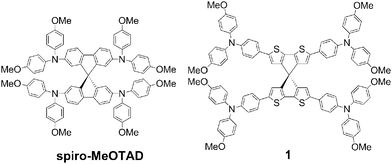
A dopant-free spirobi[cyclopenta[2,1-b:3,4-b′]dithiophene] based hole-transport material for efficient perovskite solar cells†
Marius
Franckevičius
ab,
Amaresh
Mishra
*c,
Franziska
Kreuzer
c,
Jingshan
Luo
a,
Shaik Mohammed
Zakeeruddin
*a and
Michael
Grätzel
*a
aLaboratory of Photonics and Interfaces, Institute of Chemical Sciences and Engineering, School of Basic Sciences, Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland
bCenter for Physical Sciences and Technology, Savanori Ave. 231, LT-02300 Vilnius, Lithuania
cInstitute of Organic Chemistry II and Advanced Materials, University of Ulm, Albert-Einstein-Allee 11, 89081 Ulm, Germany. E-mail: amaresh.mishra@uni-ulm.de
First published on 2nd September 2015
Abstract
We present the design and synthesis of a promising hole transporting material (HTM) using the 4,4′-spirobi[cyclopenta[2,1-b:3,4-b′]dithiophene] derivative (spiro-CPDT) as the core and triarylamines as terminal units. The implementation of the new HTM in CH3NH3PbI3-based perovskite solar cells exhibited an excellent overall power conversion efficiency (PCE) of 13.4% without the use of any dopants and additives which is comparable to 15.0% obtained using p-doped spiro-MeOTAD-based devices. Furthermore, the device based on the new HTM generated a slightly higher open circuit voltage (VOC) of 971 mV compared to a spiro-MeOTAD (VOC = 951 mV) based device. The present results demonstrate that spiro-CPDT could be an excellent building block to prepare dopant-free HTMs for perovskite solar cells and holds promise to replace the p-doped spiro-OMeTAD, which is important for the fabrication of cost-effective devices in the future.
Conceptual insightsOrganometal halide CH3NH3PbX3 (X = Cl, Br, I) perovskite has recently become the central topic in the field of solid state thin film solar cells. High power conversion efficiency up to 20% has been achieved for these light absorbing materials. The unique advantage of these absorber materials is their low cost preparation and high efficiency which enable large scale fabrication in the future. For these high performance perovskite devices hole transport materials (HTMs) play a key role. The most efficient HTM used in these devices is spiro-MeOTAD which does not perform well in its pristine form and requires p-type dopants. However, the p-type doping approach requires further optimization of the fabrication conditions, such as the solvent, dopants and doping concentrations. In this work, we demonstrate that the new spiro-cyclopentadithiophene-based HTM can be used in efficient perovskite devices without any additives and dopants yielding an efficiency of 13.4% and is comparable to that of p-doped spiro-MeOTAD (15%). |
Introduction
Organo-lead trihalide perovskites have attracted considerable attention as alternative candidates for thin-film solar cells due to their excellent absorption from the visible to near-infrared region, charge transport properties, direct band gap and particularly their easy processability.1–9 Since the first report in 2009 with power conversion efficiencies (PCEs) of 3–4%, the research on the use of perovskites as light absorbers in thin film solar cells has been intensified.10 In the last few years, with the optimization of device fabrication conditions PCEs over 19% have been reported.2,11–15 These high performances are due to the development of efficient processing and fabrication conditions of the perovskite layer.16 In these devices 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamino)-9,9′-spirobifluorene (spiro-MeOTAD) was used as the hole transport material (HTM) due to its excellent performance in solid-state dye-sensitized solar cells.17 However, due to the low conductivity of spiro-MeOTAD in the pristine form, dopants and additives are necessary to improve the device performance. In the meantime, several new molecular materials have been developed as alternating HTMs showing PCEs in the range of 12–16%.18–29 Some polymeric HTMs have also been reported among which polytriarylamine showed the highest PCEs up to 12% in mesoscopic TiO2-based devices and above 15% in planar heterojunction devices.30–34 There are only very few examples of HTMs which work efficiently (10–13%) without the use of any additives and dopants, and could also be beneficial to reduce the processing cost and device optimisation time.26,27,35–39Spiro-MeOTAD comprises two fluorene units connected by a sp3-hybridized carbon atom forming a perpendicular arrangement, which efficiently suppresses the molecular interaction between π-systems and increases glass transition temperature (Tg) and mechanical stability.40 Furthermore, the solubility of the molecule also improved due to its three-dimensional structure. The major drawback of spiro-MeOTAD is that the device works efficiently only in the presence of additives, such as tert-butylpyridine (t-BP) and lithium bis(trifluoromethanesulfonyl)imide (Li-TFSI) and cobalt complexes as p-type dopants, which require additional optimization steps. In order to bring perovskite solar cells one step further towards commercial applications, exploration of new HTMs which work efficiently without any additives and dopants is highly desirable. In this communication, we now report the synthesis, characterization, and photovoltaic performance of a new HTM based on spiro-cyclopenta[2,1-b:3,4-b′]dithiophene (spiro-CPDT) connected by p-methoxy-substituted triphenylamine groups (Fig. 1). The application of spiro-CPDT-based molecules is very rare. A D–π–A dye containing a triphenylamine donor, spiro-CPDT π-bridge and terminal cyanoacrylic acid groups was used in dye-sensitized solar cells showing a PCE of 6%.41 Recently, Wang and co-workers reported a spiro-CPDT-based HTM comprising octyl-substituted bithiophenes as terminal units, which gave a PCE of 10.4% in CH3NH3PbI3-based perovskite devices in the presence of additives.42 In this work the CH3NH3PbI3 perovskite solar cells based on s-CPDT 1 exhibited an excellent PCE of 13.4% without any further optimization of the device fabrication conditions. The improvement was further related to the initial light soaking effect of the device.43 It is also important to note that the device works efficiently even without the addition of any foreign chemicals for doping.
Result and discussion
2,2′-6,6′-Tetrabromo-4,4′-spirocyclopenta[2,1-b:3,4-b′]dithiophene was synthesized according to the literature procedure with some modifications (Scheme S1, ESI†).41 The target spiro-CPDT-based HTM 1 was prepared by Pd-catalysed Suzuki cross-coupling reaction of the tetrabromo-derivative and boronic ester of methoxy-substituted triphenylamine in a yield of 91%.Differential scanning calorimetry (DSC) measurements showed that the glass transition temperature (Tg) of HTM 1 is around 150 °C which is higher than that of spiro-MeOTAD (Tg = 125 °C)44 suggesting good thermal stability of 1 (Fig. S1, ESI†). Furthermore, HTM 1 displayed a cold crystallization at around 270 °C suggesting crystallization of the HTM when heated above its Tg. No melting temperature was observed up to 350 °C suggesting the amorphous nature of HTM 1.
The UV-Vis absorption spectrum of compound 1 in dichloromethane solution is shown in Fig. 2a and compared with spiro-MeOTAD (Table 1). The spiro-derivative 1 showed an intense absorption band at 447 nm with a molar extinction coefficient (ε) of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1. This corresponds to a red-shift of 60 nm and significantly higher molar absorptivity (Δε = 40
300 M−1 cm−1. This corresponds to a red-shift of 60 nm and significantly higher molar absorptivity (Δε = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) compared to the state-of-the art spiro-MeOTAD. This is ascribed to the introduction of a strong electron donating spiro-CPDT core unit compared to the spiro-bifluorene unit.
000 M−1 cm−1) compared to the state-of-the art spiro-MeOTAD. This is ascribed to the introduction of a strong electron donating spiro-CPDT core unit compared to the spiro-bifluorene unit.
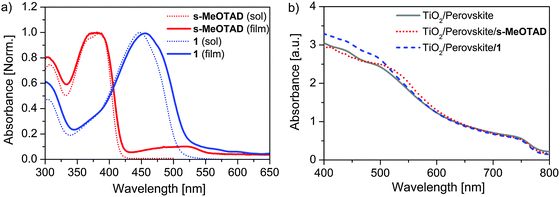 | ||
| Fig. 2 Absorption spectra of the s-CPDT based HTM 1 and spiro-MeOTAD (a) in solution and coated on TiO2 and (b) TiO2/perovskite. Perovskite films cast from PbI2 (1.2 M) and MAI (0.05 M). | ||
| HTM | λ abs sol [nm] | ε [M−1 cm−1] | ΔEopt sola [eV] | λ abs film [nm] | ΔEopt filma [eV] | E 0ox1 [V] | E 0ox2 [V] | E 0ox3 [V] | HOMOc [eV] | LUMOc [eV] |
|---|---|---|---|---|---|---|---|---|---|---|
| a Estimated using the onset of the UV-Vis spectra in solution and in thin films ΔEopt = 1240/λonset. b Determined from the time semi-derivative convoluted cyclic voltammograms. c Estimated from the onset of the redox wave, the Fc/Fc+ value set to −5.1 eV vs. vacuum. | ||||||||||
| 1 | 447 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.43 | 455 | 2.36 | −0.03 | 0.13 | 0.67 | −5.00 | −2.57 |
| spiro-MeOTAD | 387 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.98 | 385 | 2.94 | −0.03 | 0.13 | 0.34 | −5.04 | −2.05 |
When coated on the TiO2 surface the absorption band of HTM 1 is slightly red-shifted to 455 nm, whereas for spiro-MeOTAD it remains unchanged and becomes less structured (Fig. 2a). The optical band gaps estimated from the thin film absorption spectra are 2.94 eV and 2.36 eV and are slightly lower compared to the values obtained from the corresponding solution spectra. The absorption spectra of perovskite films on TiO2 with and without HTMs are presented in Fig. 2b. All films show unique spectral signatures which are similar in the longer wavelength region above 600 nm. In comparison to the films without or with spiro-MeOTAD, films with HTM 1 showed an increased absorption below 500 nm which undoubtedly comes from the contribution of HTM 1. Whereas, spiro-MeOTAD slightly affects perovskite absorption in the 520–600 nm range, probably due to increased low energy absorption of the oxidized species of spiro-MeOTAD at around 525 nm.
Cyclic voltammetry measurement was performed in order to determine the frontier molecular orbital energy levels. Spiro-derivative 1 showed reversible one-electron (1e−) and three-electron (3e−) oxidation processes with half wave potentials at −0.03 V and 0.13 V vs. Fc/Fc+ which are assigned to the oxidation of arylamine moieties (Fig. 3). The third reversible 2e− oxidation wave for 1 at 0.67 V is assigned to the s-CPDT core.45 On the other hand, spiro-MeOTAD showed two reversible 1e− and one 2e− oxidation processes at −0.03, 0.13 and 0.34 V vs. Fc/Fc+. The additional phenyl spacer between the spiro-CPDT core and arylamine units in 1 introduces conformational flexibility to the molecule, thus exhibited poor electronic communication among the redox centers. Therefore, the 210 mV difference between the second and third oxidation waves in spiro-MeOTAD merges together showing a single wave at 0.13 V in HTM 1.
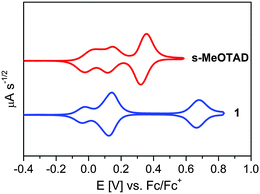 | ||
| Fig. 3 Time semi-derivative convoluted cyclic voltammograms of HTM 1 and spiro-MeOTAD measured in dichloromethane–TBAPF6 (0.1 M), scan speed 100 mV s−1, potentials vs. Fc/Fc+. | ||
By applying the space-charge limited current (SCLC) method, the hole mobilities of about 8.5 × 10−5 cm2 V−1 s−1 and 6.0 × 10−6 cm2 V−1 s−1 were determined for spiro-MeOTAD and spiro-CPDT based HTM 1, respectively (Fig. S2, ESI†). Similar mobility values for spiro-OMeTAD were also reported in the literature.44,46 Interestingly, the hole mobility for HTM 1 was improved by an order of magnitude to 3.0 × 10−5 cm2 V−1 s−1 when the same hole only device was treated under light illumination for about five minutes. No light effect was observed for the spiro-MeOTAD device. This effect of changes in the mobility could be further visible in the solar cell performance.
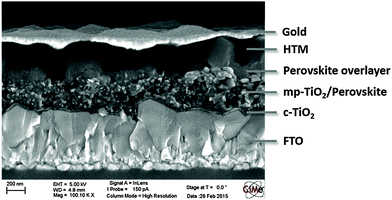
Perovskite solar cells were fabricated using device architecture FTO/compact-TiO2/mp-TiO2/CH3NH3PbI3/HTM/Au. The cross-section scanning electron microscopy (SEM) image in Fig. 4 demonstrates the typical device structure based on different TiO2 layers, perovskite and HTM. As can be seen the perovskite penetrates into the mesoporous TiO2 (mp-TiO2) layer and forms an overlayer. The hole transport material then fills in the remaining pores in the TiO2/perovskite layer and forms a thick capping layer on the top as well. Finally, the devices were completed by the evaporation of the gold electrode.
Fig. 5 presents the current density–voltage (J–V) characteristic of perovskite solar cells using 1 and spiro-MeOTAD as HTMs measured in dark and under illumination. The photovoltaic results are summarized in Table 2. It is well known that the device performance, specifically for spiro-MeOTAD, can be significantly improved by the addition of additives and dopants. Therefore, tert-butylpyridine (tBP) and Li-bis(trifluoromethanesulfonyl)imide (Li-TFSI) were used as additives and a cobalt complex FK209 is used as the p-dopant. In the presence of additives, HTM 1 based devices gave an efficiency between 9 and 10.3% with short circuit current density (JSC) up to 13.8 mA cm−2 (Fig. S3, ESI†). The PCE of 10.3% obtained using additives is very similar to the value of 10.4% reported by Wang and co-workers.42 However, in the presence of these additives, the devices also show huge decrease in the fill factor when measured in forward scan (scanning from short-circuit to open-circuit), which also caused an apparent drop in PCE from 9.6 to 7.3% (Fig. S4, ESI†). The presence of additives and dopants can slightly influence the charge transfer properties of HTM 1 and consequently, modify the hole extraction rates.17,47,48 Holes are extracted less efficiently than electrons and accumulate at the interface. This unbalanced charge distribution in the perovskite solar cell with doped HTM 1 could be the major cause for the hysteresis effect. Doping with FK209 further reduced the PCE to 6% due to the lowering of all parameters.
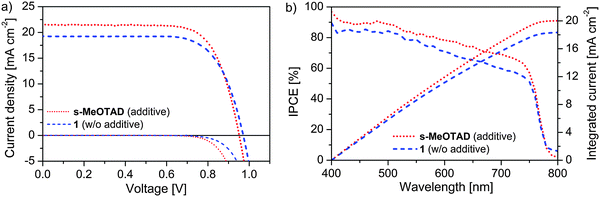 | ||
| Fig. 5 (a) J–V characteristics and (b) IPCE spectra and integrated current of the best devices based on HTM 1 without (w/o) and spiro-MeOTAD with additives and dopants. | ||
| HTM | Additives | J SC [mA cm−2] | V OC [mV] | FF [%] | PCE [%] | Average PCE ± std dev. [%] |
|---|---|---|---|---|---|---|
| 1 | — | 19.3 | 971 | 72 | 13.4 | 13.2 ± 0.28 |
| spiro-MeOTAD | — | 17.6 | 835 | 49 | 7.2 | 6.98 ± 0.19 |
| 1 | TBP + Li | 13.8 | 982 | 76 | 10.3 | 9.6 ± 0.6 |
| 1 | TBP + Li + FK209 | 10.0 | 891 | 67 | 6.0 | 5.5 ± 0.55 |
| spiro-MeOTAD | TBP + Li + FR209 | 21.6 | 951 | 73 | 15.0 | 14.74 ± 0.36 |
Most importantly, we observed that the photovoltaic performance with HTM 1 could be further improved without the use of any additives and dopants. The best device generated a PCE of 13.4% due to the improved JSC of 19.3 mA cm−2, an open circuit voltage (VOC) of 971 mV and a fill factor (FF) of 71.6%. The average PCE on a batch of five identical devices with HTM 1 is 13.2 ± 0.28% (Table S1, ESI†). Moreover, a small hysteresis was also observed in the J–V curve, where a PCE at forward scan was measured to be about 92% of the PCE at backward scan (scanning from open-circuit to short-circuit) (Fig. S5 and Table S2, ESI†). For comparison devices with state-of-the art spiro-MeOTAD were also fabricated. The best result with spiro-MeOTAD was obtained in the presence of additives and dopants giving rise to a JSC value of 21.6 mA cm−2, VOC of 952 mV and FF of 73.3% and an overall PCE of 15% with an average PCE of 14.74 ± 0.36%. In the absence of dopants spiro-MeOTAD based devices generated a PCE of only 7.2% due to the significant lowering of VOC and FF.
The IPCE spectrum of the cell with both HTMs is presented in Fig. 5b. The integrated current densities estimated from the IPCE spectra (18.3 mA cm−2 for 1 and 20.0 mA cm−2 for spiro-MeOTAD) were in good agreement with the JSC values obtained from the J–V measurements.
It should be mentioned that light-soaking plays a crucial role in the performance improvement of HTM 1-based devices. The light-soaking effect has been shown to improve the performance in dye-sensitized solar cells49–51 and organic photovoltaics52 and was explained to be due to photoinduced doping. Very recently, McGehee and co-workers have reported that light-soaking has a strong effect on the performance of thin film planar-heterojunction perovskite solar cells, however, no effect was observed in the case of mp-TiO2-based devices using spiro-MeOTAD as the HTM.43 We have performed a similar experiment using HTM 1 and the J–V curve is displayed in Fig. 6. The device performance specifically the FF improved significantly with prolonged light soaking and was saturated after about five minutes (Table S3, ESI†). We attribute this enhanced performance to the increased hole mobility of HTM 1 upon light exposure. To obtain this optimum photovoltaic performance, the devices were exposed to light only during the first measurement and no additional photo-doping is required afterwards. On the other hand, light soaking does not have any additional influence on the performance of spiro-MeOTAD-based devices. However, further research is desirable for the detailed understanding of this phenomenon.
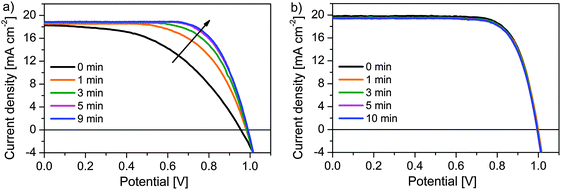 | ||
| Fig. 6 Positive effect of light-soaking at forward bias on the device performance of (a) HTM 1 and (b) spiro-MeOTAD. Light soaking was performed at 1.1 V. | ||
Conclusions
In conclusion, we have designed and synthesized a new triarylamine-substituted spiro-cyclopentadithiophene based hole transporting material for efficient perovskite solar cells. The new HTM 1 exhibited red-shifted absorption compared to spiro-MeOTAD in both solution and thin films. Our preliminary results on perovskite devices fabricated using HTM 1 gave impressive PCEs of up to 13.4% without any doping which is very close to p-doped spiro-MeOTAD devices. This is one of the highest values reported for chemically undoped perovskite solar cells fabricated using the sequential deposition method. Light soaking also plays a crucial role in the improvement of this device performance. Further research needs to be undertaken in order to understand the light soaking behavior and the hysteresis effect on the device performance. The present result provides that the spiro-CPDT is a promising alternative building block for the preparation of high efficiency additives and dopant-free HTMs and has great potential for future applications. Further studies towards this direction are in progress.Acknowledgements
Financial support from the Swiss National Science Foundation (SNF)-NRP70 (PV2050, 407040-153990 and 40740-153952) is gratefully acknowledged. M.G. thanks the Swiss Confederation for funding under the Sciex-NMS exchange program (Project Code 13.194).References
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Nature, 2015, 517, 476–480 CrossRef CAS PubMed.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed.
- N.-G. Park, Mater. Today, 2014, 18, 65–72 CrossRef PubMed.
- P. P. Boix, K. Nonomura, N. Mathews and S. G. Mhaisalkar, Mater. Today, 2014, 17, 16–23 CrossRef CAS PubMed.
- S. Shi, Y. Li, X. Li and H. Wang, Mater. Horiz., 2015, 2, 378–405 RSC.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- P. Gao, M. Gratzel and N. Mohammad, Energy Environ. Sci., 2014, 7, 2448–2463 CAS.
- S. P. Singh and P. Nagarjuna, Dalton Trans., 2014, 43, 5247–5251 RSC.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- J. H. Kim, P.-W. Liang, S. T. Williams, N. Cho, C.-C. Chueh, M. S. Glaz, D. S. Ginger and A. K.-Y. Jen, Adv. Mater., 2015, 27, 695–701 CrossRef CAS PubMed.
- Z. Xiao, Q. Dong, C. Bi, Y. Shao, Y. Yuan and J. Huang, Adv. Mater., 2014, 26, 6503–6509 CrossRef CAS PubMed.
- H. Zhou, Q. Chen, G. Li, S. Luo, T.-B. Song, H.-S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345, 542–546 CrossRef CAS PubMed.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Science, 2015, 348, 1234–1237 CrossRef CAS PubMed.
- N. Ahn, D.-Y. Son, I.-H. Jang, S. M. Kang, M. Choi and N.-G. Park, J. Am. Chem. Soc., 2015, 137, 8696–8699 CrossRef CAS PubMed.
- S. D. Stranks, P. K. Nayak, W. Zhang, T. Stergiopoulos and H. J. Snaith, Angew. Chem., Int. Ed., 2015, 54, 3240–3248 CrossRef CAS PubMed.
- J. Burschka, A. Dualeh, F. Kessler, E. Baranoff, N.-L. Cevey-Ha, C. Yi, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2011, 133, 18042–18045 CrossRef CAS PubMed.
- N. J. Jeon, J. Lee, J. H. Noh, M. K. Nazeeruddin, M. Grätzel and S. I. Seok, J. Am. Chem. Soc., 2013, 135, 19087–19090 CrossRef CAS PubMed.
- N. J. Jeon, H. G. Lee, Y. C. Kim, J. Seo, J. H. Noh, J. Lee and S. I. Seok, J. Am. Chem. Soc., 2014, 136, 7837–7840 CrossRef CAS PubMed.
- K. Do, H. Choi, K. Lim, H. Jo, J. W. Cho, M. K. Nazeeruddin and J. Ko, Chem. Commun., 2014, 50, 10971–10974 RSC.
- H. Choi, S. Paek, N. Lim, Y. Lee, M. K. Nazeeruddin and J. Ko, Chem. – Eur. J., 2014, 20, 10894–10899 CrossRef CAS PubMed.
- H. Li, K. Fu, A. Hagfeldt, M. Grätzel, S. G. Mhaisalkar and A. C. Grimsdale, Angew. Chem., Int. Ed., 2014, 53, 4085–4088 CrossRef CAS PubMed.
- P. Qin, N. Tetreault, M. I. Dar, P. Gao, K. L. McCall, S. R. Rutter, S. D. Ogier, N. D. Forrest, J. S. Bissett, M. J. Simms, A. J. Page, R. Fisher, M. Grätzel and M. K. Nazeeruddin, Adv. Energy Mater., 2014, 5, 1400980 Search PubMed.
- H. Choi, S. Park, S. Paek, P. Ekanayake, M. K. Nazeeruddin and J. Ko, J. Mater. Chem. A, 2014, 2, 19136–19140 CAS.
- K. Thirumal, K. Fu, P. P. Boix, H. Li, T. M. Koh, W. Leong, S. Powar, A. C. Grimsdale, M. Gratzel, N. Mathews and S. G. Mhaisalkar, J. Mater. Chem. A, 2014, 2, 6305–6309 Search PubMed.
- M. Cheng, B. Xu, C. Chen, X. Yang, F. Zhang, Q. Tan, Y. Hua, L. Kloo and L. Sun, Adv. Energy Mater., 2015, 1401720 Search PubMed.
- M. Cheng, C. Chen, X. Yang, J. Huang, F. Zhang, B. Xu and L. Sun, Chem. Mater., 2015, 27, 1808–1814 CrossRef CAS.
- p. Ganesan, K. Fu, P. Gao, I. Raabe, K. Schenk, R. Scopelliti, J. Luo, L. H. Wong, M. Gratzel and M. K. Nazeeruddin, Energy Environ. Sci., 2015, 8, 1986–1991 CAS.
- T. Swetha and S. P. Singh, J. Mater. Chem. A, 2015, 3, 18329–18344 CAS.
- J. H. Heo, S. H. Im, J. H. Noh, T. N. Mandal, C.-S. Lim, J. A. Chang, Y. H. Lee, H.-j. Kim, A. Sarkar, M. K. Nazeeruddin, M. Grätzel and S. I. Seok, Nat. Photonics, 2013, 7, 486–491 CrossRef CAS PubMed.
- Y. S. Kwon, J. Lim, H.-J. Yun, Y.-H. Kim and T. Park, Energy Environ. Sci., 2014, 7, 1454–1460 CAS.
- Y. Guo, C. Liu, K. Inoue, K. Harano, H. Tanaka and E. Nakamura, J. Mater. Chem. A, 2014, 2, 13827–13830 CAS.
- D. Zhao, M. Sexton, H.-Y. Park, G. Baure, J. C. Nino and F. So, Adv. Energy Mater., 2014, 1401855 Search PubMed.
- P. Nagarjuna, K. Narayanaswamy, T. Swetha, G. H. Rao, S. P. Singh and G. D. Sharma, Electrochim. Acta, 2015, 151, 21–26 CrossRef CAS PubMed.
- P. Qin, H. Kast, M. K. Nazeeruddin, S. M. Zakeeruddin, A. Mishra, P. Bäuerle and M. Grätzel, Energy Environ. Sci., 2014, 7, 2981–2985 CAS.
- P. Qin, S. Paek, M. I. Dar, N. Pellet, J. Ko, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2014, 136, 8516–8519 CrossRef CAS PubMed.
- Y. Song, S. Lv, X. Liu, X. Li, S. Wang, H. Wei, D. Li, Y. Xiao and Q. Meng, Chem. Commun., 2014, 50, 15239–15242 RSC.
- J. Liu, W. Yongzhen, C. Qin, X. Yang, T. Yasuda, A. Islam, K. Zhang, W. Peng, W. Chen and L. Han, Energy Environ. Sci., 2014, 7, 2963–2967 CAS.
- C. Steck, M. Franckevičius, S. M. Zakeeruddin, A. Mishra, P. Bäuerle and M. Grätzel, J. Mater. Chem. A, 2015, 3, 17738–17746 CAS.
- T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011–1065 CrossRef CAS PubMed.
- G. Pozzi, S. Orlandi, M. Cavazzini, D. Minudri, L. Macor, L. Otero and F. Fungo, Org. Lett., 2013, 15, 4642–4645 CrossRef CAS PubMed.
- S. Ma, H. Zhang, N. Zhao, Y.-B. Cheng, M. Wang, Y. Shen and G. Tu, J. Mater. Chem. A, 2015, 3, 12139–12144 CAS.
- E. L. Unger, E. T. Hoke, C. D. Bailie, W. H. Nguyen, A. R. Bowring, T. Heumuller, M. G. Christoforo and M. D. McGehee, Energy Environ. Sci., 2014, 7, 3690–3698 CAS.
- T. Leijtens, I. K. Ding, T. Giovenzana, J. T. Bloking, M. D. McGehee and A. Sellinger, ACS Nano, 2012, 6, 1455–1462 CrossRef CAS PubMed.
- J. Londenberg, T. P. I. Saragi, I. Suske and J. Salbeck, Adv. Mater., 2007, 19, 4049–4053 CrossRef CAS PubMed.
- H. J. Snaith and M. Grätzel, Adv. Mater., 2007, 19, 3643–3647 CrossRef CAS PubMed.
- Y. Liu, Q. Chen, H.-S. Duan, H. Zhou, Y. Yang, H. Chen, S. Luo, T.-B. Song, L. Dou, Z. Hong and Y. Yang, J. Mater. Chem. A, 2015, 3, 11940–11947 CAS.
- A. Abate, D. R. Staff, D. J. Hollman, H. J. Snaith and A. B. Walker, Phys. Chem. Chem. Phys., 2014, 16, 1132–1138 RSC.
- U. B. Cappel, T. Daeneke and U. Bach, Nano Lett., 2012, 12, 4925–4931 CrossRef CAS PubMed.
- P. Tiwana, P. Docampo, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2012, 5, 9566–9573 CAS.
- L. Yang, B. Xu, D. Bi, H. Tian, G. Boschloo, L. Sun, A. Hagfeldt and E. M. J. Johansson, J. Am. Chem. Soc., 2013, 135, 7378–7385 CrossRef CAS PubMed.
- J. Gilot, M. M. Wienk and R. A. J. Janssen, Appl. Phys. Lett., 2007, 90, 143512 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5mh00154d |
| This journal is © The Royal Society of Chemistry 2015 |