWNTs.3 Noncovalent functionalization of SWNTs4 has been recognized as one of the most promising approaches, with the current state-of-the-art techniques, in the field that allows specific types of SWNTs to be sorted out of the as-produced mixtures according to their electronic nature,5 diameter,6 and chiral index.7 Generally speaking, to effectively disperse SWNTs in a solvent, dispersing agents (dispersants) are required to have sufficient binding forces toward SWNTs so as to break apart their heavily entangled bundles. We recently discovered that relatively short phenylene ethynylene and phenylene vinylene oligomers when endcapped with dithiafulvenyl (DTF) groups, exhibited strong supramolecular interactions with SWNTs.8 The performance of these relatively short DTF-functionalized oligomers in enhancing the binding strength toward SWNTs was remarkable, which in turn prompted us to conceive that polymer systems carrying DTF groups would serve as more effective SWNT dispersants. In this vein, we have subsequently investigated two phenylene ethynylene-based polymers 1 and 2 which are grafted with DTF endgroups on their side chains (Fig. 1).
The supramolecular interactions between the designed polymers and SWNTs were first surveyed by molecular modeling.9Fig. 1B and C depict the minimum energy conformation of the trimer of 1 in interaction with a (7,7) SWNT, showing that the trimer adopts a folded conformation to tightly wrap itself around the nanotube sidewall. The phenylene ethynylene backbone of the trimer adheres to the SWNT at an angle of ca. 19° with respect to the longitudinal axis of the tube, while the DTF-appended side chains embrace the tube circumferentially at an angle of ca. 90°. In a previous molecular dynamics (MD) study, Saven and co-workers demonstrated that poly(phenylene ethynylene)s could adhere to the surface of SWNTs forming a robust helical superstructure.10 Polymers 1 and 2 were thus envisioned to take a unique “centipede-like” wrapping mode to result in highly efficient SWNT dispersion in organic solvents.
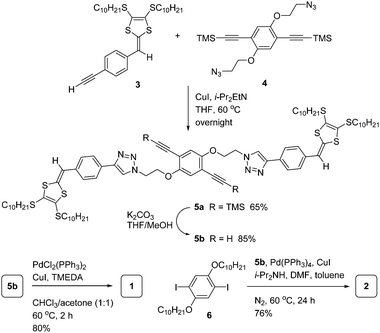
The synthesis of polymers 1 and 2 was undertaken through a modular synthetic route11,12 as outlined in Scheme 1. Acetylenic phenyl-DTF 3 was first reacted with diazido-phenylacetylene 412via the Cu-catalyzed alkyne–azide coupling reaction (Click reaction11c) to afford precursor 5a. Compound 5a was then desilylated with K2CO3 to give free terminal dialkyne 5b, which then underwent a Pd/Cu catalyzed homocoupling polymerization to afford polymer 1 in a good yield of 80%. Alternatively, precursor 5b was subjected to a Sonogashira coupling polymerization with diiodoarene 6 in the presence of a Pd/Cu catalyst and diisopropylamine as base to yield polymer 2 in 76% yield. The two polymers are brown coloured solids with good solubility in chlorinated and aromatic organic solvents, such as CHCl3, CH2Cl2, chlorobenzene, and toluene. In aliphatic hydrocarbon or polar organic solvents, however, their solubility diminished substantially. The molecular structures of 1 and 2 have been characterized by IR and NMR analysis (see ESI†). Gel permeation chromatographic (GPC) analysis shows that polymer 1 has a number-average molar mass (Mn) of 6223 g mol−1 relative to polystyrene standards and a polydispersity index (PDI) of 1.7, while for polymer 2 the Mn is measured to be 7275 g mol−1 with a PDI of 1.7.
Two types of commercially available SWNTs (HiPCO and CoMoCAT) were subjected to dispersion experiments in various organic solvents using polymers 1 and 2 as dispersants. Both polymers were found to be capable of effectively dispersing SWNTs in organic solvents, including CHCl3, CH2Cl2, chlorobenzene, toluene, and THF, wherein the polymers themselves also showed very good solubility. Fig. 2 illustrates the UV-Vis-NIR spectra of the SWNT–polymer suspensions in toluene. As a general trend, polymer 1 imparts a much better solubility to SWNTs than polymer 2, as a result of the relatively more DTF-functionalities per repeat unit in 1. The nanotubes dispersed by polymer 1 in solution are of much better quality than those dispersed by 2, which is evidenced by the less significant amorphous carbon baseline in the Vis-NIR absorption spectrum. The well-resolved peaks in the Vis-NIR region are due to the inter-band electronic transitions between the van Hove singularities in the valence and conduction bands of SWNTs.1,13 Assignments of these peaks to specific chiral indices are made according to literature data1,14,15 and photoluminescence-excitation (PLE) mapping results.
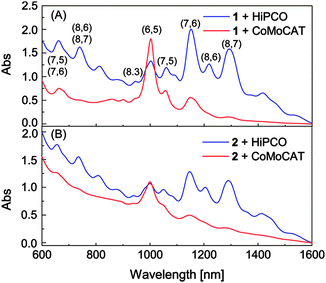 | ||
| Fig. 2 Vis-NIR absorption spectra of CoMoCAT and HiPCO nanotubes dispersed in toluene with (A) polymer 1 and (B) polymer 2. | ||
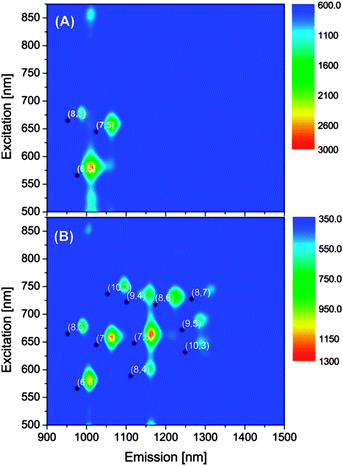
The selectivity of polymer 1 toward SWNT dispersion was manifested by the PLE maps shown in Fig. 3. For the dispersion of the CoMoCAT sample, (6,5) and (7,5) tubes are favoured with a small amount of (8,3) tubes co-existing in the suspension. For HiPCO nanotubes, the dispersion selectivity mainly goes to (6,5), (7,5), and (7,6) chiral indices, while some nanotubes with other chirality are also present in the suspension, including (8,3), (8,4), (8,6), (8,7), (9,4), (9,5), (10,2) and (10,3). In comparison with the types of SWNTs dispersed by a typical surfactant, sodium dodecylbenzenesulfonate (SDBS), in water (see Fig. S-21 in the ESI†), polymer 1 affords a very high selectivity for (6,5), (7,5) and (7,6) tubes which are of a relatively small diameter (ca. 0.75–0.90 nm). Such selectivity likely arises from the theoretically predicted “centipede-like” binding mode, wherein the electron-rich DTF endgroups act as the major driving force for effective SWNT binding. PLE mapping studies of the suspensions of SWNTs and polymer 2, however, did not yield meaningful results due to their poor fluorescence intensity.
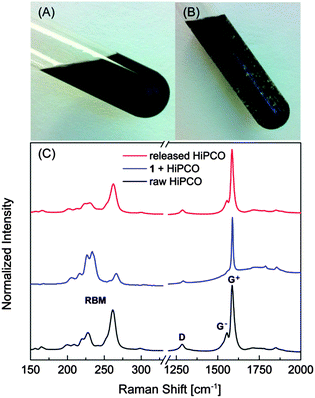
SWNTs dispersed with polymers 1 and 2 in chlorinated or aromatic organic solvents formed stable homogeneous suspensions. There was no precipitation of SWNTs observable even after long-term (several months) storage. Addition of aliphatic hydrocarbon solvents to these suspensions, however, was found to quickly induce precipitation of SWNTs out of the polymer solution. This observation is consistent with the results of DTF-endcapped π-oligomers in our previous study,8 offering a facile means to release pristine SWNTs from their polymer suspensions using solvent control. Controllable release of SWNTs from rationally designed supramolecular hosts has become a topic of growing interest over the past few years,15,16 driven by the critical demand for “additive-free” SWNTs in the fabrication of advanced electronic and optoelectronic devices. To further explore this solvent-controlled behaviour, experiments on hexane-induced release of SWNTs were undertaken. Fig. 4 demonstrates a reversible process of dispersing and releasing HiPCO SWNTs in different solvent systems, which could be potentially developed into a useful technique for applications where “dispersant-free” SWNTs17 are desired after solution-phase processing in order to maximize electronic and/or optoelectronic performance.
To further evaluate the efficiency of the solvent-controlled release of SWNTs, comparative Raman spectroscopic analyses were performed. As shown in Fig. 4C, the Raman spectrum of HiPCO SWNTs complexed with polymer 1 exhibits the characteristic G+/G− and D bands of SWNTs in the region from ca. 1200 to 2000 cm−1, which are convoluted with some broad Raman peaks attributable to polymer 1. The G− band typical of pristine SWNTs, however, disappears in the spectrum of HiPCO–polymer 1, which is indicative of strong electronic interactions18 between polymer 1 and SWNTs. The Raman spectrum of HiPCO SWNTs released from the polymer solution after addition of hexanes does not show any broad Raman bands due to the polymer. Instead, it shows a spectral pattern that closely resembles that of the raw pristine HiPCO sample. The comparative Raman study confirms that the DTF-grafted polymers are able to effectively release SWNTs out of the polymer dispersants, which in turn presents a significant advantage over other ways of releasing SWNTs (e.g., redox, pH, light)15,16 in terms of efficiency, ease of operation, and cost-effectiveness. Thermogravimetric analysis (TGA) shows that only a small amount of polymer dispersant (ca. 7.7 wt%) remains within the released SWNTs (see Fig. S-16 in the ESI† for details). Nevertheless, with the redox activity of the DTF groups in the polymer side chains, it is possible that the trace amount of polymer dispersants can be further removed by using the redox or acidity-controlled methods similar to what we devised in our recent papers on a class of tetrathiafulvalene-based polymers.15
Preliminary investigations of electrochemically controlled SWNT release were conducted on the complexes of HiPCO SWNTs and polymer 2 dispersed in CHCl3 (Bu4NBF4 was present as an electrolyte). It was found that at a certain applied voltage, usually greater than +0.8 V (vs. Ag/AgCl), pristine SWNTs began to precipitate out of the suspension. The remaining SWNT–polymer suspension was monitored by UV-Vis-NIR absorption analysis, and the results clearly indicate a depletion of the SWNT absorption bands in the NIR region (see Fig. S-23 in the ESI†), attesting the feasibility of separating SWNTs from the polymers by electrolysis.
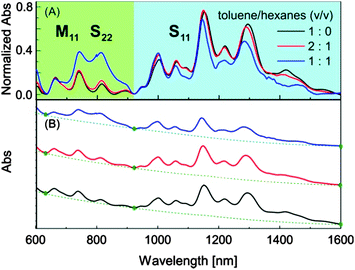
The exact mechanisms for the interactions of SWNTs with DTF-grafted conjugated polymers as well as the physical origin for solvent-controlled releasing behaviour, at this juncture, have not yet been fully unravelled. However, the presence of DTF endgroups is known to be a key factor accounting for effective SWNT dispersion. Evidence for this point comes from a comparative study where a “click-generated” phenylene ethynylene polymer,19 analogous to polymer 2 but carrying N,N-dimethylaniline instead of phenyl-DTF endgroups in the side chains, was observed to be completely ineffective at dispersing SWNTs in common organic solvents. Given the rich π-electron properties of DTF, it is proposed that two noncovalent forces, π-stacking and charge-transfer (CT) interaction, drive the polymers to wrap SWNTs.20 Our cyclic voltammetric studies showed that the oxidation potential of the DTF unit in polymer 1 shifted from +0.86 V to +1.04 V upon complexation with HiPCO SWNTs (see Fig. S-10 in the ESI†), confirming the occurrence of significant CT interactions. Compared with nonpolar aliphatic hydrocarbon solvents, chlorinated solvents and aromatic solvents in theory should engender much better solvation effects on SWNT–polymer complexes assembled via π-stacking and CT interaction. To test this hypothesis, a mixed-solvent experiment was next conducted as follows. To a suspension of HiPCO SWNTs dispersed with polymer 1 in toluene, hexanes were gradually added to induce partial precipitation of SWNTs. The mixtures were subjected to filtration, and the filtrate was then examined by Vis-NIR absorption analysis to monitor the compositional changes of SWNTs remaining in the solution.
Fig. 5 compares the normalized absorption spectra of HiPCO SWNTs suspended in toluene and/or hexanes at various ratios. The results show that more metallic tubes remained in the solution phase than semiconducting tubes with increasing addition of hexanes. It has been known that semiconducting tubes favour to interact with electron-donating molecules via CT interaction.18 In principle, CT interaction is more sensitive to solvent effects than other noncovalent forces (e.g., van der Waals, π-stacking, and dipole–dipole interactions).21 Therefore, the observation that semiconducting tubes are more readily released than metallic tubes upon addition of hexanes corroborates that CT interaction plays an important role in the solvent-controlled dispersion/release behaviour.
In conclusion, we have demonstrated a “centipede wrapping” strategy for effective dispersion of SWNTs in various organic solvents using DTF-grafted π-conjugated polymers as dispersants. The dispersion appeared to be selective for small-diameter semiconducting (6,5), (5,6), and (7,6) tubes, and almost dispersant-free SWNTs could be readily released from the SWNT–polymer suspensions under easy solvent control. Moreover, the redox activity of DTF allows the polymers to be detached from SWNTs by electrolysis. Since both the solvent mixing and electrolysis methods are simple and easy to scale up in practice, it is envisaged that our DTF-grafted polymers may find potential use in developing cost-effective and large-scale methods for preparation of high-quality SWNTs.
Notes and references
- (a) R. Saito, G. Dresselhaus and M. S. Dresselhaus, Physical Properties of Carbon Nanotubes, Imperial College Press, London, 1998 Search PubMed; (b) M. Meyyappan, Carbon Nanotubes: Science and Applications, CRC Press, Boca Raton, 2005 Search PubMed; (c) D. M. Guldi and N. Martín, Carbon Nanotubes and Related Structures: Synthesis, Characterization, Functionalization, and Applications, Wiley-VCH, Weinheim, 2010 Search PubMed; (d) R. Klingeler and R. B. Sim, Carbon Nanotubes for Biomedical Applications, Springer, Berlin, 2011 Search PubMed.
- For recent reviews on applications of SWNTs, see: (a) M. F. L. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535 CrossRef CAS PubMed; (b) Q. Zhang, J.-Q. Huang, W.-Z. Qian, Y.-Y. Zhang and F. Wei, Small, 2013, 9, 1237–1265 CrossRef CAS PubMed; (c) S. Park, M. Vosguerichian and Z. Bao, Nanoscale, 2013, 5, 1727 RSC; (d) Y. Chen, A. Star and S. Vidal, Chem. Soc. Rev., 2013, 42, 4532 RSC; (e) J. M. Schnorr and T. M. Swager, Chem. Mater., 2011, 23, 646 CrossRef CAS; (f) F. Liang and B. Chen, Curr. Med. Chem., 2010, 17, 10 CrossRef CAS; (g) N. Saito, Y. Usui, K. Aoki, N. Narita, M. Shimizu, K. Hara, N. Ogiwara, K. Nakamura, N. Ishigaki, H. Kato, S. Tarutac and M. Endoc, Chem. Soc. Rev., 2009, 38, 1897 RSC.
- (a) H. Omachi, Y. Segawa and K. Itami, Acc. Chem. Res., 2012, 45, 1378 CrossRef CAS PubMed; (b) Y. Zhang and L. Zheng, Nanoscale, 2010, 2, 1919 RSC; (c) M. C. Hersam, Nat. Nanotechnol., 2008, 3, 387 CrossRef CAS PubMed; (d) R. B. Weisman, Nat. Mater., 2003, 2, 569 CrossRef CAS PubMed and references therein.
- (a) Y.-L. Zhao and J. F. Stoddart, Acc. Chem. Res., 2009, 42, 1161 CrossRef CAS PubMed; (b) P. Bilalis, D. Katsigiannopoulos, A. Avgeropoulos and G. Sakellariou, RSC Adv., 2014, 4, 2911 RSC.
- (a) H. Wang, J. Mei, P. Liu, K. Schmidt, G. Jiménez-Osés, S. Osuna, L. Fang, C. J. Tassone, A. P. Zoombelt, A. N. Sokolov, K. N. Houk, M. F. Tone and Z. Bao, ACS Nano, 2013, 7, 2659 CrossRef CAS PubMed; (b) M. Tange, T. Okazaki and S. Iijima, ACS Appl. Mater. Interfaces, 2012, 4, 6458 CrossRef CAS PubMed; (c) M. S. Arnold, A. A. Green, J. F. Hulvat, S. I. Stupp and M. C. Hersam, Nat. Nanotechnol., 2006, 1, 60 CrossRef CAS PubMed.
- H. Wang, G. I. Koleilat, P. Liu, G. Jiménez-Osés, Y.-C. Lai, M. Vosgueritchian, Y. Fang, S. Park, K. N. Houk and Z. Bao, ACS Nano, 2014, 8, 2609 CrossRef CAS PubMed.
- (a) H. Ozawa, T. Fujigaya, Y. Niidome, N. Hotta, M. Fujiki and N. Nakashima, J. Am. Chem. Soc., 2011, 133, 2651 CrossRef CAS PubMed; (b) P. Gerstel, S. Klumpp, F. Hennrich, A. Poschlad, V. Meded, E. Blasco, W. Wenzel, M. M. Kappes and C. Barner-Kowollik, ACS Macro Lett., 2014, 3, 10 CrossRef CAS.
- K. Mulla and Y. Zhao, J. Mater. Chem. C, 2013, 1, 5116 RSC.
- Molecular modelling was done using Spartan’10 (Wavefunction Inc., Irvine, CA) running on a Mac Pro workstation.
- C. D. Von Bargen, C. M. MacDermaid, O.-S. Lee, P. Deria, M. J. Therien and J. G. Saven, J. Phys. Chem. B, 2013, 117, 12953 CrossRef CAS PubMed.
- (a) B. C. Englert, S. Bakbak and U. H. F. Bunz, Macromolecules, 2005, 38, 5868 CrossRef CAS; (b) W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2007, 28, 15 CrossRef CAS; (c) J. Lahann, Click Chemistry for Biotechnology and Materials Science, Wiley, Chichester, West Sussex, 2009 Search PubMed; (d) H. Durmaz, A. Sanyal, G. Hizal and U. Tunca, Polym. Chem., 2012, 3, 825 RSC.
- Y. Pourghaz, P. Dongare, D. W. Thompson and Y. Zhao, Chem. Commun., 2011, 47, 11014 RSC.
- M. J. O'Connell, S. M. Bachilo, C. B. Huffman, V. C. Moore, M. S. Strano, E. H. Haroz, K. L. Rialon, P. J. Boul, W. H. Noon, C. Kittrell, J. Ma, R. H. Hauge, R. B. Weisman and R. E. Smalley, Science, 2002, 297, 593 CrossRef PubMed.
- (a) S. M. Bachilo, M. S. Strano, C. Kittrell, R. H. Hauge, R. E. Smalley and R. B. Weisman, Science, 2002, 298, 2361 CrossRef CAS PubMed; (b) Y. Lian, Y. Maeda, T. Wakahara, T. Akasaka, S. Kazaoui, N. Minami, N. Choi and H. Tokumoto, J. Phys. Chem. B, 2003, 107, 12082 CrossRef CAS; (c) A. Hagen and T. Hertel, Nano Lett., 2003, 3, 383 CrossRef CAS.
- (a) S. Liang, G. Chen, J. Peddle and Y. Zhao, Chem. Commun., 2012, 48, 3100 RSC; (b) S. Liang, G. Chen and Y. Zhao, J. Mater. Chem. C, 2013, 1, 5477 RSC; (c) S. Liang, Y. Zhao and A. Adronov, J. Am. Chem. Soc., 2014, 136, 970 CrossRef CAS PubMed.
- (a) S. Chen, Y. Jiang, Z. Wang, X. Zhang, L. Dai and M. Smet, Langmuir, 2008, 24, 9233 CrossRef CAS PubMed; (b) Y. Ding, S. Chen, H. Xu, Z. Wang, X. Zhang, T. H. Ngo and M. Smet, Langmuir, 2010, 26, 16667 CrossRef CAS PubMed; (c) Z. Zhang, Y. Che, R. A. Smaldone, M. Xu, B. R. Bunes, J. S. Moore and L. Zang, J. Am. Chem. Soc., 2010, 132, 14113 CrossRef CAS PubMed; (d) X. Mao, G. C. Rutledge and T. A. Hatton, Langmuir, 2013, 29, 9626 CrossRef CAS PubMed; (e) Z. Guo, Y. Feng, S. He, M. Qu, H. Chen, H. Liu, Y. Wu and Y. Wang, Adv. Mater., 2013, 25, 584 CrossRef CAS PubMed; (f) Y. Matsuzawa, Y. Takada, T. Kodaira, H. Kihara, H. Kataura and M. Yoshida, J. Phys. Chem. C, 2014, 118, 5013 CrossRef CAS.
- (a) W.-B. Liu, S. Pei, J. Du, B. Liu, L. Gao, Y. Su, C. Liu and H.-M. Cheng, Adv. Funct. Mater., 2011, 21, 2330 CrossRef CAS; (b) E. Remy, C. Hérold, F. Valsaque, J.-F. Marêché, S. Fontana, A. Desforges, S. Cahen, J. Ghanbaja, J. Gleize and B. Vigolo, J. Phys. Chem. C, 2013, 117, 19245 CrossRef CAS.
- (a) H.-J. Shin, S. M. Kim, S.-M. Yoon, A. Benayad, K. K. Kim, S. J. Kim, H. K. Park, J.-Y. Choi and Y. H. Lee, J. Am. Chem. Soc., 2008, 130, 2062 CrossRef CAS PubMed; (b) N. Varghese, A. Ghosh, R. Voggu, S. Ghosh and C. N. R. Rao, J. Phys. Chem. C, 2009, 113, 16855 CrossRef CAS.
- Y. Pourghaz, P. Dongare, D. W. Thompson and Y. Zhao, Chem. Commun., 2011, 47, 11014 RSC.
- (a) M. Á. Herranz, N. Martín, S. Campidelli, M. Prato, G. Brehm and D. M. Guldi, Angew. Chem., Int. Ed., 2006, 45, 4478 CrossRef CAS PubMed; (b) D. Canevet, E. M. Pérez and N. Martín, Angew. Chem., Int. Ed., 2011, 50, 9248 CrossRef CAS PubMed.
- R. Voggu, C. S. Rout, A. D. Franklin, T. S. Fisher and C. N. R. Rao, J. Phys. Chem. C, 2008, 112, 13053 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The full synthetic procedures of polymer 1 and 2 as well as relevant precursors, along with their detailed spectroscopic characterization data. See DOI: 10.1039/c4cc07239a |
| This journal is © The Royal Society of Chemistry 2015 |