Partial pressure-induced growth of silicon nitride belts with tunable width and photoluminescence properties†
J.
Cai
ab,
Y. L.
Zhang
ab,
Z. Y.
Lyu
ab,
J.
Zhao
ab,
J. C.
Shen
c,
Q.
Wu
ab,
X. Z.
Wang
*ab,
X. L.
Wu
*c,
Y.
Chen
ab and
Z.
Hu
ab
aKey Laboratory of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, PR China. E-mail: wangxzh@nju.edu.cn
bHigh-Tech Research Institute of Nanjing University (Suzhou), Suzhou, Jiangsu 215123, China
cKey Laboratory of Modern Acoustics of MOE, Institute of Acoustics, Department of Physics, Nanjing 210093, Jiangsu, PR China. E-mail: hkxlwu@nju.edu.cn
First published on 3rd November 2014
Abstract
α- and β-Si3N4 belts with tunable width were synthesized by regulating the partial pressure of NH3/N2 in gaseous mixtures of Ar and NH3/N2 during the nitridation of silicon powders, which demonstrated tunable photoluminescence properties.
Introduction
Wide-bandgap semiconductor materials with novel properties have exhibited promising applications in the fields of light-emitting diodes,1,2 lasers,3,4 field emission devices,5,6 and so on. The performance of the functional devices strongly depends on the intrinsic properties and microstructures of semiconductor materials.7 Silicon nitride (Si3N4), an important wide-bandgap (5.3 eV) semiconductor, has attracted extensive attention due to its unique physical and chemical properties including high mechanical strength, remarkable thermal and chemical stability, doping capability, and tunable optoelectronic properties.8 Up to now, various morphologies of Si3N4, such as wire-like,9,10 belt-like,11–16 and ring-like,17 have been prepared via nitridation of silicon powder,12 Fe–Si alloy,13 silicon oxide,14etc. But the controllable synthesis of Si3N4 structures with specific morphologies is still a challenge to meet the demand of functional devices. It is known that the growth behaviors of materials strongly depend on the growth conditions, such as the growth temperature and the concentrations of reactants.18,19 For example, in the anisotropic epitaxial growth model, these two factors can greatly influence the nucleation rate and the lateral growth rate of crystals.20,21 Recently, it has been reported that the width of Si3N4 microribbons can be modulated in the range of 3 to 10 μm by controlling the nitridation temperatures of silicon monoxide (SiO) powders, leading to tunable photoluminescence properties in the blue-light band.22In this study, we obtained Si3N4 belts with tunable width from nanoscale to microscale by regulating the partial pressure of NH3/N2 in the gaseous mixtures of Ar and NH3/N2, i.e. the concentrations of nitrogen-containing precursors, during the nitridation of silicon powders. When the partial pressure of NH3/N2 decreases from 100% to 2%, the average width of Si3N4 belts can gradually increase from 183 to 2400 nm. The corresponding photoluminescence characterization indicates that the Si3N4 belts present tunable emission bands. Specifically, Si3N4 belts with widths of 183 to 610 nm have strong cyan–red light emission and show a progressive blue shift, and the belts with 1675 and 2400 nm width have weak near-ultraviolet–blue light emission and very weak cyan–red light emission.
Experimental
Si3N4 micro- and nano-belts were synthesized in a horizontal furnace by nitrifying silicon powders. In a typical run, silicon powders dispersed on an alumina wafer were placed at the center of the furnace. The system was vacuumed using a rotary pump to remove the O2 and moisture. Subsequently, the temperature of the system was elevated to 1400 °C in 50 sccm (standard cubic centimeter per minute) gas mixtures of NH3/N2 (4 vol.% NH3) and Ar at the rate of 10 K min−1 and then kept for 14 h. The volume ratios of NH3/N2 to Ar are specified to be 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 50
0, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 40
50, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, 10
60, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 2
90, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98, and 0
98, and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. Finally, the system was cooled to ambient temperature. The structures and morphologies of the products were characterized using an X-ray diffractometer (XRD, D8 Advance A25, Co target, λKα1 = 1.78897 Å), a scanning electron microscope (SEM, Hitachi S-4800) attached to an energy-dispersive X-ray spectroscopy instrument (EDS, Ametek, EDAX-Genesis-XM2-Imaging-60SEM), and a high-resolution transmission electron microscope (HRTEM, JEOL, JEM-2100, operating at 200 kV). The photoluminescence properties were investigated using an FLS 920 instrument with a He-Cd laser of 325 nm at room temperature.
100. Finally, the system was cooled to ambient temperature. The structures and morphologies of the products were characterized using an X-ray diffractometer (XRD, D8 Advance A25, Co target, λKα1 = 1.78897 Å), a scanning electron microscope (SEM, Hitachi S-4800) attached to an energy-dispersive X-ray spectroscopy instrument (EDS, Ametek, EDAX-Genesis-XM2-Imaging-60SEM), and a high-resolution transmission electron microscope (HRTEM, JEOL, JEM-2100, operating at 200 kV). The photoluminescence properties were investigated using an FLS 920 instrument with a He-Cd laser of 325 nm at room temperature.
Results and discussion
Fig. 1 presents the XRD patterns of the nitridation products under different ratios of P(NH3/N2) to P(Ar). As shown in Fig. 1, all five samples include the hexagonal Si3N4, indexed to α and β phases. The ratios of β to α are near 1.2 (Fig. S1, ESI†), which is basically independent of the partial pressure of NH3/N2 in the gaseous mixtures of Ar and NH3/N2. For the samples nitrified at P(NH3/N2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P(Ar) = 100
P(Ar) = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and 50
0 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, all diffraction peaks are assigned to Si3N4 without Si signals, while there are additional diffraction peaks of Si in the samples nitrified at P(NH3/N2)
50, all diffraction peaks are assigned to Si3N4 without Si signals, while there are additional diffraction peaks of Si in the samples nitrified at P(NH3/N2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P(Ar) = 40
P(Ar) = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, 10
60, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, and 2
90, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98. The Si signals come from incomplete nitridation of Si powders.
98. The Si signals come from incomplete nitridation of Si powders.
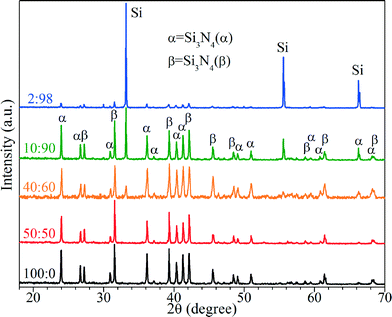 | ||
Fig. 1 XRD patterns of the nitridation products at P(NH3/N2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P(Ar) = 100 P(Ar) = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 50 0, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 40 50, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, 10 60, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, and 2 90, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98. Co target, λKα1 = 1.78897 Å. 98. Co target, λKα1 = 1.78897 Å. | ||
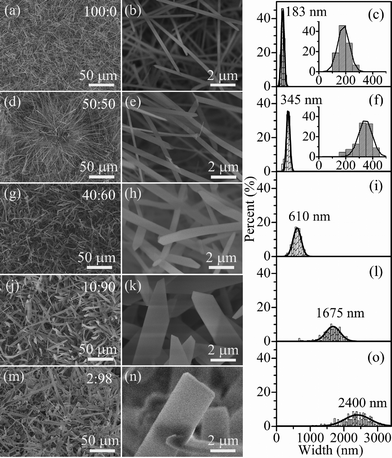
The typical morphologies of the products nitrified at different ratios of P(NH3/N2) to P(Ar) are shown in Fig. 2 and S2 (ESI†). It can be clearly seen that all five products show the belt-like morphology, whose statistical widths are centered at 183, 345, 610, 1675, and 2400 nm with full width at half maximum (FWHM) values of 102, 117, 279, 500, and 894 nm corresponding to the P(NH3/N2)-to-P(Ar) ratios of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 50
0, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 40
50, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, 10
60, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, and 2
90, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 (Fig. 2c, f, i, l, o). Namely, by decreasing the ratio of P(NH3/N2) to P(Ar), the width of the belts increases from several hundred nanometers to several microns and the width distribution becomes wider and wider. When the silicon powders were treated in pure Ar flow (P(NH3/N2)
98 (Fig. 2c, f, i, l, o). Namely, by decreasing the ratio of P(NH3/N2) to P(Ar), the width of the belts increases from several hundred nanometers to several microns and the width distribution becomes wider and wider. When the silicon powders were treated in pure Ar flow (P(NH3/N2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P(Ar) = 0
P(Ar) = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100), no wire-like or belt-like morphologies were observed (Fig. S2f, ESI†). Further EDS and element mapping evidences from a single micro-belt demonstrate that the belt consists of Si and N elements (Fig. S3, ESI†). In addition, the influence of the nitridation temperature was investigated. At the designated P(NH3/N2)
100), no wire-like or belt-like morphologies were observed (Fig. S2f, ESI†). Further EDS and element mapping evidences from a single micro-belt demonstrate that the belt consists of Si and N elements (Fig. S3, ESI†). In addition, the influence of the nitridation temperature was investigated. At the designated P(NH3/N2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P(Ar) = 50
P(Ar) = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, by elevating the nitridation temperatures from 1300 to 1400 °C, the average width of the belts increases slightly from 270 to 345 nm (Fig. S4, ESI†). Thus, following Fig. 2 and S4 (ESI†), modulating the partial pressure is an effective way to control the width of belts during the nitridation of silicon powders.
50, by elevating the nitridation temperatures from 1300 to 1400 °C, the average width of the belts increases slightly from 270 to 345 nm (Fig. S4, ESI†). Thus, following Fig. 2 and S4 (ESI†), modulating the partial pressure is an effective way to control the width of belts during the nitridation of silicon powders.
To investigate the microstructure of the nitridation products, (HR)TEM observations were carried out. The typical TEM image of the nitridation product at P(NH3/N2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P(Ar) = 50
P(Ar) = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 indicates that the belts are ca. 200–300 nm in width (Fig. 3a). The corresponding HRTEM image in Fig. 3b shows the interplanar spacings of 4.4 and 2.9 Å with an angle of ca. 83°, corresponding to the d0–11 and d201 of hexagonal α-Si3N4 with a dihedral angle of 83°. Besides, there exist some defects on the surface of the belt. In combination with XRD analyses, SEM and (HR)TEM observations, EDS and element mapping results, the belts can be assigned to Si3N4.
50 indicates that the belts are ca. 200–300 nm in width (Fig. 3a). The corresponding HRTEM image in Fig. 3b shows the interplanar spacings of 4.4 and 2.9 Å with an angle of ca. 83°, corresponding to the d0–11 and d201 of hexagonal α-Si3N4 with a dihedral angle of 83°. Besides, there exist some defects on the surface of the belt. In combination with XRD analyses, SEM and (HR)TEM observations, EDS and element mapping results, the belts can be assigned to Si3N4.
As demonstrated in Fig. 2, the width of Si3N4 belts strongly depends on the partial pressure of NH3/N2 in the gaseous mixtures of Ar and NH3/N2. To understand the relationship between the width of Si3N4 belts and the partial pressure of NH3/N2 in the gaseous mixtures of Ar and NH3/N2 at the designated nitridation temperature (e.g. 1400 °C), the formation schematic diagram of Si3N4 belts is proposed (Fig. 4). Generally, for anisotropic epitaxial growth of compound materials, e.g. Si3N4, they are firstly nucleated on the substrate and then epitaxially grown into a variety of morphologies due to the difference in axial and lateral growth rates.23,24 In our case of partial pressure-induced growth of Si3N4, by increasing the partial pressure of NH3/N2, the nucleation density of Si3N4 increases and the axial growth rate increases faster than the lateral growth rate. Hence, the higher the ratio of P(NH3/N2) to P(Ar) is, the thinner the Si3N4 belts that are formed. In other words, the size and density of Si3N4 belts can be regulated by a facile method of controlling the partial pressure of NH3/N2 in the gaseous mixtures of Ar and NH3/N2. In addition, by elevating the nitridation temperatures from 1300 to 1400 °C, the lateral growth rate increases slightly, leading to the slight increase in width of the Si3N4 belts.
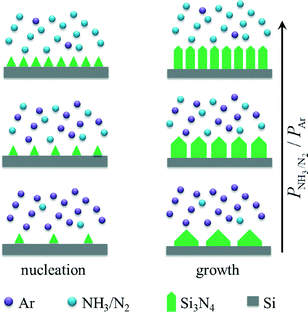 | ||
| Fig. 4 Formation schematic diagram of Si3N4 belts with adjustable width. By increasing the partial pressure of NH3/N2 in the gaseous mixtures of Ar and NH3/N2, the width of Si3N4 belts decreases. | ||
The photoluminescence properties of the five Si3N4 samples were investigated by the PL technique at room temperature under excitation of 325 nm. As shown in Fig. 5 and S5 (ESI†), all five Si3N4 samples exhibit broad emission bands in the near-ultraviolet (UV)–visible light region (350–680 nm). Specifically, for the three samples of Si3N4 belts with average widths of 183, 345, and 610 nm, there exist intense cyan–red emission bands with a progressive blue shift and weak near-UV–blue emission bands. For the other two samples of Si3N4 belts with average widths of 1675 and 2400 nm, the emission bands are mainly located at the near-UV–blue light region, while the cyan–red emission bands are very weak. The emission can be attributed to the defect energy levels in the Si3N4 belts, including Si–Si, N–N, ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si and
Si and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N.25–28 However, the mechanism of the variable emission bands in relation to the width of Si3N4 belts is still unclear and further investigation is under way.
N.25–28 However, the mechanism of the variable emission bands in relation to the width of Si3N4 belts is still unclear and further investigation is under way.
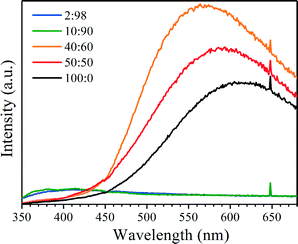 | ||
| Fig. 5 PL spectra of the Si3N4 products. The sharp peaks near 650 nm come from the multiplication frequency of 325 nm excitation. | ||
Conclusions
In summary, the width of α- and β-Si3N4 belts with good crystallinity can be effectively tuned by regulating the partial pressure of NH3/N2 in Ar–NH3/N2 flow during nitridation of silicon powders. By increasing the partial pressure of NH3/N2 in the gaseous mixture of Ar and NH3/N2, the average width of Si3N4 belts decreases from several microns to several hundred nanometers and the width distribution becomes narrower and narrower. The Si3N4 belts exhibit broad emission bands in the near-UV–visible light region (350–680 nm), attributed to the defect energy levels such as Si–Si, N–N,![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si and
Si and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. Specifically, Si3N4 belts with an average width lower than 610 nm have the intensive cyan–red emission bands, while for the belts with the average width on the micron scale, the cyan–red emission bands are very weak. This provides a facile method to synthesize compound semiconductor materials with tunable morphology and optical properties.
N. Specifically, Si3N4 belts with an average width lower than 610 nm have the intensive cyan–red emission bands, while for the belts with the average width on the micron scale, the cyan–red emission bands are very weak. This provides a facile method to synthesize compound semiconductor materials with tunable morphology and optical properties.
Acknowledgements
We acknowledge the joint financial support by the NSFC (21473089, 21173115, 51232003), the “973” program (2013CB932902), the Jiangsu Province Science and Technology Support Project (BE2012159), and the Suzhou Science and Technology Plan Projects (ZXG2013025).Notes and references
- Y. Taniyasu, M. Kasu and T. Makimoto, Nature, 2006, 441, 325–328 CrossRef CAS PubMed.
- A. Khan, K. Balakrishnan and T. Katona, Nat. Photonics, 2008, 2, 77–84 CrossRef CAS.
- M. H. Huang, S. Mao, H. Feick, H. Q. Yan, Y. Y. Wu, H. Kind, E. Weber, R. Russo and P. D. Yang, Science, 2001, 292, 1897–1899 CrossRef CAS PubMed.
- S. Arafin, X. Liu and Z. Mi, J. Nanophotonics, 2013, 7, 074599 CrossRef PubMed.
- C. C. Chen, C. C. Yeh, C. H. Chen, M. Y. Yu, H. L. Liu, J. J. Wu, K. H. Chen, L. C. Chen, J. Y. Peng and Y. F. Chen, J. Am. Chem. Soc., 2001, 123, 2791–2798 CrossRef CAS PubMed.
- D. Banerjee, S. H. Jo and Z. F. Ren, Adv. Mater., 2004, 16, 2028–2032 CrossRef CAS.
- F. Meng, S. A. Morin, A. Forticaux and S. Jin, Acc. Chem. Res., 2013, 46, 1616–1626 CrossRef CAS PubMed.
- J. Huang, Z. Huang, S. Yi, Y. G. Liu, M. Fang and S. Zhang, Sci. Rep., 2013, 3, 3504 Search PubMed.
- F. Gao, W. Yang, Y. Fan and L. An, Nanotechnology, 2008, 19, 105602 CrossRef PubMed.
- L. W. Lin and Y. H. He, CrystEngComm, 2012, 14, 3250–3256 RSC.
- J. Huang, S. Zhang, Z. Huang, Y. Wen, M. Fang and Y. Liu, CrystEngComm, 2012, 14, 7301–7305 RSC.
- F. Wang, X. F. Qin, G. Q. Jin, Y. Y. Wang and X. Y. Guo, Phys. E, 2008, 41, 120–123 CrossRef CAS PubMed.
- K. Huo, Y. Ma, Y. Hu, J. Fu, B. Lu, Y. Lu, Z. Hu and Y. Chen, Nanotechnology, 2005, 16, 2282 CrossRef CAS PubMed.
- L. W. Yin, Y. Bando, Y. C. Zhu and Y. B. Li, Appl. Phys. Lett., 2003, 83, 3584–3586 CrossRef CAS PubMed.
- J. Huang, S. Zhang, Z. Huang, Y. G. Liu and M. Fang, CrystEngComm, 2013, 15, 785–790 RSC.
- J. Cai, Y. L. Zhang, Y. Li, L. Y. Du, Z. Y. Lyu, Q. Wu, X. Z. Wang and Z. Hu, CrystEngComm, 2014, 16, 9555–9559 RSC.
- W. Yang, X. Cheng, H. Wang, Z. Xie, F. Xing and L. An, Cryst. Growth Des., 2008, 8, 3921–3923 CAS.
- W. S. Shi, H. Y. Peng, Y. F. Zheng, N. Wang, N. G. Shang, Z. W. Pan, C. S. Lee and S. T. Lee, Adv. Mater., 2000, 12, 1343–1345 CrossRef CAS.
- W. I. Park, G. Zheng, X. Jiang, B. Tian and C. M. Lieber, Nano Lett., 2008, 8, 3004–3009 CrossRef PubMed.
- S. A. Kukushkin and A. V. Osipov, Prog. Surf. Sci., 1996, 51, 1–107 CrossRef CAS.
- S. Biswas, C. O'Regan, N. Petkov, M. A. Morris and J. D. Holmes, Nano Lett., 2013, 13, 4044–4052 CrossRef CAS PubMed.
- H. Qian, Y. Zhu, Z. Mao, J. Xiao, J. Chen and L. Zhang, Micro Nano Lett., 2012, 7, 637–640 Search PubMed.
- F. Wang, X. Qin, G. Jin and X. Guo, Phys. E, 2010, 42, 2033–2035 CrossRef CAS PubMed.
- W. Yang, Z. Xie, H. Miao, L. Zhang, H. Ji and L. An, J. Am. Ceram. Soc., 2005, 88, 466–469 CAS.
- J. Robertson and M. J. Powell, Appl. Phys. Lett., 1984, 44, 415–417 CrossRef CAS PubMed.
- J. Robertson, Philos. Mag. B, 1991, 63, 47–77 CAS.
- C. M. Mo, L. D. Zhang, C. Y. Xie and T. Wang, J. Appl. Phys., 1993, 73, 5185–5188 CrossRef PubMed.
- S. V. Deshpande, E. Gulari, S. W. Brown and S. C. Rand, J. Appl. Phys., 1995, 77, 6534–6541 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ce01903b |
| This journal is © The Royal Society of Chemistry 2015 |