Synthesis, characterization, and application of Fe3O4-SA-PPCA as a novel nanomagnetic reusable catalyst for the efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones and polyhydroquinolines
Arash Ghorbani-Choghamarani* and
Gouhar Azadi
Department of Chemistry, Faculty of Science, Ilam University, Ilam, Iran. E-mail: a.ghorbani@mail.ilam.ac.ir; arashghch58@yahoo.com
First published on 23rd December 2014
Abstract
Piperidine-4-carboxylic acid (PPCA) functionalized Fe3O4 nanoparticles (Fe3O4-PPCA) were prepared by the one pot co-precipitation of iron oxide in the presence of PPCA. Grafting of chlorosulfonic acid on the synthesized Fe3O4-PPCA nanoparticles afforded sulfamic acid-functionalized magnetic nanoparticles (Fe3O4-SA-PPCA). The catalytic activity of Fe3O4-SA-PPCA as a novel catalyst was probed through one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones and polyhydroquinolines derivatives. The heterogeneous catalyst could be recovered easily and reused many times without significant loss of its catalytic activity.
1 Introduction
Nanoscale materials have been a subject of particular interest due to their properties, which differ from their bulk counterparts.1 They have been used extensively in chemistry,2 physics,3 biology4 and catalysis.5 Magnetic nanoparticles have attracted great interest because of their multifunctional physical and chemical properties. Magnetic NPs have many unique magnetic properties such as superparamagnetic, high coercivity, low Curie temperature, high magnetic susceptibility, etc. Among the nanoparticulate transition-metal oxides, magnetite is a common magnetic iron oxide that has a cubic inverse spinel structure with fcc close packed oxygen anions and Fe cations occupying interstitial tetrahedral and octahedral sites.6 Superparamagnetic magnetite (Fe3O4) NP's are getting great interest due to their unique chemical and physical properties. Moreover, the naked iron oxide NPs have high chemical activity, and are easily oxidized in air (especially), generally resulting in loss of magnetism and magnetite dispersibility. Therefore, providing proper surface coating and developing some effective protection strategies (to form core/shell structured materials) to keep the stability of magnetic iron oxide NPs is very important.7 Practically, it is worthy that in many cases the protecting shells not only stabilize the magnetic iron oxide NPs, but can also be used for further functionalization.8–11 Heterogeneous catalysts with a matrix structure generally as the magnetically recyclable catalysts (MRCs) are developed in recent years and can be very useful to assist an effective separation and recovery in a liquid-phase reaction by a magnet, especially when the catalysts are in the nanometer-sized range. Therefore, the development of the facile and rapid methods for the preparation of efficient MRCs is still a challenge.12 In particular, various magnetic nanoparticle-catalysts have been widely employed for promoting different organic reactions.13–152 Results and discussion
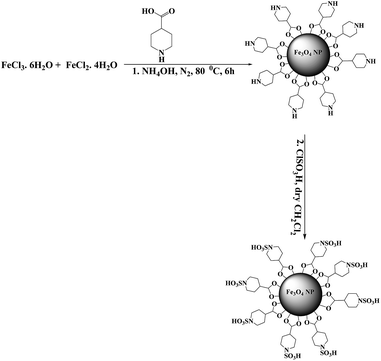
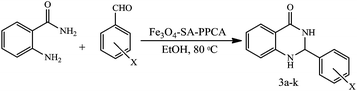
In continuation of our recent success in the introduction of new catalysts,16–20 in the present article we describe the preparation of sulfamic acid-functionalized Fe3O4 (Fe3O4-SA-PPCA) with high magnetic sensitivity and application in multicomponent reactions (Scheme 1).2.1 Catalyst characterization
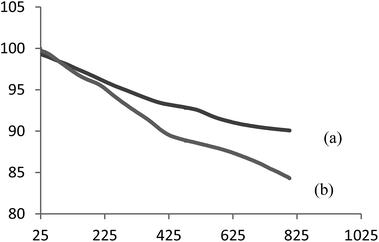
The magnetic nano catalyst, Fe3O4-SA-PPCA, is fully characterized by FT-IR, TGA, XRD, SEM, TEM and Vibrating Sample Magnetometer (VSM) analyses. The prepared Fe3O4-SA-PPCA NPs catalyst is placed in an aqueous NaCl solution (1 M, 25 mL), where the pH drops instantaneously to ≈2.95, indicating ion exchanges between –SO3H protons and sodium ions.The thermo gravimetric analysis curves of the piperidine-4-carboxylic acid coated iron oxide and sulfamic acid-functionalized Fe3O4 show the mass loss of the organic materials as they decompose upon heating (Fig. 1). Organic groups have been reported to desorb at temperatures above 260 °C. piperidine-4-carboxylic acid coated iron oxide shows three-step weight loss behavior. The initial weight loss up to 100 °C is due to residual water; the weight loss of PPCA modified magnetite NPs appears about 4.5% at 270–450 °C which is contributed to the thermal decomposition of the piperidine-4-carboxylic acid groups. For Fe3O4-SA-PPCA MNPs, there is a well-defined mass weight loss of 7.2% between 250 and 465 °C related to the breakdown of the PPCA-SA moieties. On the basis of these results, the well grafting of PPCA and SA groups on the MNPs is verified.
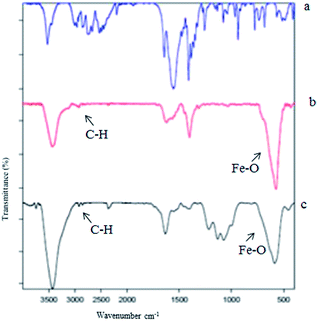
Fig. 2 shows the FT-IR spectra of both Fe3O4-PPCA and Fe3O4-SA-PPCA. The Fe–O stretching vibration near 575 cm−1 was observed and O–H stretching vibration near 3500 cm−1. In the spectrum of Fe3O4-PPCA the peak at 3423 cm−1 was probably attributed to the NH groups, which is overlapped by the O–H stretching vibration. The presence of the anchored PPCA group is confirmed by C–H stretching vibrations that appear at 2918 and 2846 cm−1 in Fe3O4-PPCA spectra. These results provided the evidences that the piperidine-4-carboxylic acid groups were successfully attached to the surface of Fe3O4 nanoparticles as a carboxylate.21 Reaction of PPCA-Fe3O4 with chlorosulfonic acid produces SA-PPCA-MNPs in which the presence of sulfonylmoiety is asserted with 1228 and 1132 cm−1 bands in FT-IR spectra. These results were in agreement with the chemical component of the materials prepared.
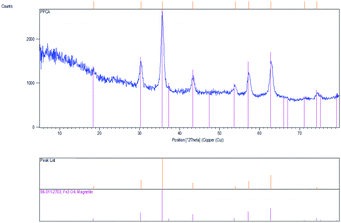
In order to investigate the crystal structure of the obtained nanoparticles (Fe3O4-SA-PPCA) XRD analysis was performed and the resultant pattern of the as-prepared sample is presented in Fig. 3. The XRD pattern indicates that the product consists of magnetite, Fe3O4, and the line profile was fitted for observed 7 peaks: (111), (220), (311), (400), (422), (511) and (440).
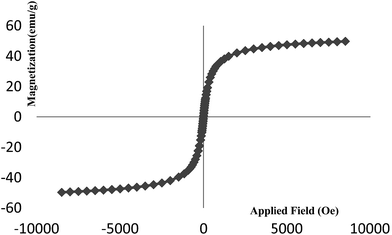
Magnetic properties of the Fe3O4-PPCA-SA were evaluated by vibrating sample magnetometer at room temperature. VSM measurements showed that the saturation magnetization (Ms) of the catalyst is 48 emu g−1 (Fig. 4).
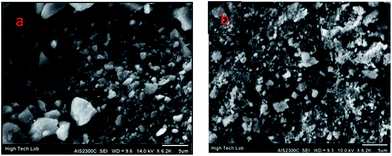
The SEM images of Fe3O4-PPCA and Fe3O4-SA-PPCA was confirmed that the catalyst was made up of uniform nanometer sized particles (Fig. 5).
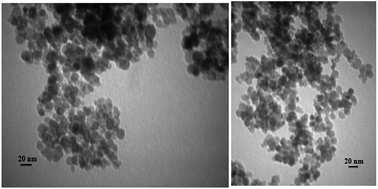
TEM micrographs of described catalyst (Fe3O4-PPCA-SA) are given in Fig. 6. Generally, the particles have spherical morphology and their sizes are varying in the range 8–18 nm.
2.2 Evaluation of the catalytic activity of Fe3O4-SA-PPCA through the synthesis of 2,3-dihydroquinazolin-4(1H)-ones and polyhydroquinolines
2,3-Dihydroquinazolin-4(1H)-ones are an important class of heterocycles with wide range of biological activities including antibacterial, antifungal, anticancer and anticonvulsant activities.22 Therefore, we investigated catalytic activity of Fe3O4-SA-PPCA as a new heterogeneous catalyst in the one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones through the reaction of anthranilamide, and aromatic aldehydes. For this purpose, the reaction of p-clbenzaldehyde and anthranilamide as a simple model reaction was probed to establish the feasibility of the strategy and optimize the reaction conditions.To investigate the effect of the catalyst, systematic studies are carried out in the presence of different amounts of the catalyst (5, 8, 11, 15 mg) in ethanol, affording 2,3-dihydroquinazolin-4(1H)-ones with 62%, 63%, 70% and 95% isolated yields, respectively (Table 1, entry 2–5). Thus, the best yield is found in the presence of just 15 mg (6.7 × 10−3 mmol) Fe3O4-SA-PPCA, and the use of higher amounts of catalyst (18 mg, 7.7 × 10−3 mmol) does not improve the result to an appreciable extent (Table 1, entry 6).
| Entry | Catalyst (mg) | Time (min) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: anthranilamide (1 mmol), p-clbenzaldehyde (1 mmol) in EtOH under reflux condition. | |||
| 1 | None | 30 | 0 |
| 2 | 5 (2.1 × 10−3 mmol) | 30 | 62 |
| 3 | 8 (3.4 × 10−3 mmol) | 30 | 63 |
| 4 | 11 (4.7 × 10−3 mmol) | 30 | 70 |
| 5 | 15 (6.7 × 10−3 mmol) | 30 | 95 |
| 6 | 18 (7.7 × 10−3 mmol) | 30 | 96 |
Also, this reaction was checked under different temperatures including 25, 40, 60, and 80 °C. The greatest yield in the shortest reaction time was obtained at 80 °C in the presence of 15 mg (6.7 × 10−3 mmol) of catalyst. Using the optimized reaction conditions, this process was demonstrated by the wide range of substituted divers aldehydes to synthesize the corresponding products in excellent yields (Scheme 2, Table 2).
| Entry | Aldehyde | Product | Time (min) | Yielda (%) | M. p. (°C) |
|---|---|---|---|---|---|
| a Isolated yield.b Reaction conditions: anthranilamide (2 mmol), terephthalaldehyde (1 mmol) and Fe3O4-SA-PPCA (30 mg, 13.4 × 10−3 mmol). | |||||
| 1 | 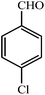 |
3a | 30 | 95 | 201–203 (ref. 22) |
| 2 | 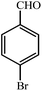 |
3b | 50 | 95 | 196–199 (ref. 22) |
| 3 | 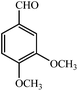 |
3c | 30 | 94 | 212–214 (ref. 22) |
| 4 | 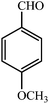 |
3d | 35 | 95 | 191–192 (ref. 22) |
| 5 | 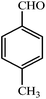 |
3e | 30 | 95 | 229–232 (ref. 22) |
| 6 | 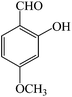 |
3f | 80 | 95 | 195–198 (ref. 23) |
| 7 | 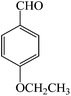 |
3g | 70 | 93 | 171–173 (ref. 23) |
| 8 | 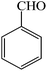 |
3h | 30 | 91 | 226–228 (ref. 22) |
| 9 | 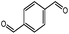 |
3i | 75 | 92b | 245–248 (ref. 24) |
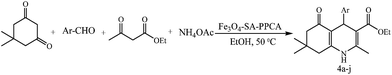
After successfully synthesizing a series of affording 2,3-dihydroquinazolin-4(1H)-ones in excellent yields, we turned our attention towards the synthesis of polyhydroquinolines derivatives. In view of the biological as well as medicinal applications of polyhydroquinoline derivatives, development of efficient and economic method of synthesizing these compounds is a continuous challenge to the chemists. For these reasons, we applied Fe3O4-SA-PPCA for the one-pot synthesis of polyhydroquinoline derivatives from four components coupling of aromatic aldehydes, ethyl acetoacetate, dimedone and ammonium acetate [Scheme 3].
To obtain the optimal reaction conditions, we evaluated the influence of temperature and different amounts of catalyst on the time and product yield in the reaction of 3,4-dimetoxybenzaldehyde (1 mmol), dimedone (1 mmol), ethyl acetoacetate (1 mmol), and NH4OAc (1.2 mmol) which was used as a model. 10 mg (4.3 × 10−3 mmol) of Fe3O4-SA-PPCA in ethanol at 50 °C was optimal for the desired reaction. Subsequently, a series of differently polyhydroquinoline derivatives were prepared successfully under optimal conditions. The results obtained in the current method are illustrated in Table 3.
| Entry | Aldehyde | Product | Time (min) | Yieldb (%) | M. p. (°C) |
|---|---|---|---|---|---|
| a Reaction conditions: arylaldehyde (1 mmol), dimedone (1 mmol), ethyl acetoacetate (1 mmol), NH4OAc (1.2 mmol) and Fe3O4-SA-PPCA (10 mg, 4.3 × 10−3 mmol).b Isolated yield. | |||||
| 1 | 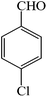 |
4a | 120 | 95 | 237–238 (ref. 25) |
| 2 | 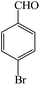 |
4b | 90 | 97 | 248–250 (ref. 26) |
| 3 | 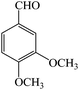 |
4c | 90 | 93 | 204–206 (ref. 25) |
| 4 | 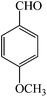 |
4d | 120 | 93 | 247–248 (ref. 27) |
| 5 | 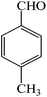 |
4e | 110 | 91 | 252–254 (ref. 26) |
| 6 | 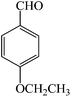 |
4f | 90 | 97 | 179–181 |
| 7 | 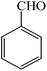 |
4g | 120 | 97 | 216–218 (ref. 26) |
| 8 | 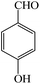 |
4h | 120 | 97 | 232–234 (ref. 25) |
| 9 | 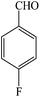 |
4i | 90 | 85 | 184–186 (ref. 25) |
| 10 | 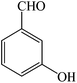 |
4j | 100 | 92 | 221–223 (ref. 26) |
The reusability of the catalysts is an important advantage and makes them useful for commercial applications. Thus the recovery and reusability of Fe3O4-SA-PPCA were investigated in the synthesis of 2,3-dihydroquinazolin-4(1H)-ones. In these experiments, the reaction mixture was dilute with ethanol and the catalyst was easily and rapidly separated from the product by exposure to an external magnet and decantation of the reaction solution. The remaining magnetic nanocatalyst was further washed with ethanol to remove residual product. Then, the reaction vessel was charged with fresh substrate and subjected to the next. As shown in Fig. 7, the catalyst can be recycled up to 5 runs without any significant loss of its catalytic activity. In addition, one of the attractive features of this novel catalyst system is the rapid and efficient separation of the catalyst by using an appropriate external magnet, which minimizes the loss of catalyst during separation.
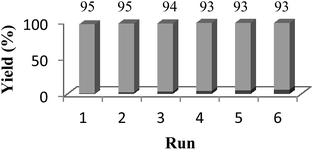 | ||
| Fig. 7 Recyclability of Fe3O4-SA-PPCA in the reaction of anthranilamide (1 mmol) and 3,4-dimethoxy benzaldehyde (1 mmol) at reflux condition in EtOH. | ||
In order to investigate the efficiency of this new procedure in comparison with known reported procedures in the literature, the results for the synthesis of 2,3-dihydroquinazolin-4(1H)-one and PHQ derivatives, compared with the best of the well-known data from the literature as outlined in the Table 4. As shown in Table 4, the previously reported procedures suffer from one or more disadvantages such as longer reaction time, using a transition metal catalysts and low yields of products.
| Entry | Catalyst | Condition | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a Reaction condition: 4-methylbenzaldehyde (1 mmol) and anthranilamide (1 mmol).b Reaction condition: 4-methylbenzaldehyde, dimedone, ethyl acetoacetate and NH4OAc. | |||||
| 1 | Beta-cyclodextrin | H2O, reflux | 150 | 91 | 28 |
| 2 | SO42−/ZrO2 | EtOH, reflux | 7 | 87 | 22 |
| 3 | Succinimide-N-sulfonic acid | H2O, 70 °C | 60 | 87 | 29 |
| 4 | CuCl2/Fe3O4-TEDETA | EtOH, reflux | 60 | 95 | 23 |
| 5 | NH2SO3H | H2O, 70 °C | 30 | 86 | 30 |
| 6 | Fe3O4-SA-PPCA | EtOH, reflux | 30 | 95 | This work |
| 7 | Yb(OTf)3 | EtOH, r.t | 120 | 94 | 31 |
| 8 | MCM-41 | 90 °C, solvent free | 20 | 87 | 27 |
| 9 | PdCl2 | THF, reflux | 270 | 86 | 32 |
| 10 | L-Proline | H2O, r.t. | 210 | 72 | 33 |
| 11 | Fe3O4-SA-PPCA | EtOH, 50 °C | 110 | 91 | This work |
3 Conclusions
In this study Fe3O4-SA-PPCA nanocomposite was synthesized for the first time on a novel one-pot reflux route as a new heterogeneous catalyst. The catalytic activity of Fe3O4-SA-PPCA was probed through one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones and polyhydroquinoline compounds. The heterogeneous catalyst showed very high conversion rates and could be recovered easily and reused many times without significant loss of its catalytic activity, which makes it useful and attractive for synthesis of these classes of compounds for economic availability and greater selectivity.4 Experimental
4.1 Materials and instruments
The reagents and solvents used in this work were all purchased from Aldrich and Merck and used without further purification. FT-IR measurements were performed using KBr disc using a NICOLET impact 410 spectrometer. The known products were characterized by comparison of their spectral (1H-NMR, and 13C-NMR, Bruker NMR-Spectrometer FX 400Q) and physical data with those of authentic samples. Powder XRD was collected with a Rigaku-Dmax 2500 diffractometer with nickel filtered Cu Kα radiation (λ = 1.5418 Å, 40 kV). TEM of the NPs were recorded using a Zeiss-EM10C TEM. Supermagnetic properties of catalyst was measured on Vibrating Sample Magnetometer (VSM) MDKFD.4.2 General procedure for preparation of 2,3-dihydroquinazolin-4(1H)-ones
The Fe3O4-SA-PPCA (15 mg, 6.7 × 10−3 mmol) was added to a mixture of anthranilamide (1 mmol, 0.136 g) and aldehyde (1 mmol) in ethanol as solvent. Then the mixture was stirred for the appropriate time under reflux condition. The progress was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature and the catalyst was separated by an external magnet. The products were extracted with ethanol, then EtOH was evaporated under reduced pressure to afford the essentially pure products. In some cases, the product was recrystalized from ethanol for further purification.4.3 General procedure for the synthesis of polyhydroquinolines
A mixture of dimedone (1 mmol), aldehyde (1 mmol), ethyl acetoacetate (1 mmol), ammonium acetate (1.2 mmol) and Fe3O4-SA-PPCA as catalyst (10 mg, 4.3 × 10−3 mmol) in ethanol were stirred at 50 °C for an appropriate time. The progress of reaction was monitored by TLC. After completion of the reaction, the catalyst was separated by an external magnet and solvent was evaporated. The residue was extracted by ethyl acetate (3 × 10 mL) and the combined organic extract was washed with water (3 × 10 mL), organic layer was dried over anhydrous sodium sulfate and evaporated to dryness. A crude solid was obtained. The pure product was obtained through crystallization from ethanol.4.4 Representative NMR data
Acknowledgements
This work was supported by the research facilities of Ilam University, Ilam, Iran.References
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed.
- C. Burda, X. B. Chen, R. B. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS PubMed.
- J. T. Hu, T. W. Odom and C. M. Lieber, Acc. Chem. Res., 1999, 32, 435 CrossRef CAS.
- V. Sokolova and M. Epple, Angew. Chem., Int. Ed., 2008, 47, 1382 CrossRef CAS PubMed.
- A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef PubMed.
- R. M. Cornell and U. Schwertmann, The Iron Oxides: Structure, Properties, Reactions Occurrence and Uses, VCH, New York, 1996 Search PubMed.
- S. Bahceci, B. Unal, A. Baykal, H. Sozerid, E. Karaoglua and B. Esata, J. Alloys Compd., 2011, 509, 8825 CrossRef CAS PubMed.
- A. Wei Wu, A. Quanguo He and J. Changzhong, Nanoscale Res. Lett., 2008, 3, 397 CrossRef PubMed.
- K. Singh, A. Ohlan, A. K. Bakhshia and S. K. Dhawan, Mater. Chem. Phys., 2010, 119, 201 CrossRef CAS PubMed.
- M. Aydm, Z. Durmus, H. Kavas, B. Esat, H. Sözeri, A. Baykal, F. Yılmaz and M. S. Toprak, Polyhedron, 2011, 30, 1120 CrossRef PubMed.
- Y. Li, G. Chen, Q. Li, G. Qiu and X. Liu, J. Alloys Compd., 2011, 509, 4104 CrossRef CAS PubMed.
- E. Karaoglu, U. Ozel, C. Caner, A. Baykal, M. M. Summak and H. Sozeri, Mater. Res. Bull., 2012, 47, 4316 CrossRef CAS PubMed.
- K. K. Senapati, S. Roy, C. Borgohain and P. Phukan, J. Mol. Catal. A: Chem., 2012, 352, 128 CrossRef CAS PubMed.
- S. C. Tsang, V. Caps, I. Paraskevas, D. Chadwick and D. Thompsett, Angew. Chem., Int. Ed., 2004, 43, 5645 CrossRef CAS PubMed.
- J. Liu, X. Peng, W. Sun, Y. Zhao and C. Xia, Org. Lett., 2008, 10, 3933 CrossRef CAS PubMed.
- M. A. Zolfigol, K. Amani, M. Hajjami and A. Ghorbani-Choghamarani, Monatsh. Chem., 2008, 139, 895 CrossRef CAS.
- A. Ghorbani-Choghamarani and P. Zamani, J. Iran. Chem. Soc., 2012, 9, 607 CrossRef CAS PubMed.
- A. Ghorbani-Choghamarani and G. Azadi, J. Iran. Chem. Soc., 2011, 8, 1082 CrossRef CAS.
- A. Ghorbani-Choghamarani and M. Norouzi, Bull. Korean Chem. Soc., 2011, 32, 1399 CrossRef CAS.
- A. Ghorbani-Choghamarani, M. Hajjami, H. Goudarziafshar, M. Nikoorazm, S. Mallakpour, F. Sadeghizadeh and G. Azadi, Monatsh. Chem., 2009, 140, 607 CrossRef CAS PubMed.
- E. Karaoglu, A. Baykal, M. Şenel, H. Sozeri and M. S. Toprak, Mater. Res. Bull., 2012, 47, 2480 CrossRef CAS PubMed.
- M. Abdollahi-Alibeik and E. Shabani, J. Iran. Chem. Soc., 2014, 11, 351 CrossRef CAS PubMed.
- A. Ghorbani-Choghamarani and M. Norouzi, J. Mol. Catal. A: Chem., 2014, 395, 172 CrossRef CAS PubMed.
- A. Rostami, B. Tahmasbi, H. Gholami and H. Taymorian, Chin. Chem. Lett., 2013, 24, 211 CrossRef CAS PubMed.
- M. Tajbakhsh, H. Alinezhad, M. Norouzi, S. Baghery and M. Akbari, J. Mol. Liq., 2013, 177, 44 CrossRef CAS PubMed.
- S. B. Sapkal, K. F. Shelke, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2009, 50, 1754 CrossRef CAS PubMed.
- L. Nagarapu, M. D. Kumari, N. V. Kumari and S. Kantevari, Catal. Commun., 2007, 8, 1871 CrossRef CAS PubMed.
- D. R. Patil, P. G. Ingole, K. Singh and D. S. Dalal, J. Inclusion Phenom. Macrocyclic Chem., 2013, 76, 327 CrossRef CAS.
- M. Ghashang, S. S. Mansoor and K. Aswin, Res. Chem. Intermed., 2013, 6168, 695 Search PubMed.
- A. Rostami and A. Tavakoli, Chin. Chem. Lett., 2011, 22, 3117 Search PubMed.
- L. M. Wang, J. Sheng, L. Zhang, J. W. Han, Z. Y. Fan, H. Tian and C. T. Qian, Tetrahedron, 2005, 61, 1539 CrossRef CAS PubMed.
- M. Saha and A. K. Pal, Tetrahedron Lett., 2011, 52, 4872 CrossRef CAS PubMed.
- A. Kumar and R. A. Maurya, Tetrahedron, 2007, 63, 1946 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |