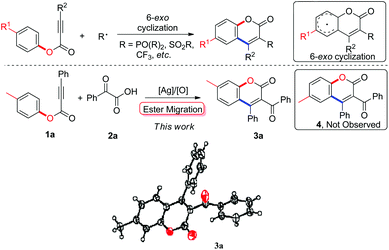
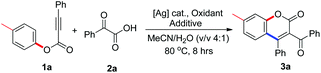Radical 5-exo cyclization of alkynoates with 2-oxoacetic acids for synthesis of 3-acylcoumarins†
Tong
Liu
a,
Qiuping
Ding
*a,
Qianshou
Zong
b and
Guanyinsheng
Qiu
*b
aCollege of Chemistry and Chemical Engineering, Jiangxi Normal University, Nanchang 330013, China. E-mail: dqpjxnu@gmail.com
bCollege of Biological, Chemical Science and Engineering, Jiaxing University, 118 Jiahang Road, Jiaxing 314001, China. E-mail: 11110220028@fudan.edu.cn
First published on 31st March 2015
Abstract
A silver-promoted decarboxylative annulation of alkynoates with 2-oxoacetic acids leading to the formation of 3-acyl-4-arylcoumarins is reported. The process involves radical decarboxylative acylation, 5-exo cyclization, and ester migration.
The coumarin scaffold, which is found in many bioactive natural products and pharmaceuticals, is generally accepted as one of the most important classes of heterocyclic compounds.1 Consequently, much attention has been paid to developing methodology for facile, mild, and efficient syntheses of coumarin derivatives.2 One notable achievement has been the development of alkynoate-based radical 6-endo cyclization, which has been used to install many functional groups, such as phosphonates and arylsulfonyl groups, into the coumarin skeleton with high efficiency and broad substrate scope (Scheme 1).3 Very recently, we developed a novel radical pathway to introduce a trifluoromethyl group into the coumarin core at the 3-position, in which alkynoates and Togni's reagent were employed as starting materials, and an array of 3-trifluoromethylcoumarins were produced efficiently under mild conditions.3c In this process, radical trifluoromethylation of alkynoates and 6-endo cyclization produced the desired 3-trifluoromethylcoumarins. As a continuation of our interest in methodology development for the synthesis of privileged heterocycles,4 we wished to introduce other versatile building blocks into the coumarin structure with the aim of synthesizing various 3-functionalized coumarins in a radical fashion. We thus focused our attention on 3-acylcoumarin architectures, a class of coumarins with bioactivities including antioxidant, antitumor, and anti-inflammatory activity.5 Traditional routes to 3-acylcoumarins have mainly focused on substrate-based condensation.6 Understandably, the development of new synthetic methodology is highly desirable.
It is well-known that carbonyl radicals can be readily generated from aldehydes.7 Possibly due to the fact that aldehydes are readily oxidized, an excess of aldehyde has always been necessary for this type of reaction. As an elegant alternative source, 2-oxoacetic acids can provide acyl radicals through silver-catalyzed decarboxylation.8 Therefore, we anticipated that 3-acylcoumarins could be obtained through radical silver-promoted decarboxylative annulation of alkynoates with 2-oxoacetic acids.
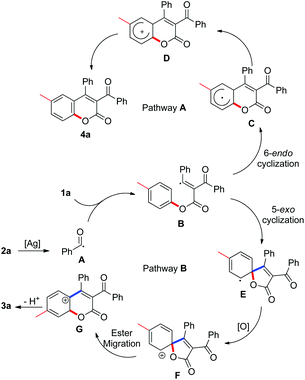
In our preliminary tests, we were pleased to find that the model reaction of alkynoate 1a with 2-phenyl-2-oxoacetic acid 2a resulted in a 38% yield of product in the presence of 20 mol% Ag2CO3 and 2.0 equiv. K2S2O8 in DMF at 80 °C. To our surprise, structural identification by NMR and X-ray diffraction suggested that the product was not the anticipated one (4a), but an unexpected compound 3a (Scheme 1). Structural comparison of compound 3a with 4a revealed that ester migration probably took place in the reaction to give 3a.
However, very recent findings by Wu indicated that acyl radicals, which were derived from aldehydes, reacted with alkynoates to form products of the 4a variety.9 The process involved radical acylation and 6-endo cyclization (pathway A, Scheme 2). From these results, we were convinced that our transformation proceeded through another pathway distinctive from Wu's. In the light of previous work by our group and others, a tandem process combining radical acylation, 5-exo annulation,10 and ester migration was proposed to explain the formation of the unexpected product 3a (pathway B, Scheme 2). As illustrated in Scheme 2, acyl radical species A, generated from silver-catalyzed decarboxylation of 2-phenyl-2-oxoacetic acid 2a, is involved in the radical acylation of alkynoate 1a, leading to intermediate B. The following 5-exo cyclization affords a spirocyclic species E, which is readily converted into intermediate F in the presence of oxidant. Ester migration and de-protonation then yields the desired 3-acylcoumarin 3a. The discrepancy with Wu's results can probably be attributed to the relatively slow rate of acyl radical formation through silver-catalyzed decarboxylation. As a result of this, the concentration of intermediate B remains low in our method, such that the reaction proceeds through 5-exo cyclization to form intermediate E specifically.
We next optimized the reaction conditions. All result-influencing factors including the silver source, oxidant, additive, solvent, and temperature were evaluated for the reaction. The results are presented in Table 1. From the results of the solvent screening, it seemed that moisture was critical for the reaction, and a solvent mixture of acetonitrile and water (v/v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was the best choice, providing the desired product 3a in 45% yield (entry 4, Table 1). This is probably due to the fact that water could improve the solubility of the silver salt and oxidant. Changing the catalyst to a different silver salt (silver nitrate) with better solubility in water did not bring about a better result (entry 5, Table 1). Other oxidants including (NH4)2S2O8, Oxone, PhI(OAc)2, and TBHP were also explored, but no improved outcomes were observed (entries 6–9, Table 1). The evaluation of additives revealed that the appropriate choice of base could drastically improve the reaction efficiency. When sodium acetate was used as the additive, the reaction afforded 3a in 74% yield (entry 11, Table 1). Reducing the loading of silver carbonate to 10 mol% gave an inferior yield in the reaction (entry 14, Table 1). Temperature and reaction time only slightly affected the reaction results (data not shown in Table 1). No reaction was observed in the absence of silver carbonate (entry 15, Table 1). A very inefficient reaction occurred when 2.0 equiv. TEMPO was added (entry 16, Table 1), producing the desired molecule 3a in only 8% yield.
1) was the best choice, providing the desired product 3a in 45% yield (entry 4, Table 1). This is probably due to the fact that water could improve the solubility of the silver salt and oxidant. Changing the catalyst to a different silver salt (silver nitrate) with better solubility in water did not bring about a better result (entry 5, Table 1). Other oxidants including (NH4)2S2O8, Oxone, PhI(OAc)2, and TBHP were also explored, but no improved outcomes were observed (entries 6–9, Table 1). The evaluation of additives revealed that the appropriate choice of base could drastically improve the reaction efficiency. When sodium acetate was used as the additive, the reaction afforded 3a in 74% yield (entry 11, Table 1). Reducing the loading of silver carbonate to 10 mol% gave an inferior yield in the reaction (entry 14, Table 1). Temperature and reaction time only slightly affected the reaction results (data not shown in Table 1). No reaction was observed in the absence of silver carbonate (entry 15, Table 1). A very inefficient reaction occurred when 2.0 equiv. TEMPO was added (entry 16, Table 1), producing the desired molecule 3a in only 8% yield.
| Entry | Catalyst | Oxidant | Additive | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
a Reaction conditions: alkynoate 1a (1.0 equiv.), 2-oxoacetic acid 2a (1.2 equiv.), silver carbonate (20 mol%), K2S2O8 (2.0 equiv.), NaOAc (2.5 equiv.), MeCN–H2O (v/v 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C, 8 h.
b Isolated yield based on alkynoate 1a.
c In the presence of 10 mol% of Ag2CO3.
d 2.0 equiv. TEMPO was added. 1), 80 °C, 8 h.
b Isolated yield based on alkynoate 1a.
c In the presence of 10 mol% of Ag2CO3.
d 2.0 equiv. TEMPO was added.
|
|||||
| 1 | Ag2CO3 | K2S2O8 | — | DMF | 38 |
| 2 | Ag2CO3 | K2S2O8 | — | MeCN | 21 |
| 3 | Ag2CO3 | K2S2O8 | — | DMF–H2O | 16 |
| 4 | Ag2CO3 | K2S2O8 | — | MeCN–H2O | 45 |
| 5 | AgNO3 | K2S2O8 | — | MeCN–H2O | 38 |
| 6 | Ag2CO3 | (NH4)2S2O8 | — | MeCN–H2O | 40 |
| 7 | Ag2CO3 | Oxone | — | MeCN–H2O | NR |
| 8 | Ag2CO3 | PhI(OAc)2 | — | MeCN–H2O | NR |
| 9 | Ag2CO3 | TBHP | — | MeCN–H2O | NR |
| 10 | Ag2CO3 | K2S2O8 | Na2CO3 | MeCN–H2O | 52 |
| 11 | Ag 2 CO 3 | K 2 S 2 O 8 | NaOAc | MeCN–H 2 O | 74 |
| 12 | Ag2CO3 | K2S2O8 | KHCO3 | MeCN–H2O | 68 |
| 13 | Ag2CO3 | K2S2O8 | KOH | MeCN–H2O | 71 |
| 14c | Ag2CO3 | K2S2O8 | NaOAc | MeCN–H2O | 62 |
| 15 | — | K2S2O8 | NaOAc | MeCN–H2O | NR |
| 16d | Ag2CO3 | K2S2O8 | NaOAc | MeCN–H2O | 8 |
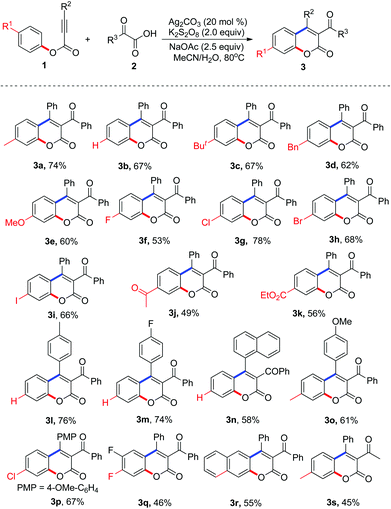
With the optimized conditions in hand (20 mol% Ag2CO3, 2.0 equiv. K2S2O8, 2.5 equiv. NaOAc in MeCN–H2O at 80 °C), we proceeded to examine the scope and generality of the radical decarboxylative annulation of alkynoates 1 with 2-oxoacetic acids 2. The corresponding results are presented in Table 2. From the results shown in Table 2, a series of substituted 3-acylcoumarins 3 were obtained as expected. Electronic effects exerted minimal influence on the yield of the reaction. In the reaction, both electron-rich and electron-deficient groups were tolerated as the R1 substituent. For example, the reaction produced the corresponding products 3c–3e in good yields when R1 was tert-butyl, benzyl, and methoxyl, respectively. Additionally, substrates with electron-withdrawing groups such as halide, acetyl and ester groups proved to be excellent reaction partners, affording the corresponding products 3f–3k in 49–78% yields. Surprisingly, the substituent R2 was limited to aryl groups. When a compound with an alkyl group at the R2 position was used as the substrate, the reaction did not deliver the target molecule (data not shown in Table 2). When R2 was an aryl group, the electronic effect of substituents on the aryl group did not have a great impact on the result, and all the reactions provided the corresponding products in moderate to good yields. Double fluoro-substituted substrate 1q and naphthalenyl alkynoate 1r were compatible with the reaction, forming the expected 3-acylcoumarins 3q and 3r in 46% and 55% yields, respectively. Methyl-substituted 2-oxoacetic acid was also an efficient source of acyl group, giving the expected molecule 3s in 45% yield (Table 3).
| a Isolated yields are given based on alkynoates 1. |
|---|
|
| a Isolated yields are given based on alkynoate 1t. |
|---|
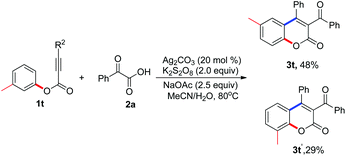
|
Interestingly, when m-methyl alkynoate 1t was employed as the substrate, the reaction afforded a mixture of products 3t and 3t′. The ratio of these two compounds was about 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. To our delight, these two compounds could be isolated and purified by column chromatography.
3. To our delight, these two compounds could be isolated and purified by column chromatography.
In conclusion, we have developed a novel silver-promoted decarboxylative annulation for the synthesis of 3-acylcoumarins with high efficiency and good functional group tolerance. It is believed that radical acylation, 5-exo cyclization and ester migration are involved in this process. This protocol represents the first example of the preparation of coumarin derivatives from alkynoates via radical 5-exo annulation and ester migration. Its application to the synthesis of other 3-substituted coumarins is under way in our lab. The results will be published in due course.
Financial support from the National Natural Science Foundation of China (no. 21262016) is gratefully acknowledged. Additionally, we thank Prof. Jie Wu (Fudan University, China) for valuable discussion.
Notes and references
- For selected examples, see: (a) B. Naser-Hijazi, B. Stolze and K. Zanker, Second Proceedings of the International Society of Coumarin Investigators, Springer, Berlin, 1994 Search PubMed; (b) L. Santana, E. Uriarte, F. Roleira, N. Milhazes and F. Borges, Curr. Med. Chem., 2004, 11, 3239 CrossRef CAS; (c) F. Borges, F. Roleira, N. Milhazes, L. Santana and E. Uriarte, Curr. Med. Chem., 2005, 12, 887 CrossRef CAS; (d) B. M. Trost and F. Tost, J. Am. Chem. Soc., 1996, 118, 6305 CrossRef CAS; (e) X. Peng, G. Damu and C. Zhou, Curr. Pharm. Des., 2013, 19, 3884 CrossRef CAS.
- For selected reviews on the synthesis of coumarins, see: (a) R. Vekariya and H. Patel, Synth. Commun., 2014, 44, 2756 CrossRef CAS; (b) S. Trenor, A. Shulty, B. Love and T. Long, Chem. Rev., 2004, 104, 3059 CrossRef CAS PubMed; (c) S. Sethna and N. Shah, Chem. Rev., 1945, 36, 1 CrossRef CAS.
- For selected examples, see: (a) W. Wie, J. D. Wen, M. Guo, Y. Wang, J. You and H. Wang, Chem. Commun., 2015, 51, 768 RSC; (b) X. Mi, C. Wang, M. Huang, J. Zhang and Y. Wu, Org. Lett., 2014, 16, 3356 CrossRef CAS PubMed; (c) Y. Li, Y. Lu, G. Qiu and Q. Ding, Org. Lett., 2014, 16, 4240 CrossRef CAS PubMed; (d) A. Mantovani, T. Goulart, D. Back, P. Menezes and G. Zeni, J. Org. Chem., 2014, 79, 10526 CrossRef CAS PubMed; (e) M. Yoon, J. Kim, D. Choi, U. Shin, J. Lee and C. Song, Adv. Synth. Catal., 2007, 349, 1725 CrossRef CAS PubMed; (f) C. Jia, D. Piao, T. Kitamura and Y. Fujiwara, J. Org. Chem., 2000, 65, 7516 CrossRef CAS PubMed.
- For selected recent examples, see: (a) J. Song, C. Fan, G. Liu and G. Qiu, Org. Chem. Front., 2014, 1, 1045 RSC; (b) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC.
- For selected recent examples, see: (a) S. Sandhu, Y. Bansal, O. Silakari and G. Bansal, Bioorg. Med. Chem., 2014, 22, 3806 CrossRef CAS PubMed; (b) B.-C. Raju, A.-K. Tiwari, J.-K. Kumar, A.-Z. Ali, S.-B. Agawane, G. Saidahary and K. Madhusudana, Bioorg. Med. Chem., 2010, 18, 358 CrossRef CAS PubMed; (c) M.-M. Heravi, N. Poormohammd, Y.-S. Beheshtiha, B. Baghernejad and R. Malakooti, Chin. J. Chem., 2009, 27, 968 CrossRef CAS PubMed.
- For selected examples, see: (a) H. Sun, Y. Zhang, F. Guo, Y. Yan, C. Wan, Z. Zha and Z. Wang, Eur. J. Org. Chem., 2012, 480 CrossRef CAS PubMed; (b) H. Yuan, M. Wang, Y. Liu, L. Wang, J. Liu and Q. Liu, Chem. – Eur. J., 2010, 16, 13450 CrossRef CAS PubMed; (c) O.-O. Ajania and O.-C. Niwinyi, J. Heterocycl. Chem., 2010, 47, 179 Search PubMed; (d) P. R. Surya and S. Sivakumar, J. Org. Chem., 2006, 71, 8715 CrossRef PubMed; (e) B.-C. Ranu and R. Jana, Eur. J. Org. Chem., 2006, 3767 CrossRef CAS PubMed; (f) D.-P. Specht, P.-A. Martic and S. Farid, Tetrahedron, 1982, 38, 1203 CrossRef CAS.
- For selected examples, see: (a) B.-X. Tang, R.-J. Song, C.-Y. Wu, Y. Liu, M.-B. Zhou, W.-T. Wei, G.-B. Deng, D.-L. Yin and J.-H. Li, J. Am. Chem. Soc., 2010, 132, 8900 CrossRef CAS PubMed; (b) K. Matcha and A. Antonchick, Angew. Chem., Int. Ed., 2013, 52, 2082 CrossRef CAS PubMed; (c) J. Wang, C. Liu, J.-W. Yuan and A.-W. Lei, Angew. Chem., Int. Ed., 2013, 52, 2256 CrossRef CAS PubMed; (d) J.-Y. Luo, H.-L. Hua, Z.-S. Chen, Z.-Z. Zhou, Y.-F. Yang, P.-X. Zhou, Y.-T. He, X.-Y. Liu and Y.-M. Liang, Chem. Commun., 2014, 50, 1564 RSC; (e) Z. Shi and F. Glorius, Chem. Sci., 2013, 4, 829 RSC; (f) H.-H. Rao, X.-Y. Ma, Q.-Z. Liu, Z.-F. Li, S.-L. Cao and C.-J. Li, Adv. Synth. Catal., 2013, 355, 2191 CrossRef CAS PubMed.
- For selected examples, see: (a) H. Wang, L.-N. Guo and X.-H. Duan, Chem. Commun., 2014, 50, 7382 RSC; (b) H. Li, P. Li, Q. Zhao and L. Wang, Chem. Commun., 2013, 49, 9170 RSC; (c) J. Yao, R. Feng, Z. Wu, Z. Liu and Y. Zhang, Adv. Synth. Catal., 2013, 355, 1517 CrossRef CAS PubMed; (d) P. Fang, M. Li and H. Ge, J. Am. Chem. Soc., 2010, 132, 11898 CrossRef CAS PubMed; (e) L. Goossen, B. Zimmermann and T. Knauber, Angew. Chem., Int. Ed., 2008, 47, 7103 CrossRef CAS PubMed; (f) L. Goossen, F. Rudolphi, C. Oppel and N. Rodriguez, Angew. Chem., Int. Ed., 2008, 47, 3043 CrossRef CAS PubMed; (g) F. Fontana, F. Minisci, M. Claudia, N. Barbosa and E. Vismara, J. Org. Chem., 1991, 56, 2866 CrossRef CAS; (h) J. Anderson and J. Kochi, J. Am. Chem. Soc., 1970, 92, 1651 CrossRef CAS.
- X. Mi, C. Wang, M. Huang, Y. Wu and Y. Wu, J. Org. Chem., 2015, 80, 148 CrossRef CAS PubMed.
- For selected examples of 5-exo-trig cyclizations of alkynoates, see: (a) M. Aparece and P. Vadola, Org. Lett., 2014, 16, 6008 CrossRef CAS PubMed; (b) W.-T. Wei, R.-J. Song, X.-H. Ouyang, Y. Li, H.-B. Li and J.-H. Li, Org. Chem. Front., 2014, 1, 484 RSC; (c) B.-X. Tang, D.-J. Tang, S. Tang, Q.-F. Yu, Y.-H. Zhang, Y. Liang, P. Zhong and J.-H. Li, Org. Lett., 2008, 10, 1063 CrossRef CAS PubMed; (d) B.-X. Tang, Y.-H. Zhang, R.-J. Song, D.-J. Tang, G.-B. Deng, Z.-Q. Wang, Y.-X. Xie, Y.-Z. Xia and J.-H. Li, J. Org. Chem., 2012, 77, 2837 CrossRef CAS PubMed; (e) T. Dohi, T. Nakae, Y. Ishikado, D. Kato and Y. Kita, Org. Biomol. Chem., 2011, 9, 6899 RSC; (f) X. Zhang and R. C. Larock, J. Am. Chem. Soc., 2005, 127, 12230 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data, 1H and 13C NMR spectra of compounds 3. CCDC 1043097 (compound 3a). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00029g |
| This journal is © the Partner Organisations 2015 |