Copper-catalyzed direct decarboxylative hydrosulfonylation of aryl propiolic acids with sulfonylhydrazides leading to vinylsulfones†
Siyu
Li
,
Xiang
Li
,
Fan
Yang
* and
Yangjie
Wu
*
The College of Chemistry and Molecular Engineering, Henan Key Laboratory of Chemical Biology and Organic Chemistry, Key Laboratory of Applied Chemistry of Henan Universities, Zhengzhou University, Zhengzhou 450052, People's Republic of China. E-mail: yangf@zzu.edu.cn; wyj@zzu.edu.cn
First published on 10th July 2015
Abstract
A simple and facile protocol for copper-catalyzed direct decarboxylative hydrosulfonylation of aryl propiolic acids with sulfonylhydrazides was developed, establishing an attractive approach to obtain (E)-vinylsulfones under base-free conditions. Remarkable features of this reaction include using a simple copper catalyst in air and good tolerance to aromatic and aliphatic sulfonylhydrazides.
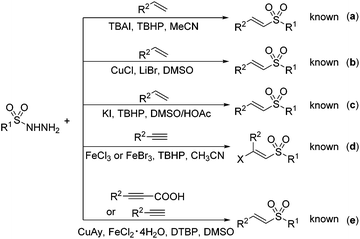
Vinyl sulfone derivatives as versatile building blocks or key structural motifs have wide applications in various organic transformations, pharmaceutical intermediates, biologically active compounds and materials science.1 Over the past several decades, various classic synthetic routes to the vinyl sulfone skeleton have been developed such as the Knoevenagel condensation,2 the Wittig reaction,3 and the Horner–Emmons reaction,4 although suffering from the drawback of obtaining the Z/E mixture isomers of vinyl sulfones. To cope with this drawback, the direct cross-coupling of the sulfonyl derivative (e.g., RSO2Cl and RSO2NHNH2) with an alkene source (e.g., alkene or alkyne) has emerged as a robust and reliable tool for the synthesis of vinyl sulfones and has drawn much attention in recent years (Scheme 1).5 In particular, sulfonyl hydrazides are not sensitive to air and moisture, easy to prepare and store, and more importantly, their byproducts: i.e., nitrogen and water, are environmentally benign. Therefore, many research efforts have been devoted to the use of sulfonyl hydrazides as a sulfonyl source.5a–d The pioneering work on this type of coupling belongs to Li, who reported the first cross-coupling of 4-methylbenzenesulfonohydrazide with styrene in MeCN under metal-free conditions in 2012, albeit affording the vinyl sulfone in a relatively low yield (31%) (Scheme 1a).5a Subsequently, Jiang et al. described a copper-catalyzed version of styrene with 4-methylbenzenesulfonohydrazide in DMSO using LiBr as the additive in 2014, and vinyl sulfones could be generated in a high yield (Scheme 1b).5c Remarkably, Lei developed another facile and elegant synthesis of vinyl sulfones via the iodine-catalyzed reaction of styrene and 4-methylbenzenesulfonohydrazide in the absence of any metal catalysts (Scheme 1c).5d Moreover, Xu established a simple and efficient approach to vinyl sulfones by iron-catalyzed chlorosulfonylation addition using terminal alkynes as an alternative alkene source (Scheme 1d).5b
Aryl propiolic acids are usually solid-state without pungent smell and easy to prepare, store, and transport.6 Just recently, Mao developed a Cu/Fe co-catalyzed sulfonylation of aromatic propiolic acids with sulfonyl hydrazides using DTBP as an oxidant via a combined decarboxylative and dehydrazine process, which was not suitable for aliphatic sulfonylhydrazides (Scheme 1e).5e And our research interest is to demonstrate a simple and environmental synthesis of vinyl sulfones through the copper-catalyzed direct decarboxylative coupling of aryl propiolic acids with sulfonyl hydrazides using an environmentally benign oxidant. And we envisioned developing another reaction pattern of aryl propiolic acids with sulfonylhydrazides, possibly in a different hydrosulfonylation process.
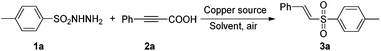
The initial optimization process of decarboxylative coupling of 4-methylbenzenesulfonohydrazide (1a) with phenylpropiolic acid (2a) was investigated, and the results are displayed in Table 1. After the effect of various copper catalysts was checked, we found that 10 mol% of CuI gave the best result, affording the vinylsulfone product in a moderate yield of 57% under air in DMF (Table 1, entry 3); CuCl, CuBr, Cu2O and Cu(OAc)2·H2O also showed catalytic activity, generating the desired product in relatively lower yields of 25–51% (Table 1, entries 1, 2, 4, and 5); other copper species such as CuCl, CuCl2, CuBr2 and CuF2·2H2O could not facilitate the reaction (see ESI†). Then, other solvents (e.g., DMA, THF, toluene, DMSO, dioxane, and p-xylene) were also examined, and among them the desired product could be obtained in DMSO, dioxane and p-xylene in yields of 41%, 25% and 21%, respectively (Table 1, entries 6–8). Moreover, the ligand effect played an important role in this hydrosulfonylation process, and notably 2,2′-bpy (2,2′-bipyridine) exhibited excellent activity affording the desired product in a high yield of 85% (Table 1, entry 9). However, other nitrogen ligands such as pyridine, phen (1,10-phenanthroline), and DMEDA (N,N′-dimethyl-1,2-ethanediamine) did not show any catalytic activity (see ESI†). Finally, some controlling experiments were also explored. For example, when the reaction was performed under copper-free conditions, we almost did not obtain the desired product 3a (Table 1, entry 10). When the reaction temperature was increased to 120 °C or decreased to 80 °C, no much better results were observed and the coupling products were afforded in 87% and 64% yield, respectively (Table 1, entries 11 and 12). If the reaction was performed under a nitrogen atmosphere instead of air, only a trace of the desired product could be achieved (Table 1, entry 13).
| Entry | Catalyst | Solvent | Ligand | Yieldb |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.24 mmol), catalyst (10 mol%) and base (0.4 mmol) in solvent (1 mL) at 100 °C under air for 18 h. b Isolated yield. c Under 5 mol% of Cu2O catalyst loading. d At 120 °C. e At 80 °C. f Under a nitrogen atmosphere. | ||||
| 1 | CuCl | DMF | — | 28% |
| 2 | CuBr | DMF | — | 51% |
| 3 | CuI | DMF | — | 57% |
| 4c | Cu2O | DMF | — | 25% |
| 5 | Cu(OAc)2·H2O | DMF | — | 36% |
| 6 | CuI | DMSO | — | 41% |
| 7 | CuI | Dioxane | — | 25% |
| 8 | CuI | p-Xylene | — | 21% |
| 9 | CuI | DMF | 2,2′-bpy | 85% |
| 10 | — | DMF | — | <5% |
| 11d | CuI | DMF | 2,2′-bpy | 87% |
| 12e | CuI | DMF | 2,2′-bpy | 64% |
| 13f | CuI | DMF | 2,2′-bpy | <5% |
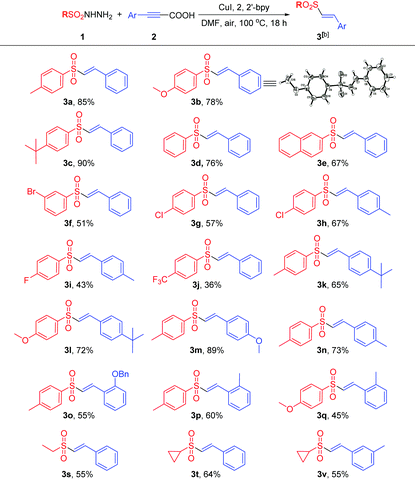
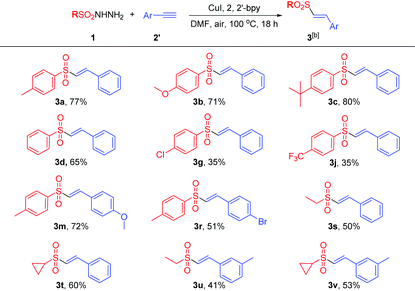
Under the optimized reaction conditions, the substrate scope was then explored and the results are summarized in Tables 2 and 3. Generally, either arylsulfonylhydrazides or alkynes possessing an electron-donating group at the benzene ring would be beneficial for this coupling reaction, while the substrates bearing an electron-withdrawing group at the benzene ring would lead to the coupling products in slightly lower yields. For example, arylsulfonylhydrazides possessing an electron-donating group such as alkyl and alkoxy groups would accelerate the reaction process, generating the products in excellent yields (3a–3c). The neutral arylsulfonylhydrazides were well tolerated in this reaction, affording the corresponding products in good yields (3d–3e).
Various electron-withdrawing groups such as halogen atoms and CF3 were also suitable for this process, albeit delivering the corresponding vinylsulfones in relatively lower to moderate yields (3f–3j). Similarly, aryl propiolic acids or terminal alkynes bearing electron-donating groups such as nBu, OBn methoxy and methyl groups at the benzene ring also afford the corresponding products in moderate to excellent yields (3k–3q). The steric effect played an important role in this reaction, and arylpropiolic acid bearing an ortho-methyl or OBn group led to an evident decrease of reaction yields (3o–3q). Moreover, an alkyne possessing an electron-withdrawing bromo atom could also give the product in a moderate yield of 51% (3r). Finally, the reaction of aliphatic sulfonylhydrazides with aryl propiolic acids or terminal alkynes was also performed, and the corresponding vinylsulfone products were obtained in moderate yields (3s–3v). The molecular structure of the vinylsulfone product (3b) was unambiguously determined by the single crystal X-ray diffraction study.7
 | (1) |
In order to understand the mechanism of the transformations, a number of controlling experiments were carried out to gain more insights into the mechanism (eqn (1)). When the decarboxylative hydrosulfonylation of phenylpropiolic acid 2a with 4-methylbenzenesulfonohydrazide 1a was performed under a nitrogen atmosphere instead of air, only a trace amount of the desired product 3a could be observed; when the reaction was performed under copper-free conditions, the reaction could not occur at all, which indicates that the copper species and oxygen are essential to the reaction. The addition of the radical scavenger TEMPO could dramatically inhibit the formation of 3a, demonstrating that a radical process may be involved in this reaction.
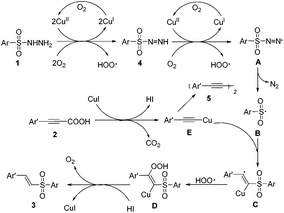
On the basis of these experimental results and previous reports, a plausible mechanism for direct decarboxylative hydrosulfonylation of aryl propiolic acids with arylsulfonylhydrazides leading to vinylsulfones is illustrated in Scheme 2. Initially, the Cu(I) catalyst was oxidized to Cu(II) species in the presence of oxygen in air. The arylsulfonylhydrazide 1 was oxidized to (arylsulfonyl)diazene 4 by Cu(II) species formed in situ and O2 to release a hydrogen peroxide radical. In this way, (arylsulfonyl)diazene 4 was further oxidized to an active azo radical intermediate A. This oxidation of an arylsulfonylhydrazide 1 into an azo radical A proceeds with the assistance of a Cu(II) species in air, which is quite different from the Mao and Zhang's version of Fe(II)/Cu(II) species in the presence of DTBP.5e Then, the intermediate A was transferred to a sulfonyl radical B with the release of a molecular nitrogen via a single electron transfer process. Then, the sulfonyl radical B reacted with alkynylcopper(I) intermediate E generated from aryl propiolic acid 2 and copper(I) iodide to afford an active intermediate radical C. The active intermediate C coupled with the hydrogen peroxide radical to form the copper(I) intermediate D. In the presence of HI, the copper(I) intermediate D was decomposed to the vinylsulfone product 3, along with the regeneration of a molecular oxygen and CuI to fulfill the catalytic cycle. Note that the homocoupling of alkynylcopper(I) intermediate E could occur to afford diyne 5 as a byproduct.
In conclusion, we have developed a copper-catalyzed direct decarboxylative hydrosulfonylation of aryl propiolic acids with sulfonylhydrazides, providing a new and convenient route to (E)-vinylsulfone. This reaction features a simple catalytic system and good tolerance of substrates including aliphatic sulfonylhydrazides. Moreover, the decarboxylative hydrosulfonylation shows high regio- and stereo-selectivity, generating (E)-vinylsulfone as the only product.
We are grateful to the National Natural Science Foundation of China (no. 21102134 and 21172200) for financial support.
Notes and references
- (a) J. J. Reddick, J. Cheng and W. R. Roush, Org. Lett., 2003, 5, 1967 CrossRef CAS PubMed; (b) G. Wang, U. Mahesh, G. Y. J. Chen and S. Q. Yao, Org. Lett., 2003, 5, 737 CrossRef CAS PubMed; (c) D. C. Meadows, T. Sanchez, N. Neamati, T. W. North and J. Gervay-Hague, Bioorg. Med. Chem., 2007, 15, 1127 CrossRef CAS PubMed; (d) B. A. Frankel, M. Bentley, R. G. Kruger and D. G. McCafferty, J. Am. Chem. Soc., 2004, 126, 3404 CrossRef CAS PubMed; (e) M. N. Noshi, A. El-awa, E. Torres and P. L. Fuchs, J. Am. Chem. Soc., 2007, 129, 11242 CrossRef CAS PubMed; (f) S. Sulzer-Mossé, A. Alexakis, J. Mareda, G. Bollot, G. Bernardinelli and Y. Filinchuk, Chem. – Eur. J., 2009, 15, 3204 CrossRef PubMed.
- (a) S. Chodroff and W. F. Whitmore, J. Am. Chem. Soc., 1950, 72, 1073 CrossRef CAS; (b) D. A. R. Happer and B. E. Steenson, Synthesis, 1980, 806 CrossRef CAS.
- (a) M. Mikolajczyk, W. Perlikowska, J. Omelanczuk, H. Cristau and A. Perraud-Darcy, J. Org. Chem., 1998, 63, 9716 CrossRef CAS; (b) J. H. van Steenis, J. J. G. S. van Es and A. van der Gen, Eur. J. Org. Chem., 2000, 2787 CrossRef CAS.
- I. C. Popoff and J. L. Denver, J. Org. Chem., 1969, 34, 1128 CrossRef CAS.
- (a) X.-Q. Li, X.-S. Xu and C. Zhou, Chem. Commun., 2012, 48, 12240 RSC; (b) X.-Q. Li, X.-H. Shi, M.-W. Fang and X.-S. Xu, J. Org. Chem., 2013, 78, 9499 CrossRef CAS PubMed; (c) X.-W. Li, Y.-L. Xu, W.-Q. Wu, C. Jiang, C.-R. Qi and H.-F. Jiang, Chem. – Eur. J., 2014, 20, 7911 CrossRef CAS PubMed; (d) S. Tang, Y. Wu, W.-Q. Liao, R.-P. Bai, C. Liu and A.-W. Lei, Chem. Commun., 2014, 50, 4496 RSC; (e) G.-W. Rong, J.-C. Mao, H. Yan, Y. Zheng and G.-Q. Zhang, J. Org. Chem., 2015, 80, 4697 CrossRef CAS PubMed.
- (a) D. Yu and Y. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20184 CrossRef CAS PubMed; (b) Y. Hajbi, C. Neagoie, B. Biannic, A. Chilloux, E. Vedrenne, B. Baldeyrou, C. Bailly, J.-Y. Merour, S. Rosca, S. Routier and A. Lansiaux, Eur. J. Med. Chem., 2010, 45, 5428 CrossRef CAS PubMed; (c) D. Yu and Y. Zhang, Green Chem., 2011, 13, 1275 RSC; (d) X. Zhang, W.-Z. Zhang, X. Ren, L.-L. Zhang and X.-B. Lu, Org. Lett., 2011, 13, 2402 CrossRef CAS PubMed; (e) K. Park, T. Palani, A. Pyo and S. Lee, Tetrahedron Lett., 2012, 53, 733 CrossRef CAS PubMed; (f) M. Arndt, E. Risto, T. Krause and L. J. Goossen, ChemCatChem, 2012, 4, 484 CrossRef CAS PubMed; (g) T. Ponpandian and S. Muthusubramanian, Tetrahedron Lett., 2012, 43, 4248 CrossRef PubMed; (h) L. J. Gooßen, N. Rodríguez, F. Manjolinho and P. P. Lange, Adv. Synth. Catal., 2010, 352, 2913 CrossRef PubMed; (i) D. Yu, M.-X. Tan and Y. Zhang, Adv. Synth. Catal., 2012, 354, 969 CrossRef CAS PubMed; (j) K. Park, J.-M. You, S. Jeon and S. Lee, Eur. J. Org. Chem., 2013, 1973 CrossRef CAS PubMed; (k) X. Li, F. Yang and Y.-J. Wu, J. Org. Chem., 2013, 78, 4543 CrossRef CAS PubMed; (l) X. Li, F. Yang, Y.-J. Wu and Y.-S. Wu, Org. Lett., 2014, 16, 992 CrossRef CAS PubMed.
- CCDC 1059933 (3b).† Crystal data for compound 3b: C15H14O3S, M = 274.32, triclinic, a = 8.5030(6) Å, α = 76.750(5)°, b = 10.6762(5) Å, β = 81.107(5)°, c = 15.8607(10) Å, γ = 88.437(5)°, V = 1384.60(15) Å3, T = 291.15 K, space group = P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 4, number of reflections = 9993, independent reflections = 4932, [R(int) = 0.0186], final R indices [I > 2σ(I)] R1 = 0.0630, wR2 = 0.1737, R indices (all data) R1 = 0.0743, wR2 = 0.1857.
, Z = 4, number of reflections = 9993, independent reflections = 4932, [R(int) = 0.0186], final R indices [I > 2σ(I)] R1 = 0.0630, wR2 = 0.1737, R indices (all data) R1 = 0.0743, wR2 = 0.1857.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1059933. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00212e |
| This journal is © the Partner Organisations 2015 |