Planar trinuclear complexes with linear arrays of metal ions†
Shotaro
Terashima
,
Graham N.
Newton
,
Takuya
Shiga
* and
Hiroki
Oshio
*
Graduate School of Pure and Applied Sciences, University of Tsukuba, Tennodai 1-1-1, Tsukuba 305-8571, Japan. E-mail: oshio@chem.tsukuba.ac.jp
First published on 26th December 2014
Abstract
Two trinuclear complexes, [CuII3(L)2](BF4)2 (1) and [NiII3(L)2(MeOH)4](BF4)2 (2), supported by the multidentate ligand L2− (H2L = 5,5′-pyridyl-3,3′-bi-1H-pyrazole) were obtained and their structures determined. Both complexes have linear trinuclear structures composed of two ligands and three metal ions. Cryomagnetic studies reveal that both complexes show intramolecular antiferromagnetic interactions between the metal ions.
Coordination architectures with varied topologies can be constructed through the self-assembly of transition metals and multidentate ligands, and their structure-dependent physical properties and functionalities have been intensively investigated.1 The assembly of metal ions into specific shapes, structures and arrays can be achieved through the considered choice of ‘pre-programmed’ ligands with clearly defined binding sites and orientations.2 When using rigid ligands with linear bridging coordination modes, network compounds are commonly formed. For example, metal–organic-frameworks (MOFs) are often constructed from rigid ligands, which can linearly connect between metal ions.1a In contrast, flexible bridging ligands can effectively form discrete polynuclear cluster complexes, such as oxo-bridged polynuclear clusters which can be supported by multidentate alkoxo ligands.3 Polynuclear complexes with regular arrays of metal ions are of particular interest to chemists due to the synergy between their physical properties and their clearly defined specific structures.4 For example, molecular wires composed of metal ions with metal–metal bonds were investigated,1c and may have applications in molecular nano-electronics, while triangle and grid-like cluster molecules exhibit specific electronic structures and spin ground states.5,6
To date, we have studied ring, helix, and grid molecules supported by rigid and planar multidentate ligands.7 Based on our previous research, it is clear that pyridine–pyrazolate ligands can be very useful in the construction of multinuclear complexes through self-assembly based on their preprogrammed transition metal bridging behaviour and propensity for supramolecular π–π stacking interactions. We previously reported the synthesis and characterization of a [3 × 3] grid-like nonanuclear cobalt single-molecule magnet and multiredox active [3 × 3] copper grids.7b The complexes are constructed using a pyridine–pyrazolate ligand with one tridentate and two bidentate binding sites. Ligands with such defined coordination abilities have great possibilities for the formation of coordination architectures with predictable, stable structures and tuneable physical properties. As a result we are now extending our study of the complex structures accessible with planar and rigid multidentate ligands.
In this work, we use a pyridine–pyrazolate ligand H2L (5,5′-pyridyl-3,3′-bi-1H-pyrazole)8 with two types of bidentate binding sites. Two novel complexes with the bridging ligand L2−, [CuII3(L)2](BF4)2 (1) and [NiII3(L)2(MeOH)4](BF4)2 (2), are isolated, their structures determined by single-crystal X-ray structural analyses and their magnetic properties investigated.
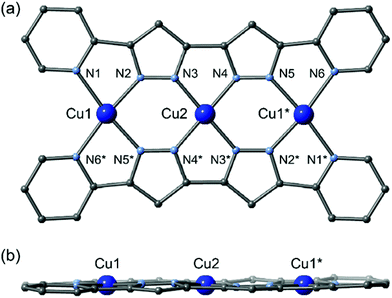
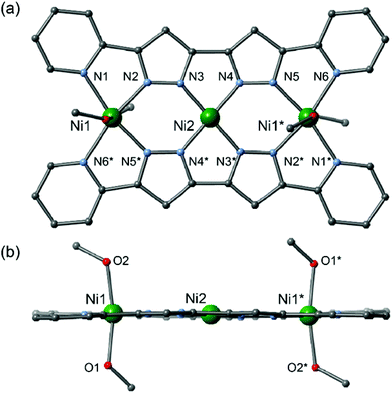
The planar multidentate pyridine–pyrazole ligand H2L was synthesized by a modified version of a previously reported synthetic method.8 Reactions of the planar ligand H2L with metal sources afford the linear trinuclear complexes 1 and 2.9,10 X-ray structural analyses reveal that both complexes have linear trinuclear structures composed of two L2− ligands and three metal ions arranged to form planar molecules. The three metal ions can be categorized into two types: terminal metal ions located at the edge of the molecule with four nitrogen donor atoms from two pyridine and two pyrazole donor groups; and central metal ions with four nitrogen donors from four pyrazole groups (Fig. 1).
1 crystalized in the monoclinic space group P21/n,11 with an inversion centre on the Cu2 ion. All copper ions have the same square-planar four-coordinate geometry without any solvent coordination, and their equatorial planes lie on the plane described by the two supporting ligands. The BF4− counterion weakly interacts with the Cu1 ion in its apical position. While Cu2 in the central coordination site has an average bond length of 1.884(7) Å, the Cu1 ions have an average bond distance of 2.026(8) Å. The Cu1 ion deviates from the equatorial plane defined by N1, N2, N5*, and N6* atoms by 0.030(4) Å, while the Cu2 ion lies on the N3, N4, N3*, and N4* plane. Significant π–π stacking interactions can be observed, and a one-dimensional columnar structure is formed (Fig. S1†). The intramolecular metallic separations of Cu1⋯Cu2 and Cu1⋯Cu1* are 3.865(5) and 7.731(11) Å, respectively. The shortest intermolecular metal–metal distance (Cu1⋯Cu2#1) is 3.756(4) Å (symmetry code #1: +x + 1, +y, +z).
2 crystalized in the monoclinic space group P21/c,12 with an inversion centre located on the central Ni2 ion. The Ni2 ion is coordinated by four N-donating pyrazole moieties, resulting in a square-planar geometry, and an average bond length of 1.843(4) Å. The Ni1 ions in the terminal sites have distorted octahedral geometries, with four nitrogen donors from the ligands in the equatorial sites and two oxygen donors from solvent methanol molecules in the apical sites, and an average bond length of 2.073(5) Å. Complex 2 has a planar structure similar to that of 1, but the π-systems of neighbouring complexes are prevented from overlapping due to the coordination of the axial methanol molecules. The intracluster Ni1⋯Ni2 and Ni2⋯Ni2* metal–metal separations are 3.9491(14) and 7.898(3) Å, respectively, while the shortest intermolecular metal–metal distance is 8.004(3) Å, for Ni1#1⋯Ni1#2 (symmetry code #1: −x, −y, −z, #2: +x + 1, +y, +z). Elemental analyses of the air-dried samples of 1 and 2 gave compositions of [Cu3(L)2](BF4)2·1.5H2O (1′) and [Ni3(L)2(MeOH)2(H2O)2](BF4)2·2H2O (2′), respectively. The formula changes arise from solvent exchange and moisture absorption on the crystal surfaces. As no crystal colour changes were observed, the following magnetic studies were discussed based on the assumption that no significant changes in coordination geometry had occurred (Fig. 2).
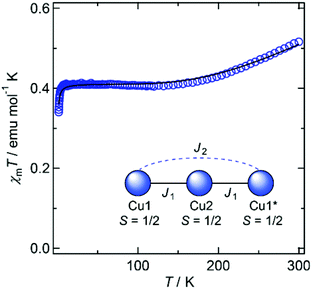
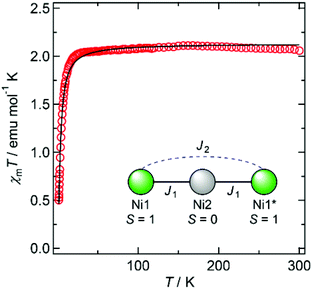
Magnetic susceptibility data for 1′ and 2′ are shown in Fig. 3 and 4, respectively. The χmT values of both compounds decrease as the temperature is lowered, indicating the occurrence of antiferromagnetic interactions between metal ions in both species.
In 1′, all copper ions lie on the molecular plane defined by the two ligands, and share their equatorial planes. The strong overlap of magnetic orbitals on the molecular plane ensures that substantial coupling constants can be expected. A spin model of the linear trinuclear core was applied to the magnetic analyses,13 and the data fitted to give intramolecular coupling constants of J1 = −194 cm−1 and J2 = −4.5 cm−1 with gCu = 2.09, and an intermolecular component of zJ′ = −0.178 cm−1.
Considering the coordination geometry of 2′, it is suggested that the central Ni2 ion is diamagnetic, while the terminal Ni1 (and Ni1*) ions are paramagnetic. The magnetic behaviour of 2′ can therefore be analysed using a dinuclear spin model,14 in which J1 can be fixed at zero, to give J2 = −1.01 cm−1 and gNi = 2.06.
In summary, two planar trinuclear complexes, [CuII3(L)2](BF4)2 (1) and [NiII3(L)2(MeOH)4](BF4)2 (2), were synthesized and their structures and magnetic properties studied. The synthesis of planar clusters is important for the development of novel functional molecules.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (“Coordination Programming” Area 2107, no. 21108006) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by a Grant-in-Aid for Scientific Research (no. 25248014) from the Japan Society for the Promotion of Science (JSPS).
Notes and references
- (a) MOFs: S. L. James, Chem. Soc. Rev., 2003, 32, 276–288 RSC; S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 Search PubMed; A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58–67 Search PubMed; (b) Nano-sheets: T. Kambe, R. Sakamoto, K. Hoshiko, K. Takada, M. Miyachi, J.-H. Ryu, S. Sasaki, J. Kim, K. Nakazato, M. Takata and H. Nishihara, J. Am. Chem. Soc., 2013, 135, 2462–2464 CrossRef CAS PubMed; (c) Wires: C. Coulon, H. Miyasaka and R. Clerác, Struct. Bonding, 2006, 122, 163–206 CrossRef CAS; I. P.-C. Liu, W.-Z. Wang and S.-M. Peng, Chem. Commun., 2009, 4323–4331 Search PubMed; P. R. Andres and U. S. Schubert, Adv. Mater., 2004, 16, 1043–1068 Search PubMed; J. K. Bera and K. R. Dunbar, Angew. Chem., Int. Ed., 2002, 41, 4453–4457 Search PubMed; T. Murahashi, K. Shirato, A. Fukushima, K. Takase, T. Suenobu, S. Fukuzumi, S. Ogoshi and H. Kurosawa, Nat. Chem., 2012, 4, 52–58 Search PubMed.
- (a) J.-M. Lehn, Supramolecular Chemistry – Concepts and Perspectives, VCH, Weinheim, 1995 Search PubMed; (b) R. W. Saalfrank, H. Maid and A. Scheurer, Angew. Chem., Int. Ed., 2008, 47, 8794–8824 CrossRef CAS PubMed; (c) T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed.
- T. C. Stamatatos and G. Christou, Inorg. Chem., 2009, 48, 3308–3322 CrossRef CAS PubMed.
- (a) G. Mezei, C. M. Zaleski and V. L. Pecoraro, Chem. Rev., 2007, 107, 4933–5003 CrossRef CAS PubMed; (b) E. K. Brechin, Chem. Commun., 2005, 5141–5153 RSC.
- K. Y. Choi, Y. H. Matsuda, H. Nojiri, U. Kortz, F. Hussain, A. C. Stowe, C. Ramsey and N. S. Dalal, Phys. Rev. Lett., 2006, 96, 107202 CrossRef PubMed.
- (a) L. N. Dawe, K. V. Shuvaev and L. K. Thompson, Chem. Soc. Rev., 2009, 38, 2334–2359 RSC; (b) M. Ruben, J. Rojo, F. J. Romero-Salguero, L. H. Uppadine and J.-M. Lehn, Angew. Chem., Int. Ed., 2004, 43, 3644–3662 CrossRef CAS PubMed; (c) L. K. Thompson, O. Waldmann and Z. Xu, Coord. Chem. Rev., 2005, 249, 2677–2690 CrossRef CAS.
- (a) T. Shiga, M. Noguchi, H. Sato, T. Matsumoto, G. N. Newton and H. Oshio, Dalton Trans., 2013, 42, 16185–16193 RSC; (b) H. Sato, L. Miya, K. Mitsumoto, T. Shiga, G. N. Newton and H. Oshio, Inorg. Chem., 2013, 52, 9714–9716 CrossRef CAS PubMed; (c) G. N. Newton, T. Onuki, T. Shiga, M. Noguchi, T. Matsumoto, J. S. Mathieson, M. Nihei, L. Cronin and H. Oshio, Angew. Chem., Int. Ed., 2011, 50, 4844–4848 CrossRef CAS PubMed; (d) T. Shiga, T. Matsumoto, M. Noguchi, T. Onuki, N. Hoshino, G. N. Newton, M. Nakano and H. Oshio, Chem. – Asian J., 2009, 4, 1660–1663 CrossRef CAS PubMed.
- J. Janculev and B. Podolesov, God. Zb., Filoz. Fak. Univ. Skopje, Prir.-Mat. Oddel, 1958, 11, 47–49 CAS.
- Synthesis of [CuII3(L)2](BF4)2 (1): To a methanolic solution (20 mL) of Cu(BF4)2·nH2O (286 mg, 0.90 mmol) was added a methanolic suspension (15 mL) of H2L (43.0 mg, 0.15 mmol) and Et3N (41.3 μL, 0.30 mmol). After stirring for several minutes, 35 mL of acetonitrile was added to the reaction mixture. The resulting brown solution was filtered and the filtrate was diffused by diethyl ether. Yellow needles of [CuII3(L)2](BF4)2 (1) were crystalized. The crystals were collected in vacuo and exposed to air to obtain [CuII3(L)2](BF4)2·1.5H2O (1′). Yield: 27 mg (39%). Anal. calcd for 1′ [Cu3(L)2](BF4)2·1.5H2O (C32H23N12B2F8O1.5Cu3) (%): C, 39.88; H, 2.41; N, 17.44. Found (%) C, 39.90; H, 2.21; N, 17.25.
- Synthesis of [NiII3(L)2(MeOH)4](BF4)2 (2): To a methanolic solution (7.5 mL) of Ni(BF4)2·6H2O (61 mg, 0.18 mmol) was added a methanolic suspension (5 mL) of H2L (8.6 mg, 0.03 mmol) and Et3N (8.3 μL, 0.06 mmol). The resulting green solution was filtered and the filtrate was diffused by diethyl ether. Green needles of [NiII3(L)2(MeOH)4](BF4)2 (2): were crystalized. The crystals were collected in vacuo and exposed to air to obtain [NiII3(L)2(MeOH)2(H2O)2](BF4)2·2H2O (2′). Yield: 21.5 mg (28%). Anal. calcd for 2′ [NiII3(L)2(MeOH)2(H2O)2](BF4)2·2H2O (C34H36N12B2F8O6Ni3) (%) : C, 38.58; H, 3.43; N, 15.88. Found (%) C, 38.78; H, 3.38; N, 16.15.
- Crystallographic data for 1 (C32H20N12B2F8Cu3 = 936.84 g mol−1): monoclinic, P21/n, a = 6.262(11) Å, b = 23.89(4) Å, c = 11.132(19) Å, β = 104.46(3)°, V = 1612(5) Å3, Z = 2, Dcalcd = 1.930 g cm−3, R1 = 0.0868 (I > 2σ(I)), wR2 = 0.2268 (all data) (CCDC 1020899).
- Crystallographic data for 2 (C36H32N12B2F8O4Ni3 = 1046.48 g mol−1): monoclinic, P21/c, a = 9.771(4) Å, b = 14.196(6) Å, c = 14.513(6) Å, β = 90.374(8)°, V = 2013.1(15) Å3, Z = 2, Dcalcd = 1.726 g cm−3, R1 = 0.0827 (I > 2σ(I)), wR2 = 0.299 (all data) (CCDC 1020900).
- Spin Hamiltonian of the {Cu3} system: H = −2J1(SCu1SCu2 + SCu1*SCu2) − 2J2(SCu1SCu1*).
- Spin Hamiltonian of the {Ni3} system: H = −2J1(SNi1SNi2 + SNi1*SNi2) − 2J2(SNi1SNi1*).
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of ligands, packing diagrams and crystallographic details. CCDC 1020899 (1) and 1020900 (2). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00172a |
| This journal is © the Partner Organisations 2015 |