Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A faux hawk fullerene with PCBM-like properties†
Long K.
San
a,
Eric V.
Bukovsky
a,
Bryon W.
Larson
ab,
James B.
Whitaker
a,
S. H. M.
Deng
c,
Nikos
Kopidakis
*b,
Garry
Rumbles
*b,
Alexey A.
Popov
*d,
Yu-Sheng
Chen
*e,
Xue-Bin
Wang
*c,
Olga V.
Boltalina
*a and
Steven H.
Strauss
*a
aDepartment of Chemistry, Colorado State University, Fort Collins, CO 80523, USA. E-mail: olga.boltalina@colostate.edu; steven.strauss@colostate.edu
bNational Renewable Energy Laboratory, Golden, CO 80401, USA. E-mail: nikos.kopidakis@nrel.gov; garry.rumbles@nrel.gov
cPhysical Sciences Division, Pacific Northwest National Laboratory, MS K8-88, P.O. Box 999, Richland, WA 99352, USA. E-mail: xuebin.wang@pnnl.gov
dLeibniz Institute for Solid State and Materials Research, 01069 Dresden, Germany. E-mail: a.popov@ifw-dresden.de
eChemMatCARS Beamline, University of Chicago Advanced Photon Source, Argonne, IL 60439, USA. E-mail: yschen@cars.uchicago.edu
First published on 16th December 2014
Abstract
Reaction of C60, C6F5CF2I, and SnH(n-Bu)3 produced, among other unidentified fullerene derivatives, the two new compounds 1,9-C60(CF2C6F5)H (1) and 1,9-C60(cyclo-CF2(2-C6F4)) (2). The highest isolated yield of 1 was 35% based on C60. Depending on the reaction conditions, the relative amounts of 1 and 2 generated in situ were as high as 85% and 71%, respectively, based on HPLC peak integration and summing over all fullerene species present other than unreacted C60. Compound 1 is thermally stable in 1,2-dichlorobenzene (oDCB) at 160 °C but was rapidly converted to 2 upon addition of Sn2(n-Bu)6 at this temperature. In contrast, complete conversion of 1 to 2 occurred within minutes, or hours, at 25 °C in 90/10 (v/v) PhCN/C6D6 by addition of stoichiometric, or sub-stoichiometric, amounts of proton sponge (PS) or cobaltocene (CoCp2). DFT calculations indicate that when 1 is deprotonated, the anion C60(CF2C6F5)− can undergo facile intramolecular SNAr annulation to form 2 with concomitant loss of F−. To our knowledge this is the first observation of a fullerene-cage carbanion acting as an SNAr nucleophile towards an aromatic C–F bond. The gas-phase electron affinity (EA) of 2 was determined to be 2.805(10) eV by low-temperature PES, higher by 0.12(1) eV than the EA of C60 and higher by 0.18(1) eV than the EA of phenyl-C61-butyric acid methyl ester (PCBM). In contrast, the relative E1/2(0/−) values of 2 and C60, −0.01(1) and 0.00(1) V, respectively, are virtually the same (on this scale, and under the same conditions, the E1/2(0/−) of PCBM is −0.09 V). Time-resolved microwave conductivity charge-carrier yield × mobility values for organic photovoltaic active-layer-type blends of 2 and poly-3-hexylthiophene (P3HT) were comparable to those for equimolar blends of PCBM and P3HT. The structure of solvent-free crystals of 2 was determined by single-crystal X-ray diffraction. The number of nearest-neighbor fullerene–fullerene interactions with centroid⋯centroid (⊙⋯⊙) distances of ≤10.34 Å is significantly greater, and the average ⊙⋯⊙ distance is shorter, for 2 (10 nearest neighbors; ave. ⊙⋯⊙ distance = 10.09 Å) than for solvent-free crystals of PCBM (7 nearest neighbors; ave. ⊙⋯⊙ distance = 10.17 Å). Finally, the thermal stability of 2 was found to be far greater than that of PCBM.
1. Introduction
We1 and others2 have been investigating homoleptic perfluoroalkylfullerenes (PFAFs, fullerene(RF)n) such as 1,7-C60(RF)2 (RF = CF3, C2F5, n-C3F7, i-C3F7, n-C4F9, 2-C4F9, and n-C8F17),3,4 C74(CF3)12,5 C84(CF3)12,2,6,7 7,24-C70(C2F5)2,8 and C3–C60(i-C3F7)6 (ref. 9) since 2003. This very large class of fullerene(X)n derivatives has fostered an understanding of the relationships between fullerene addition patterns, LUMO shapes and relative energies, perfluoroalkyl chain lengths, and electrochemical/electron affinity properties4,10,11 and has afforded a range of structurally similar PFAFs with E1/2(0/−) values that vary by as much as 0.5 V to be used for fundamental organic photovoltaic (OPV) active-layer studies.12 We have recently turned our attention to (i) fullerenes with perfluoroaryl derivatives (e.g., perfluorobenzyl)13 and (ii) hydro-PFAFs with one or more H atom substituents,14,15 the latter so that their deprotonation and subsequent treatment with electrophiles E+ would result in a variety of fullerene(E)(RF)n−1 derivatives for fundamental and applied studies.We herein report the synthesis of 1,9-C60(CF2C6F5)H (1), shown in Fig. 1, and its unexpected transformation upon deprotonation or one-electron reduction to the exocyclic “fullerene with a faux hawk” product 1,9-C60(cyclo-CF2(2-C6F4)) (2), also shown in Fig. 1 (see also Fig. S-1; ESI figures and tables, available in the ESI,† are numbered T-1, T-2, S-1, S-2, etc.). We propose a reaction sequence for the transformation 1 → 2 + HF that is supported by DFT calculations. The gas-phase electron affinities, solution reduction potentials, thermal stabilities, X-ray diffraction molecular structures, and solid-state packing in solvent-free crystals of 2, PCBM (phenyl-C61-butyric acid methyl ester), and C60 are compared and contrasted. Finally, we show that OPV active-layer thin films made from blends of 2 with poly-3-hexylthiophene (P3HT), when studied using time-resolved microwave photoconductivity, exhibit photoinduced charge-carrier yield × mobility figures of merit that rival the OPV active-layer standard blend of PCBM with P3HT, which demonstrates the potential of 2 as an electron acceptor in OPV and other optoelectronic devices.
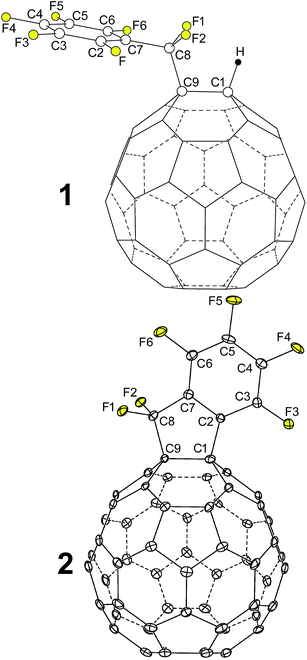 | ||
| Fig. 1 OLYP DFT-optimized structure of 1,9-C60(CF2C6F5)H (1) and the X-ray structure of 1,9-C60(cyclo-CF2(2-C6F4)) (2; 50% probability ellipsoids). Only the major twin portion of the X-ray structure is shown. The shape of compound 2 is reminiscent of a hairstyle known as the faux hawk, as shown in Fig. S-1.† | ||
2. Results and discussion
2.1. Synthesis of 1,9-C60(CF2C6F5)H (1) and 1,9-C60(cyclo-CF2(2-C6F4)) (2)
In 1996 Yoshida, Suzuki, and Iyoda reported that the reaction of C60, perfluoroalkyliodides (RFI), SnH(n-Bu)3, and a catalytic amount of the radical initiator AIBN in refluxing benzene for 30 h produced 1,9-C60(RF)H derivatives in moderate yields depending on the ratio of the reagents.16 For example, with 12 equiv. n-C6F13I, 5 equiv. SnH(n-Bu)3, and 0.1 equiv. AIBN (based on C60), the yield of 1,9-C60(n-C6F13)H was 31% and 64% of the original C60 was recovered. With 12 equiv. n-C12F25I, 14 equiv. SnH(n-Bu)3, and 0.1 equiv. AIBN, the yield of 1,9-C60(n-C12F25)H was 26% and 67% of the original C60 was recovered. However, no fullerene products containing RF groups were obtained in the absence of AIBN.16In our hands, no AIBN was necessary to prepare 1 when the solvent was 1,2-C6H4Cl2 (oDCB) and the temperature was 160 °C. Furthermore, reaction times of only 1 or 2 h were sufficient to form appreciable amounts of 1, as shown in Fig. 2 and Table 1. This is probably due to the higher temperature for the reaction and a lower C–I bond energy for C6F5CF2I than for n-C6F13I or n-C12F25I, both of which will result in more C6F5CF2˙ radicals present than the number of RF˙ radicals in the reactions of Yoshida et al. The mol% values in Table 1 are based on HPLC peak relative integrations and are only approximate. They are listed so that trends in product ratios at various reaction temperatures, reaction times, and reagent mole ratios can be easily understood.
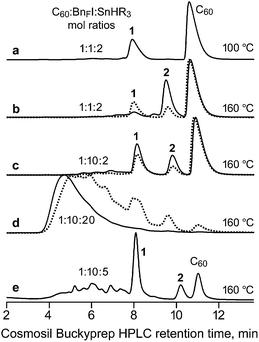 | ||
| Fig. 2 HPLC traces of C60 + C6F5CF2I + SnH(n-Bu)3 reaction mixtures (BnFI = C6F5CF2I; R = n-Bu). The dotted-line traces are for 1 h reactions; the solid-line traces are for 2 h reactions. See Table 1 for additional details. Compounds 1 and 2 are 1,9-C60(CF2C6F5)H and 1,9-C60(cyclo-CF2(2-C6F4)), respectively. | ||
| Fig. 2 HPLC trace | Temp., °C | Equiv. BnFIb | Equiv. SnHR3c | Product mixture mol% by HPLC integrationd | ||
|---|---|---|---|---|---|---|
| 1 | 2 | Unreacted C60 | ||||
| a All reactions in 1,2-C6H4Cl2 (oDCB). All volatiles (oDCB, I2) were removed under vacuum. The solid residue was redissolved in toluene, injected into a COSMOSIL Buckyprep HPLC column, and eluted with 80/20 (v/v) toluene/heptane. The HPLC traces are shown in Fig. 2. b Per equiv. C60; BnFI = C6F5CF2I. c Per equiv. C60; R = n-Bu. d The mol% values in parentheses are for 1 h reactions; all other mol% values are for 2 h reactions. The mol% values do not add up to 100% because other, unidentified fullerene byproducts were also present. | ||||||
| a | 100(2) | 1 | 2 | 24 | ca. 0 | 70 |
| b | 160(5) | 1 | 2 | (15)6 | (8)30 | (70)55 |
| c | 160(5) | 10 | 2 | (18)22 | (7)13 | (64)51 |
| d | 160(5) | 10 | 20 | (14)3 | (5)1 | (31) ca. 0 |
| e | 160(5) | 10 | 5 | 29 | 7 | 13 |
We propose that the formation of 1 from C6F5CF2I and SnH(n-Bu)3 in oDCB at elevated temperatures is best represented by the following balanced equation (BnFI = C6F5CF2I; R = n-Bu):
C60 + BnFI + SnHR3 → 1,9-C60(CF2C6F5)H (1) + 1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I2 + 1/2 I2 + 1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn2R6 Sn2R6 |
At 100 °C and C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RFI
RFI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnHR3 reagent mole ratios of 1
SnHR3 reagent mole ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (HPLC trace a in Fig. 2), compound 1 and C60 were virtually the only fullerene species present in the reaction mixture after 2 h. The same amount of unreacted C60 was also present with the same reagent ratios when the temperature was 160 °C for 1 h (HPLC trace b, dotted line), but in this case both 1 and 2 were present (in a ca. 2
2 (HPLC trace a in Fig. 2), compound 1 and C60 were virtually the only fullerene species present in the reaction mixture after 2 h. The same amount of unreacted C60 was also present with the same reagent ratios when the temperature was 160 °C for 1 h (HPLC trace b, dotted line), but in this case both 1 and 2 were present (in a ca. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol ratio). After 2 h (trace b, solid line), significantly less 1 and significantly more 2 were present (now in a ca. 1
1 mol ratio). After 2 h (trace b, solid line), significantly less 1 and significantly more 2 were present (now in a ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mol ratio). The HPLC traces labeled c and d show the results of changing the reagent mole ratios for 1 h (dotted lines) and 2 h (solid lines) reactions. HPLC traces d indicate that a large excess of SnHR3 produces many other fullerene derivatives (presumably various hydrofullerenes) and much less 1 and 2 than when less SnHR3 was used. We conclude that 1 is an intermediate in the formation of 2 under the reaction conditions. It is possible that SnR3˙ radicals are involved, as shown in the following speculative balanced equation, but SnFR3 has not been positively identified:
5 mol ratio). The HPLC traces labeled c and d show the results of changing the reagent mole ratios for 1 h (dotted lines) and 2 h (solid lines) reactions. HPLC traces d indicate that a large excess of SnHR3 produces many other fullerene derivatives (presumably various hydrofullerenes) and much less 1 and 2 than when less SnHR3 was used. We conclude that 1 is an intermediate in the formation of 2 under the reaction conditions. It is possible that SnR3˙ radicals are involved, as shown in the following speculative balanced equation, but SnFR3 has not been positively identified:
| 1,9-C60(CF2C6F5)H (1) + 2SnR3˙ → 1,9-C60(cyclo-CF2(2-C6F4)) (2) + SnHR3 + SnFR3 |
HPLC trace e in Fig. 2 represents a compromise set of reaction conditions that produced significant amounts of 1 and 2, relatively less unreacted C60, and relatively small amounts of the other fullerene byproducts. This reaction resulted in a 35% isolated yield of 1 and a 7% isolated yield of 2 after HPLC purification (both yields based on C60).
An alternate synthesis of 2 is the reaction of 1 with excess Proton Sponge (PS, 1,8-bis(dimethylamino)naphthalene) in CH2Cl2 at 23(1) °C for 24 h. This reaction, which resulted in a 76% isolated yield of 2 based on 1, will be discussed in detail in Section 2.3. We also explored photochemical syntheses, but these invariably showed lower yields of 1 and 2 and will not be discussed further.
2.2. Characterization of 1 and 2
The negative-ion (NI) APCI mass spectrum of 1 exhibited an m/z species at 937, which is consistent with C60(CF2C6F5)−, or [1 − H]−. The UV-vis spectrum of 1 (Fig. S-2†) exhibited absorption maxima at 324, 431, and 698 nm. The 431 nm band in particular is characteristic of 1,9-C60X2 or 1,9-C60XY derivatives.17 In contrast, C60XY derivatives with the substituents on the para positions on a C60 hexagon (i.e., 1,7-C60XY) generally exhibit a prominent band at 450 nm.4 The singlet at δ 7.2 in the 1H NMR spectrum of 1 in CDCl3 is characteristic of a C60–H species18–20 (cf. δ 6.65 for 1,9-C60(CH2C6H5)H20).The NI-APCI mass spectrum of 2 exhibited an m/z species at 918, which is consistent with the formula C60(CF2C6F4)−. The UV-vis spectrum of 2 (Fig. S-2†) exhibited bands at 331, 430, and 687 nm, which supports a 1,9-addition pattern for this compound as well (verified by X-ray crystallography). No resonance was observed in a 1H NMR spectrum of 2.
The structure of 2, determined by X-ray diffraction, is shown in Fig. 1. The five-membered carbocycle substituent is essentially planar, with out-of-plane displacements (OOPs) for C1, C2, C7, C8, and C9 that range from 0.003 Å to 0.066 Å (average ±0.042 Å). In fact, C1, C9, and all seven of the perfluorinated substituent's C atoms are also co-planar (the nine OOPs range from 0.003 to 0.089 Å and average ±0.032 Å). The long C1–C9 bond distance of 1.611(3) Å is typical of C60 derivatives with 3-, 4-, 5-, and 6-membered exocyclic rings.21,22
The molecule has idealized Cs symmetry, with the essentially planar faux hawk substituent nearly perpendicular (i.e., 84°) to a plane tangent to the idealized fullerene surface at the C1–C9 midpoint (the two C2–C1–Ccage angles only differ by ca. 2°; the same is true for the two C8–C9–Ccage angles). This gives the molecule its “faux-hawk-hairstyle” appearance, as shown in Fig. S-1.† In the OLYP DFT-optimized structure of 2, the faux hawk substituent is rigorously planar (except for F1 and F2) and rigorously perpendicular to the C60 surface. See Table S-1† for a comparison of relevant interatomic distances and angles for the X-ray and OLYP DFT-optimized structures of 2 and Fig. S-3† for a side-by-side comparison of the two structures. Note that the faux hawk substituent in 2 is attached to the type of C60 C–C bond that is common to two hexagons. Table S-1† also includes the distances and angles for the OLYP DFT-optimized structure of the isomer with the faux hawk substituent attached a C60 C–C bond that is common to a pentagon and a hexagon, showing that the faux hawk substituent is sterically congruent in both isomers. Nevertheless, the DFT-predicted relative energy of the unobserved alternate isomer is 62 kJ mol−1 above the energy of the observed isomer. This difference is, therefore, fullerene based and not faux hawk-substituent based. As indicated above, the faux hawk substituent in the unobserved and observed isomers is attached to a 5,6-pentagon–hexagon and a 6,6-hexagon–hexagon C60 edge, respectively. Attachment of substituent atoms to a 5,6-edge of C60 introduces two C⋯C double bonds in pentagons, each of which is predicted to raise the energy of the C60 core by 33.5 ± 4.2 kJ mol−1.23
There are several other examples of C60 derivatives with five-membered carbocyclic rings (these are formed by 3 + 2 cycloadditions of trimethylenemethanes to C60),24,25 but 2 is the only structurally-characterized example in which the carbocycle contains a C⋯C double bond and is therefore planar. It is also the only example in which the carbocycle is perfluorinated.
Fluorine-19 NMR spectra of 1 and 2 are shown in Fig. 3 and 4, respectively. Chemical shifts and coupling constants are listed in Table S-2.† The J(FF) coupling constants were determined by simulating the experimental spectra using the program MestReNova 8.1.1. The free rotation about the F2C–Cipso bond in 1 and the presumed time-averaged Cs symmetry of 2 render the F atoms in the CF2 moiety magnetically equivalent in both compounds. The aromatic moieties in 1 and 2 exhibited bb′cc′d and bcde patterns, respectively (the notation here conforms to the F atom labels in Fig. 3 and 4).
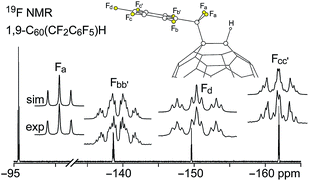 | ||
| Fig. 3 Experimental and simulated 376.5 MHz 19F NMR spectra of HPLC-purified 1,9-C60(CF2C6F5)H (1) in CDCl3. Chemical shifts and coupling constants are listed in Table S-2.† | ||
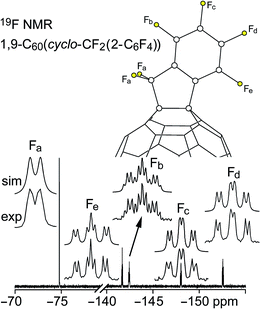 | ||
| Fig. 4 Experimental and simulated 376.5 MHz 19F NMR spectra of HPLC-purified 1,9-C60(cyclo-CF2(2-C6F4)) (2) in CDCl3. Chemical shifts and coupling constants are listed in Table S-2.† | ||
The meta coupling constants J(FbFb′), J(FcFc′), and J(Fbb′Fd) in 1 and J(FbFd) and J(FcFe) in 2 are all 5–6 Hz. The ortho values, J(FbFc)/J(Fb′Fc′) and J(Fcc′Fd) in 1 and J(FbFc), J(FcFd), and J(FdFe) in 2, are, as expected,26 significantly larger, 18–26 Hz. The para coupling constants, however, are substantially different for the two compounds; J(FbFc′) = J(Fb′Fc) is 7 Hz in 1 and J(FbFe) is 23 Hz in 2. The 7 Hz value for 1 is the same as the ca. 7 Hz coupling constants for F atoms para to one another in perfluorophenyl groups.27 The 23 Hz value for 2 can be compared with the 18–26 Hz range for F atoms para to one another in tri- and tetrafluorobenzo[b]thiophenes,28 the 14–19 Hz range in polyfluoroindenes,29 and the 12–16 Hz range in tetrafluorobenzo[b]thiazoles,30 compounds that, like 2, have a polyfluorobenzo moiety fused to a coplanar five-membered ring. The origin of the difference in magnitude for para J(FF) values for polyfluorophenyl vs. polyfluorobenzo compounds is not well understood.
On the other hand, the substantial difference in 4J(FaFbb′) in 1 and 4J(FaFb) in 2, 30 Hz and 5.5 Hz, respectively, has a compelling explanation (the 4J(FaFbb′) value for C6F5CF2I is also 30 Hz). In both cases the F atoms are separated by a C(sp2)–C(sp3) single bond as well as a C(sp2)–C(sp2) bond, and the 4J(FF) values are almost certainly dominated by Fermi-contact through-space interactions,31–40 which are strongly dependent on the F⋯F distance, the F–C⋯C(F) angle, and the F–C⋯C–F torsion angle. The two Fa⋯Fb distances in the X-ray structure of 2 (these are F1⋯F6 and F2⋯F6 in Fig. 1) are 2.998(6) and 3.151(6) Å, respectively, near the limit of ca. 3.2 Å for observable Fermi-contact through-space coupling between proximal F atoms (the corresponding distances in the Cs-symmetric DFT-optimized structure of 2 are both 3.088 Å).31–40 In contrast, the short Fa⋯Fb distances in the Cs-symmetric lowest-energy DFT-optimized structure of 1 are both 2.587 Å, a distance which is comparable to the 2.60–2.65 Å F⋯F distances in compounds previously shown to exhibit 4J(FF) values of 19, 25, 27, or 48 Hz depending on the aforementioned angles.39
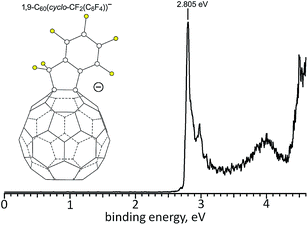
The gas-phase electron affinity (EA) of 2 was determined to be 2.805(10) eV by low-temperature photoelectron spectroscopy (LT-PES) of the 2− radical anion, as shown in Fig. 5 (cf. 2.683(8) eV for C60 (ref. 41) and 2.63(1) eV for PCBM42). Therefore, 2 is a stronger electron acceptor (in the gas phase) than C60 and PCBM by 0.12(1) and 0.18(1) eV, respectively. The LT-PES spectrum of 1− could not be observed because of the rapid loss of the H atom to form the closed-shell species [1 − H]− (i.e., C60(CF2C6F5)−). Photodetachment of an electron from this anion allowed the 3.75(3) eV EA of the neutral radical C60(CF2C6F5)˙ to be determined, but the EA of 1 remains unknown. It is well known that the EA values for fullerene radicals are ca. 1–2 eV higher than for closed-shell fullerene derivatives of similar composition. For example, the EA values for closed-shell C60F46, the C60F47˙ radical, and closed-shell C60F48 are 4.06(25),43 5.66(10),44 and 4.06(30) eV,43 respectively.
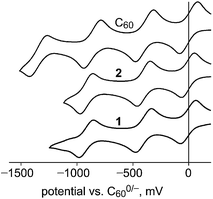
Square-wave and cyclic voltammograms (SWVs and CVs, respectively) of 1, 2, C60, and PCBM were recorded under identical conditions in oDCB containing 0.1 M N(n-Bu)4BF4 and Fe(Cp)2 as an internal standard. In all cases the reduction potentials determined by SWV and by CV were the same within the ±0.01 V uncertainty of the individual measurements. The potentials are listed in Table 2 along with E1/2(0/−) values for the related compounds 1,9-C60H2,45,46 1,9-C60(CH3)2,47 and 1,9-C60(cyclo-C2F4).48 Our E1/2(0/−) values for C60 and PCBM were first reported in 2013 in the same paper reporting the EA of PCBM.42 The CVs for 1, 2, and C60 are shown in Fig. 6. The similarity of E1/2(0/−) values for 2 and C60 is at odds with the 0.12(1) eV difference in their EAs. However, differences in E1/2(0/−) values for fullerene derivatives are generally smaller, and sometimes much smaller, than the corresponding differences in their EAs.4,11
| Compound | 0/− potential, V vs. C600/− | −/2− potential, V vs. C600/− | 2−/3− potential, V vs. C600/− | 3−/4− potential, V vs. C600/− |
|---|---|---|---|---|
| a All potentials from cyclic voltammograms unless otherwise indicated. Conditions (unless otherwise noted): purified dinitrogen atmosphere glovebox; 1,2-C6H4Cl2 (oDCB) solutions at 23(1) °C; 0.1 M N(n-Bu)4BF4 electrolyte; Fe(Cp)2 internal standard; scan rate 100 mV s−1; Pt working and counter electrodes; Ag wire quasi-reference electrode. The uncertainty for each measurement is ±0.01 V. b 1,9-C60(CF2C6F5)H = 1; 1,9-C60(cyclo-CF2(2-C6F4)) = 2. c Potential from square-wave voltammetry. d ref. 105. e At 25 °C in benzonitrile; ref. 20. f At −50 °C in 90/10 (v/v) toluene/dimethylformamide; ref. 46. g At 25 °C in benzonitrile; ref. 47. h ref. 48. | ||||
| 1,9-C60(CF2C6F5)Hb | −0.02 | −0.45 | −0.98 | — |
| 1,9-C60(cyclo-CF2(2-C6F4))b | −0.01 | −0.40 | −0.92 | −1.36c |
| C60 | 0.00 | −0.39 | −0.85 | −1.31 |
| PCBM | −0.09 | −0.48 | −0.99 | |
| iso-PCBMd | −0.08 | |||
| 1,9-C60(CH2C6H5)He | −0.08 | −0.48 | ||
| 1,9-C60H2f | −0.13 | |||
| 1,9-C60(CH3)2g | −0.13 | |||
| 1,9-C60(cyclo-C2F4)h | 0.03 | |||
Removing one of the double bonds of C60 by addition of substituents or a cycloadduct to C1 and C9 generally lowers the E1/2(0/−) by ca. 0.1 V. For example, E1/2(0/−) values for 1,9-C60(CH2C6H5)H,20 PCBM,42 1,9-C60(CH3)2,47 and 1,9-C60H2,46 are −0.08, −0.09, −0.12, and −0.13 V vs. C600/−, respectively (in each case the comparison with C60 was made under the same conditions of solvent, electrolyte, and temperature). If the cycloadduct is fluorinated and therefore electron withdrawing, as in 2 and 1,9-C60(cyclo-C2F4), the E1/2(0/−) values, −0.01 and 0.03, respectively, have increased by ca. 0.1 V from PCBM-like potentials, resulting in C60-like potentials. The E1/2(0/−) values of 2 and C60 are the same because the offsetting effects of (i) reducing the fullerene π system by one double bond and (ii) changing the substituent(s) from hydrocarbyl groups or a hydrocarbyl cycloadduct to a perfluorocarbon cycloadduct cancel each other in this case.
The foregoing analysis is the reason that we were surprised that the three E1/2 values for 1 and 2 are so similar. We expected the E1/2(0/−) value for 1 to be ca. halfway between 0.03 and −0.13 V based on the E1/2(0/−) values in Table 2, but clearly this is not the case (an 19F NMR spectrum of 1 in the electrolyte solution used for the CV experiments, to which 10% C6D6 was added, verified that 1 does not react with the electrolyte solution on the timescale of the CV experiment). We also expected 1− to undergo loss of the H atom to form [1 − H]−, as it did in the LT-PES experiment discussed above and in the 1 + CoCp2 reaction discussed below. Furthermore, hydrofullerenes such as 1,9-C60H2,45,46 1,9-C60(CH2C6H5)H,49 and isomers of C70(CH2C6H5)H50 are known to undergo observable H-atom loss upon one-electron reduction unless the CV scan speed is extremely high or the solution is cooled to a low temperature. Nevertheless, our expectations notwithstanding, and in the absence of additional electrochemical experiments, the redox potentials for 1 listed in Table 2 are correctly assigned.
2.3. Understanding the transformation 1 → 2 + “HF”
According to O3LYP//OLYP DFT calculations, the transformation 1 → 2 + HF is exothermic by 42 kJ mol−1 in the gas phase and 60 kJ mol−1 in a PhCN-like dielectric continuum. However, 1 was unchanged after heating an oDCB solution at 160(5) °C for 2 h. Therefore, this reaction does not occur rapidly by a thermally-activated intramolecular pathway in a non-basic solvent. Nevertheless, the synthesis of 1 resulted in the formation of significant amounts of 2 depending on the reaction conditions. To test the idea that 2 can be produced from 1 as an intermediate (although not necessarily as an obligate intermediate), we performed the following series of reactions.The reagent SnH(n-Bu)3 and byproduct Sn2(n-Bu)6 that are present during the synthesis of 1 and 2 can form Sn(n-Bu)3˙ radicals. In a separate experiment, we heated 1 in oDCB at 160 °C with added Sn2(n-Bu)6. Unlike the 160 °C experiment described in the previous paragraph, complete conversion of 1 to 2 occurred within 2 h in the presence of Sn2(n-Bu)6. Since the reagents SnH(n-Bu)3 and Sn2(n-Bu)6 are not “simple” one-electron reducing agents, we also studied the reaction of 1 with 1 equiv. of CoCp2 in PhCN at 23(1) °C. This also caused the conversion of 1 to 2, as shown by 19F NMR spectroscopy.
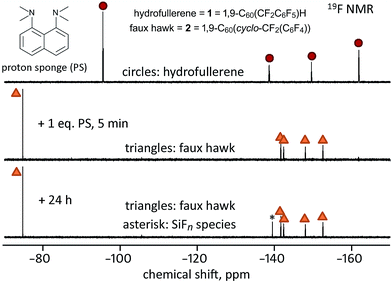
If the one-electron reduced species 1− loses an H atom, as do other one-electron reduced hydrofullerenes (see above), the intermediate would be CoCp2+C60(CF2C6F5)− (i.e., CoCp2+[1 − H]−), which would react further to form 2 and CoCp2+F−. A simpler way to generate [1 − H]− is by deprotonation. When 1.0 equiv. of the strong base PS was added to a 90/10 (v/v) PhCN/C6D6 solution of 1 at 23(1) °C, the formation of 2 was complete within 5 min, as shown in Fig. 7. At longer times, a new 19F peak appeared at δ −139.6. Based on the chemical shift, the magnitude of the coupling constant (145 Hz), and the abundance of the I = 1/2 species to which the F atoms are coupled (ca. 5%), the new peak is assigned to an “SiFn” species,51–56 indicating that HF, or species with HF-like reactivity towards glass, such as ion-paired [H(PS)]+F− and/or HF2−, were byproducts of the reaction. Rapid exchange between HF, F−, and HF2− is probably the reason why 19F peaks due to one or more of these species were not observed during or after the reaction, only an SiFn species due to reaction of the HF-like species with the walls of the NMR tube. When 1 was treated with excess PS in CDCl3 for 24 h, the reaction mixture contained 24% 1, 76% 2, and a precipitate (the amounts of 1 and 2 were determined by integrating the 19F NMR spectrum of the reaction mixture). The precipitate was soluble in CD3CN and exhibited a δ −155.8 19F NMR singlet and a broad δ 19.0 1H NMR singlet, both of which are commensurate with H(PS)+F−.57
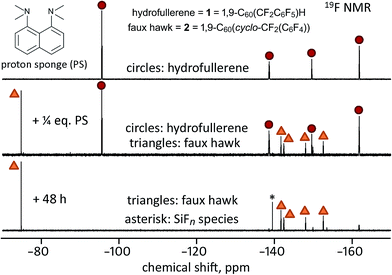
On the basis of the experiments just described, we propose that the treatment of hydrofullerene 1 with PS resulted in deprotonation to give H(PS)+ and C60(CF2C6F5)− as first-formed intermediates and that C60(CF2C6F5)− formed faux hawk fullerene 2 and “F−” within minutes. At longer times, [H(PS)]+F− or an equivalent fluoride-like species present reacted with the glass NMR tube to form the SiFn species. Even though the putative intermediate C60(CF2C6F5)− disappeared too rapidly to observe before an 19F NMR spectrum could be recorded, its presence can be proposed because simple deprotonation of hydrofullerenes to give anionic fullerene species is well documented (i.e., hydrofullerenes are known to be Brønsted acids: the pKa values for C60(CN)H,58 C60H2,59 and C60(t-Bu)H60 were found to be 2.5, 4.7, and 5.7, respectively). Interestingly, when 1 was treated with only 0.25 equiv. of PS in 90/10 (v/v) PhCN/C6D6 solution, the complete conversion to 2 also occurred, but only after 48 h, as shown in Fig. 8. This autocatalytic transformation of 1 into 2 presumably results from the first-formed 0.25 equiv. byproduct F− (or [H(PS)]+F− or HF2−), which formed rapidly, acting as a base and continuing to deprotonate, albeit more slowly, additional molecules of 1 until it is completely converted to 2. In a control experiment to inhibit the proposed catalytic effect of F− as a general base, a few drops of saturated aqueous Ca(NO3)2 were added to a similar NMR-scale reaction containing ca. 0.3 equiv. of PS (based on 1). In this case, the conversion of 1 to 2 was only 30–40% complete after 48 h, a white gelatinous precipitate formed in the aqueous layer (presumably CaF2), and the 19F NMR peak assigned to the SiFn species was absent even after 48 h.
The rapid conversion of deprotonated 1 (i.e., [1 − H]−) to 2 most likely occurs by an intramolecular SNAr mechanism whereby the [1 − H]− fullerene carbanion attacks one of the ortho-C–F bonds of the CF2C6F5 substituent. Fullerenes aside, intermolecular SNAr reactions involving aromatic C–halogen bonds have been extensively studied.61–65 In contrast, the scope of intramolecular SNAr reactions that result in breaking an aromatic C–F bond and concomitant loss of F− is limited.66–68 In the examples most relevant to this work, Hughes and co-workers showed that perfluorobenzyl ligands on either Co67 or Rh68 can undergo intramolecular SNAr substitution of an ortho-F atom to form either six- or five-membered chelate rings, respectively. There is general agreement that, all other things being equal, aromatic C–F bonds undergo SNAr substitution much faster than aromatic C–Cl, C–Br, or C–I bonds.61–65 However, there is still controversy about whether a true Meisenheimer69 intermediate is formed (even if it cannot be detected spectroscopically)70–76 or whether the reaction involves a single Meisenheimer-like transition state.77–80
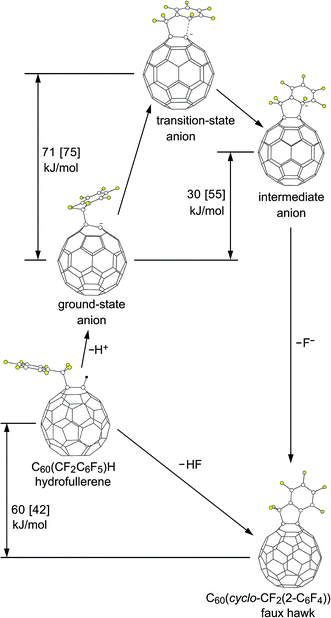
Reactions of C60R− carbanions with electrophilic substrates EX to form new C60(E)R species and X− are well known,22,81,82 but to our knowledge there is no previous example of an SNAr reaction involving a fullerene cage carbanion (i.e., not including examples such as the negatively-charged N atom of a deprotonated cyclo-pyrrolidinofullerene undergoing an intermolecular SNAr reaction with an aryl chloride83), let alone an intramolecular SNAr reaction of a fullerene cage carbanion attacking an Ar–F bond. Therefore, we decided to test the intramolecular SNAr hypothesis for the observed transformation [1 − H]− → 2 + F− by determining DFT-optimized structures and relative energies for 1 and 2 as well as for three different states of [1 − H]−. Fig. 9 shows the OLYP DFT-optimized structures and the O3LYP//OLYP relative energies of these five species. Both gas-phase and PhCN-like dielectric continuum relative energies were calculated. Drawings of the upper fragments of the gas-phase optimized structures are shown in Fig. 10 and relevant interatomic distances and angles are listed in Table 3.84 Larger drawings of the optimized species are shown in Fig. S-7 to S-12.† The calculated solvation energies for the ground-state (GS), transition-state (TS), and Meisenheimer-like intermediate-state (IS) structures of the deprotonated [1 − H]− anion are listed in Table S-3.† This table also lists the gas-phase relative energies using other DFT functionals for the three [1 − H]− states along the proposed SNAr reaction coordinate.
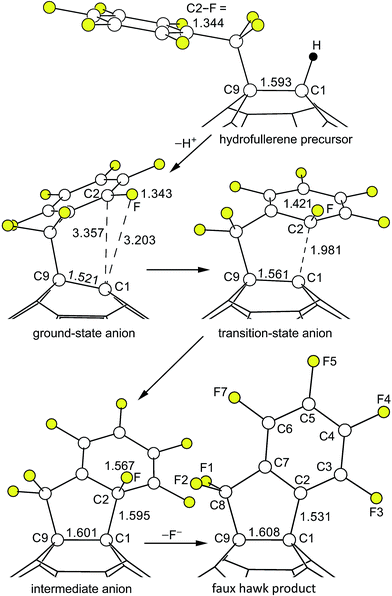 | ||
| Fig. 10 Parts of the OLYP DFT-optimized structures and O3LYP//OLYP relative energies of 1,9-C60(CF2C6F5)H (1; hydrofullerene), 1,9-C60(cyclo-CF2(2-C6F4)) (2; faux hawk), and the three [1 − H]− anions proposed for the SNAr transformation [1 − H]− → 2 + F− (i.e., the ground-state, transition-state, and intermediate C60(CF2C6F5)− anions). Additional distances and angles are listed in Table 3 and are shown in Fig. S-7 through S-12.† | ||
| Distance or angle | 1 | Ground-state [1 − H]− anion | Transition state [1 − H]− anion | Intermediate [1 − H]− anion | 2 |
|---|---|---|---|---|---|
| a OLYP DFT-optimized structures. 1 = 1,9-C60(CF2C6F5)H; 2 = 1,9-C60(cyclo-CF2(2-C6F4)). b A comparison of the DFT-predicted and experimental X-ray diffraction distances and angles for 2 is shown in Table S-1. c These four distances are listed in the order C3–C4, C4–C5, C5–C6, and C6–C7. d The π-orbital axis vector (POAV) for a fullerene C atom is defined as the vector that makes equal angles to the three Ccage atoms to which it is attached (see ref. 84). The common angle is denoted θσπ and θp = θσπ − 90°. The angle θp denotes the degree of pyramidalization of a fullerene cage C atom. For an idealized trigonal-planar C(sp2) atom, θp = 0°; for an idealized tetrahedral C(sp3) atom, θp = 19.5°. | |||||
| C1–C2 | — | 3.357 | 1.981 | 1.595 | 1.531 |
| C1–C9 | 1.593 | 1.521 | 1.561 | 1.601 | 1.608 |
| Other C1–Ccage | 1.527, 1.527 | 1.424, 1.425 | 1.474, 1.484 | 1.528, 1.533 | 1.541, 1.541 |
| C2–F | 1.344 | 1.343 | 1.421 | 1.567 | — |
| Other CAr–F | 1.342 × 2, 1.339, 1.344 | 1.345 × 2, 1.347 × 2 | 1.354, 1.356, 1,358, 1.359 | 1.356, 1.357, 1.360, 1.364 | 1.340, 1.341, 1.342, 1.348 |
| C2–C3 | 1.397 | 1.398 | 1.431 | 1.456 | 1.395 |
| C2–C7 | 1.406 | 1.406 | 1.444 | 1.457 | 1.395 |
| Other CAr–CArc | 1.395 × 2, 1.397, 1.406 | 1.393 × 2, 1.397, 1.405 | 1.380, 1.407, 1.392, 1.396 | 1.375, 1.390, 1.392, 1.413 | 1.395, 1.396, 1.399, 1.401 |
| C1–C2–C3 | — | 136.3 | 117.1 | 120.1 | 128.1 |
| C1–C2–C7 | — | 67.6 | 98.2 | 106.2 | 112.9 |
| C3–C2–C7 | 121.8 | 121.7 | 116.5 | 113.3 | 119.0 |
| F–C2–C1 | — | 71.9 | 94.9 | 101.0 | — |
| F–C2–C3 | 116.5 | 116.1 | 111.6 | 105.6 | — |
| F–C2–C7 | 121.7 | 122.2 | 116.1 | 109.7 | — |
| C1 POAV θpd | 18.2 | 9.6 | 16.2 | 19.6 | 19.3 |
| C9 POAV θpd | 19.5 | 22.0 | 20.0 | 19.0 | 19.1 |
The DFT results show that an SNAr mechanism is energetically viable for the unimolecular intramolecular annulation reaction [1 − H]− → 2 + F−, even without the probable stabilizing effect of hydrogen bonding of either H(PS)+ or HF to the three [1 − H]− structures. The transition state structure of [1 − H]− is only ca. 70 kJ mol−1 above the ground-state structure; transition states of 45–130 kJ mol−1 have been calculated for non-fullerene SNAr transition states involving nitrogen or sulfur nucleophiles and aromatic C–F bonds.74–76 This is consistent with the observed reaction time of only minutes when 1 was mixed with 1 equiv. of PS in 90/10 (v/v) PhCN/C6D6 at 23(1) °C. Apparently, there is sufficient conformational flexibility in the CF2C6F5 substituent in [1 − H]− to accommodate the nascent five-membered ring in the transition state.
The structural changes in the C1–C9 moiety of five fullerene species along the proposed SNAr reaction coordinate can be appreciated using Fig. 10 and the results listed in Table 3. There is a significant change in the degree of pyramidalization (θp; see Table 3) of C1 and in the set of three C1–C distances for the first step in the reaction sequence, the deprotonation of 1. The former changes from 18.2° for 1 to 9.6° for GS [1 − H]− and the latter from {1.59, 1.53, 1.53 Å} for 1 to {1.52, 1.42, 1.43} for GS [1 − H]−, signaling a change in hybridization of C1 from sp3 in 1 to a blend of sp3 and sp2 in GS [1 − H]−. The ground-state anion is a carbanion, but the negative charge and the putative “lone pair” are delocalized throughout the C60 cage. Significantly, the 9.6° θp degree of pyramidalization for C1 in GS [1 − H]− is smaller, not larger, than the 11.6° θp value for the cage C atoms in C60 (ref. 84) (the delocalization of the negative charge in C60R− carbanions was previously proposed by Van Lier, Geerlings, and coworkers based on computational results85−87). As expected, the C6F5 rings in 1 and GS [1 − H]− are virtually congruent. Even the C8–C9 bond distance is unaffected by the deprotonation.
In the second step, GS [1 − H]− is transformed into TS [1 − H]−. Even though the C1⋯C2 distance, at 1.981 Å, is very long, the C1 θp value increases from 9.6° to 16.2°, which is 90% of its original value in 1. Accordingly, the three C1–Ccage distances increase from {1.52, 1.42, 1.43} in GS [1 − H]− to {1.56, 1.47, 1.48} in TS [1 − H]−. At the same time, C2 is developing sp3 character: the C2–C3 and C2–C7 distances increase from 1.40 and 1.41 Å in GS [1 − H]− to 1.43 and 1.44 Å in TS [1 − H]−, and the sum of the three angles at C2 involving C3, C7, and F is 344° in TS [1 − H]− whereas this sum is 360° in GS [1 − H]−. Another way to depict the distortion in the C6F5 group in TS [1 − H]− is as follows. The 10 atoms C2–C7 and F3–F6 are coplanar to within ±0.02 Å in both GS [1 − H]− and TS [1 − H]−. However, in GS [1 − H]− atom F (i.e., the F atom bonded to C2) is also in that plane whereas in TS [1 − H]− it is displaced 0.86 Å from that plane. As expected, the C2–F bond in TS [1 − H]−, at 1.42 Å, is significantly longer than the 1.34 Å distance in both hydrofullerene precursor 1 and the GS [1 − H]− anion.
The Meisenheimer-like intermediate, denoted IS [1 − H]−, exhibits further repyramidalization of C1 and further pyramidalization of C2. Both of these atoms are essentially tetrahedral in the intermediate, with four single bonds. In fact, the C1 θp value, 19.6°, is only 0.1° different than the ideal θp tetrahedral angle (19.5°), and the sum of the three angles at C2 involving C3, C7, and F is 328.6°, within 0.1° of the expected sum for a tetrahedral C atom (i.e., 3 × 109.5° = 328.5°). Furthermore, the C2–F bond, at 1.567 Å, is exceptionally long and is clearly developing a significant amount of F− character. Note that all C–F bond distances measured by X-ray crystallography (as of 1987) are shorter than 1.4 Å.88
Finally, in the last step of the reaction sequence shown in Fig. 9 and 10, F− dissociates from the intermediate and the C6F4 ring undergoes rearomatization (i.e., the C2–C3 and C2–C7 bond distances shorten from 1.46 Å in IS [1 − H]− to 1.40 Å in 2 (therefore all six CAr–CAr distances in 2 are 1.40 Å)).
2.4. Molecular structure and solid-state packing of 2 and comparison with single-crystal X-ray structures of PCBM
There are two solvent-free X-ray structures of PCBM: a single-crystal structure determined using data collected at 100(2) K (ref. 89) and a structure determined by powder X-ray diffraction data collected at 298(2) K.90 The molecular structures of 2 and the 100 K single-crystal structure PCBM89 are shown side-by-side in Fig. S-13.† The two substituents have nearly the same number of non-hydrogen atoms, 13 for 2 and 14 for PCBM, but the faux hawk substituent is clearly the more compact. The 1.632(2) Å C1–C9 bond in PCBM is only marginally longer than the 1.610(5) Å distance in 2, and fullerene cage atoms C1 and C9 are only slightly less pyramidalized in PCBM (POAV θp = 17.1° × 2) than in 2 (θp = 18.9 and 19.1°).The solvent-free solid-state packing of 2 and PCBM89 are analyzed in detail and discussed in the ESI,† along with comparisons to PCBM X-ray structures containing solvent molecules and a related structure (see page S-17 and Fig. S-14 through S-18†). The result of this analysis is that there are only seven (7) nearest neighbor fullerene molecules in crystalline solvent-free PCBM, with C60 centroid⋯centroid (⊙⋯⊙) distances of 9.95–10.28 Å. The mean distance is 10.17 Å. On the other hand, there are ten (10) nearest neighbors in the structure of 2, with ⊙⋯⊙ distances of 9.74–10.34 Å. The mean distance is 10.09 Å. The result is that the density of crystalline 2, 1.885 g cm−3, is 15.6% higher than the 1.631 g cm−3 density of solvent-free PCBM, even though the molar masses of the two compounds, 918.67 g mol−1 for 2 and 910.83 g mol−1 for PCBM, differ by only 1.1%. The significance of this is that the aggregation behavior of OPV acceptor fullerenes in the solid state, especially the number of electronically coupled nearest neighbors and their three-dimensional arrangement, is widely believed to be among the key factors that determine charge transport properties in the fullerene domains in Type II heterojunction solar cells.89–100
2.5. Microwave conductivity experiments
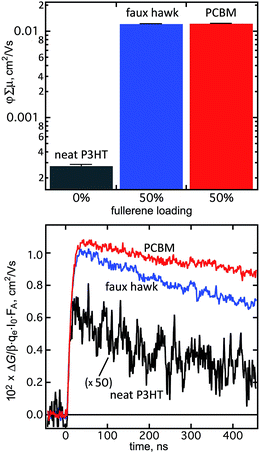
The denser packing of 2 relative to PCBM and the nearly-equal E1/2(0/−) values for 2 and C60 suggested that 2 might be an efficacious electron acceptor in OPV bulk heterojunction thin films. To test this hypothesis, we probed the charge generation and decay dynamics of 2 when blended with regioregular poly-3-hexylthiophene (rr-P3HT) using time-resolved microwave conductivity (TRMC).101 There are two advantages to measuring photoconductance with TRMC: (i) it is a contactless method and is therefore specific to processes occurring in an OPV active-layer film under illumination; and (ii) the ns–μs timescale of TRMC measurements is the same as the timescale of charge-carrier dynamics in an OPV device.95Fig. 11 shows the φ∑μ TRMC figure of merit for three thin-film samples (φ is the quantum yield of mobile-charge-carrier generation (i.e., electrons and holes) and ∑μ is the sum of charge-carrier mobilities at the limit of low excitation intensity).102The φ∑μ value for a blend of rr-P3HT and 2 is nearly two orders of magnitude higher than for a neat rr-P3HT thin film and is comparable to the φ∑μ value for an rr-P3HT/PCBM blend, as shown in Fig. 11. The latter observation is indicative of efficient free-charge-carrier generation in the rr-P3HT/2 blend, a combination of a high φ value as well as a large ∑μ contribution due to electron mobility in domains of 2 within the bulk heterojunction thin film, as previously observed for rr-P3HT blends with other high-performance OPV acceptors.95,102,103
The decay profiles of the transients for the rr-P3HT/2 and rr-P3HT/PCBM blends are nearly identical, as also shown in Fig. 11. The signals are longer lived than for the neat donor polymer, which is normally attributed to high electron mobility in the fullerene phase.102 Taken together, the TRMC results indicate that 2 is a promising acceptor for OPV. Its higher electron affinity relative to PCBM suggests that it may be better to blend 2 with “push–pull” low-bandgap donor polymers with HOMO and LUMO energies deeper than P3HT in order to offset open-circuit-voltage losses,104 and the perfluorinated nature of its substituent suggests and it may be better to blend 2 with fluorinated donor polymers. These experiments are currently underway and will be reported in a future publication.
2.6. Thermal stability of 1,9-C60(cyclo-CF2(2-C6F4)) (2)
The final comparison we wish to report is the thermal stability of 2vs. PCBM. It was recently shown that PCBM undergoes substantial decomposition in only 20 min at 340 °C.105 An HPLC trace of 340 °C-treated PCBM, taken from a figure in ref. 105, is shown in Fig. 12. Part of the 340 °C-treated PCBM sample was a charred residue that did not dissolve in toluene. Of the portion of the sample that did dissolve, only ca. 22% was intact PCBM. The most abundant decomposition product was identified as a new five-membered ring cycloadduct isomer of PCBM that was named iso-PCBM and that is virtually a hydrocarbyl equivalent of 2 (see Fig. S-19† for the structure of iso-PCBM; see also Table 2).105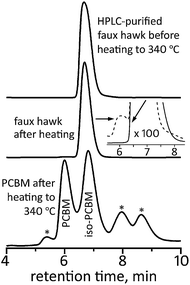 | ||
| Fig. 12 HPLC traces of faux hawk fullerene 1,9-C60(cyclo-CF2(2-C6F4)) (2) before and after heating to 340 °C for 20 min and HPLC trace of PCBM after heating to 340 °C for 20 min. The PCBM HPLC data were reported in ref. 105. The inset for the middle HPLC trace has been expanded 100 times on the vertical axis (the after-heating trace in the inset is the dashed line). The asterisks in the PCBM after-heating trace are unidentified thermal decomposition products. For all three HPLC traces, a COSMOSIL Buckyprep column was used with a toluene eluent rate of 5 mL min−1 and 300 nm detection. | ||
In contrast, the HPLC trace of 340 °C-treated 2, also shown in Fig. 12, shows no evidence of decomposition unless the traces are vertically expanded 100 times. In the expanded trace, the unambiguous presence of one yet-unidentified new species with an abundance of ca. 0.6 mol% based on HPLC relative intensities can be seen. In addition, no new peaks were observed in the 19F NMR spectrum of 340 °C-treated 2. Based on the signal/noise ratio of that spectrum, the upper limit of any fluorine-containing compound other than 2 is ca. 0.5 mol%. Significantly, there was no insoluble residue after 2 was heated at 340 °C.
These results are important because post-fabrication thermal annealing of fullerene-containing OPV devices can, in some cases, improve device efficiency and therefore have become common practice in OPV research106,107 and because thin films of PCBM or similar fullerene derivatives used for photophysical or electronic property investigations were prepared by high-temperature vacuum sublimation108–110 (see also additional references cited in ref. 105). It is possible that the thin films and other materials/devices studied in the papers just cited contained iso-PCBM as well as PCBM and possibly other PCBM thermal decomposition products. How well faux hawk fullerene 2 performs not only in OPV but in other organic electronic applications, especially those that involve thermal annealing and/or thermal evaporation at temperatures up to and including 340 °C, remains to be seen.
3. Experimental section
3.1. General methods, reagents, and solvents
An inert-atmosphere glovebox and/or standard benchtop inert-atmosphere techniques111 (dioxygen and water vapor levels ≤1 ppm) were used to perform reactions and, in general, to prepare samples for spectroscopic, electrochemical, and microwave conductivity analysis. Following filtration through silica gel, reaction mixtures were exposed to air, in most cases with minimal exposure to light. HPLC purifications were also performed in the presence of air.The following reagents and solvents were obtained from the indicated sources and were used as received or were purified/treated/stored as indicated: C60 (MTR Ltd., 99.5+%); phenyl-C61-butyric acid methyl ester (PCBM, Nano-C, 99+%); regioregular (rr) poly-3-hexylthiophene (rr-P3HT, Sigma-Aldrich, 90+% rr); heptafluorobenzyl iodide (C6F5CF2I, SynQuest, 90%); tri-n-butyltin hydride (SnH(n-Bu)3, Strem Chemicals, 95+%), hexabutylditin(Sn–Sn) (Sn2(n-Bu)6, Alfa Aesar, 98%); 1,2-dichlorobenzene (oDCB, Acros Organics, 99%, dried over and distilled from CaH2); dichloromethane (DCM, Fisher Scientific, ACS grade); benzonitrile (PhCN, Aldrich, 99+%, dried over 3 Å molecular sieves); chloroform-d (CDCl3, Cambridge Isotope Labs, 99.8%); benzene-d6 (C6D6, Cambridge Isotope Labs, dried over 3 Å molecular sieves), hexafluorobenzene (Oakwood Products); 1,4-bis(trifluoromethyl)benzene (C8H4F6, Central Glass Co., 99%); ferrocene (FeCp2, Acros Organics, 98%); cobaltocene (CoCp2, Strem Chemical, purified by sublimation and stored in the glovebox); silica gel (Sigma-Aldrich, 70−230 mesh, 60 Å); 1,8-bis(dimethylamino)naphthalene (Proton Sponge (PS), C14H18N2, Sigma-Aldrich, purified by sublimation and stored in the glovebox); toluene (Fisher Scientific, ACS grade); heptane (Mallinckrodt, ACS grade); acetonitrile (Mallinckrodt Chemicals, ACS grade); and tetra-n-butylammonium tetrafluoroborate (N(n-Bu)4BF4, TBABF4, Fluka, puriss grade, dried under vacuum at 70 °C for 24 h and stored in the glovebox).
3.2. Synthesis of compounds
Alternatively, 1 (5.0 mg) was treated with excess Proton Sponge (PS) in CH2Cl2 at 23(1) C for 24 h. The brown reaction mixture was filtered through silica gel to remove [H(PS)]+F− and unreacted PS. The filtrate was evaporated to dryness under vacuum. The solid residue was redissolved in toluene, added to the semi-preparative-scale Buckyprep HPLC column by injection (see below), and eluted with toluene at 5 mL min−1 (the HPLC trace is shown in Fig. S-20†). The fraction that eluted from 6.8 to 7.9 min was collected and evaporated to dryness under vacuum, yielding 3.9 mg of 2 (76% yield based on 1).
3.3. Physicochemical methods
| ΔG(t) = −(K(ΔP(t)/P)−1 |
| ΔGpeak = βqeI0FAφ∑μ |
Acknowledgements
The authors thank the U.S. National Science Foundation (CHE-1012468 and 1362302 to CSU and CHE-0822838 to APS), the Office of Basic Energy Sciences, U.S. Department of Energy (DE-AC02-06CH11357 to APS), and the Deutsche Forschungsgemeinschaft (Project PO1602/I-1 to IFW Dresden) for funding this research and the Research Computing Center at Moscow State University for computing time on the SKIF-Chebyshev supercomputer. The low-temperature photoelectron spectroscopy work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences & Biosciences and was performed at EMSL, a national scientific user facility sponsored by the U.S. Department of Energy's Office of Biological and Environmental Research and located at PNNL. The TRMC experiments are based upon work supported by the Solar Photochemistry Program of the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences through Grant DE-AC36-0-8GO28308 to NREL. We also thank U. Nitzsche for computational assistance, Dr Brian Newell for determining the unit cell parameters of 2 at 120 K, and the late Prof. Dr Lothar Dunsch for his unfailing friendship, guidance, and generous support.References
- O. V. Boltalina, I. V. Kuvychko, N. B. Shustova and S. H. Strauss, in Handbook of Carbon Materials, Volume 1, Syntheses and Supramolecular Systems, ed. F. Desouza and K. M. Kadish, World Scientific, Singapore, 2011, p. 101 Search PubMed.
- N. A. Romanova, M. A. Fritz, K. Chang, N. B. Tamm, A. A. Goryunkov, L. N. Sidorov, C. Chen, S. Yang, E. Kemnitz and S. I. Troyanov, Chem.–Eur. J., 2013, 17, 11707 CrossRef PubMed.
- A. A. Goryunkov, I. V. Kuvychko, I. N. Ioffe, D. L. Dick, L. N. Sidorov, S. H. Strauss and O. V. Boltalina, J. Fluorine Chem., 2003, 124, 61 CrossRef CAS.
- I. V. Kuvychko, J. B. Whitaker, B. W. Larson, T. C. Folsom, N. B. Shustova, S. M. Avdoshenko, Y. Chen, H. Wen, X. Wang, L. Dunsch, A. A. Popov, O. V. Boltalina and S. H. Strauss, Chem. Sci., 2012, 3, 1399 RSC.
- N. B. Shustova, A. A. Popov, B. S. Newell, S. M. Miller, O. P. Anderson, K. Seppelt, R. D. Bolskar, O. V. Boltalina and S. H. Strauss, Angew. Chem., Int. Ed., 2007, 46, 4111 CrossRef CAS PubMed.
- I. E. Kareev, I. V. Kuvychko, N. B. Shustova, S. F. Lebedkin, V. P. Bubnov, O. P. Anderson, A. A. Popov, S. H. Strauss and O. V. Boltalina, Angew. Chem., Int. Ed., 2008, 47, 6204 CrossRef CAS PubMed.
- I. E. Kareev, A. A. Popov, I. V. Kuvychko, N. B. Shustova, S. F. Lebedkin, V. P. Bubnov, O. P. Anderson, K. Seppelt, S. H. Strauss and O. V. Boltalina, J. Am. Chem. Soc., 2008, 130, 13471 CrossRef CAS PubMed.
- A. A. Popov, N. B. Shustova, O. V. Boltalina, S. H. Strauss and L. Dunsch, ChemPhysChem, 2008, 9, 431 CrossRef CAS PubMed.
- N. B. Shustova, I. V. Kuvychko, O. V. Boltalina and S. H. Strauss, Acta Crystallogr., 2007, E63, o4575 Search PubMed.
- A. A. Popov, I. E. Kareev, N. B. Shustova, S. F. Lebedkin, S. H. Strauss, O. V. Boltalina and L. Dunsch, Chem.–Eur. J., 2008, 14, 107 CrossRef CAS PubMed.
- A. A. Popov, I. E. Kareev, N. B. Shustova, E. B. Stukalin, S. F. Lebedkin, K. Seppelt, S. H. Strauss, O. V. Boltalina and L. Dunsch, J. Am. Chem. Soc., 2007, 129, 11551 CrossRef CAS PubMed.
- D. C. Coffey, B. W. Larson, A. W. Hains, J. B. Whitaker, N. Kopidakis, O. V. Boltalina, S. H. Strauss and G. Rumbles, J. Phys. Chem. C, 2012, 116, 8916 CAS.
- I. V. Kuvychko, B. W. Larson, S. H. Strauss and O. V. Boltalina, in Efficient Preparation of Fluorine Compounds, ed. H. Roesky, Wiley, New York, 2012, p. 19 Search PubMed.
- T. T. Clikeman, I. V. Kuvychko, N. B. Shustova, Y.-S. Chen, A. A. Popov, O. V. Boltalina and S. H. Strauss, Chem.–Eur. J., 2013, 19, 5070 CrossRef CAS PubMed.
- I. V. Kuvychko, N. B. Shustova, S. M. Avdoshenko, A. A. Popov, S. H. Strauss and O. V. Boltalina, Chem.–Eur. J., 2011, 17, 8799 CrossRef CAS PubMed.
- M. Yoshida, D. Suzuki and M. Iyoda, Chem. Lett., 1996, 1097 CrossRef CAS.
- K. M. Kadish, X. Gao, E. Van Caemelbecke, T. Suenobu and S. Fukuzumi, J. Phys. Chem. A, 2000, 104, 3878 CrossRef CAS.
- M. Yoshida, A. Morishima, Y. Morinaga and M. Iyoda, Tetrahedron Lett., 1994, 35, 9045 CrossRef CAS.
- B. Jousselme, G. Sonmez and F. Wudl, J. Mater. Chem., 2006, 16, 3478 RSC.
- W.-W. Yang, Z.-J. Li and X. Gao, J. Org. Chem., 2010, 75, 4086 CrossRef CAS PubMed.
- I. S. Neretin and Y. L. Slovokhotov, Russ. Chem. Rev., 2004, 73, 455 CrossRef CAS.
- A. Hirsch and M. Brettreich, Fullerenes - Chemistry and Reactions, Wiley-VCH, Weinheim, 2005 Search PubMed.
- D. A. Dixon, N. Matsuzawa, T. Fukunaga and F. N. Tebbe, J. Phys. Chem., 1992, 96, 6107 CrossRef CAS.
- M. Prato, T. Suzuki, H. Foroudian, Q. Li, K. Khemani, F. Wudl, J. Leonetti, R. D. Little, T. White, B. Rickborn, S. Yamago and E. Nakamura, J. Am. Chem. Soc., 1993, 115, 1594 CrossRef CAS.
- E. Nakamura and S. Yamago, Acc. Chem. Res., 2002, 35, 867 CrossRef CAS PubMed.
- J. W. Emsley, L. Phillips and V. Wray, Prog. Nucl. Magn. Reson. Spectrosc., 1976, 10, 83 CrossRef.
- K. Bynum, R. Prip, R. Callahan and R. Rothchild, J. Fluorine Chem., 1998, 90, 39 CrossRef CAS.
- M. D. Castle, E. F. Mooney and R. G. Plevey, Tetrahedron, 1968, 24, 5457 CrossRef CAS.
- R. S. Matthews and W. E. Preston, Org. Magn. Reson., 1980, 14, 258 CrossRef CAS.
- F. E. Herkes, J. Fluorine Chem., 1978, 12, 1 CrossRef.
- L. Petrakis and C. H. Sederholm, J. Chem. Phys., 1961, 35, 1243 CrossRef CAS.
- K. Hirao, H. Nakatsuji and H. Kato, J. Am. Chem. Soc., 1972, 95, 31 CrossRef.
- F. B. Mallory, J. Am. Chem. Soc., 1973, 95, 7747 CrossRef CAS.
- J. E. Peralta, R. H. Contreras and J. P. Snyder, Chem. Commun., 2000, 2025 RSC.
- W. D. Arnold, J. Mao, H. Sun and E. Oldfield, J. Am. Chem. Soc., 2000, 122, 12164 CrossRef CAS.
- T. Tuttle, J. Grafenstein and D. Cremer, Chem. Phys. Lett., 2004, 394, 5 CrossRef CAS.
- L. Krcsmar, J. Grunenberg, I. Dix, P. G. Jones, K. Ibrom and L. Ernst, Eur. J. Org. Chem., 2005, 5306 CrossRef CAS.
- I. E. Kareev, I. V. Kuvychko, S. F. Lebedkin, S. M. Miller, O. P. Anderson, K. Seppelt, S. H. Strauss and O. V. Boltalina, J. Am. Chem. Soc., 2005, 127, 8362 CrossRef CAS PubMed.
- I. E. Kareev, G. Santiso-Quinones, I. V. Kuvychko, I. N. Ioffe, I. V. Goldt, S. F. Lebedkin, K. Seppelt, S. H. Strauss and O. V. Boltalina, J. Am. Chem. Soc., 2005, 127, 11497 CrossRef CAS PubMed.
- N. B. Shustova, I. V. Kuvychko, R. D. Bolskar, K. Seppelt, S. H. Strauss, A. A. Popov and O. V. Boltalina, J. Am. Chem. Soc., 2006, 128, 15793 CrossRef CAS PubMed.
- X. B. Wang and L. S. Wang, Rev. Sci. Instrum., 2008, 79, 073108 CrossRef PubMed.
- B. W. Larson, J. B. Whitaker, X.-B. Wang, A. A. Popov, G. Rumbles, N. Kopidakis, S. H. Strauss and O. V. Boltalina, J. Phys. Chem. C, 2013, 117, 14958 CAS.
- R. Hettich, C. M. Jin and R. Compton, Int. J. Mass Spectrom. Ion Processes, 1994, 138, 263 CrossRef CAS.
- X.-B. Wang, C. Chi, M. Zhou, I. V. Kuvychko, K. Seppelt, A. A. Popov, S. H. Strauss, O. V. Boltalina and L.-S. Wang, J. Phys. Chem. A, 2010, 114, 1756 CrossRef CAS PubMed.
- T. F. Guarr, M. S. Meier, V. K. Vance and M. Clayton, J. Am. Chem. Soc., 1993, 115, 9862 CrossRef CAS.
- P. Boulas, F. D'Souza, C. C. Henderson, P. A. Cahill, M. T. Jones and K. Kadish, J. Phys. Chem., 1993, 97, 13435 CrossRef CAS.
- C. Caron, R. Subramanian, F. D'Souza, J. Kim, W. Kutner, M. T. Jones and K. Kadish, J. Am. Chem. Soc., 1993, 115, 8505 CrossRef CAS.
- I. V. Kuvychko, Ph.D. dissertation, Colorado State Univ., 2012.
- W.-W. Yang, Z.-J. Li and X. Gao, J. Org. Chem., 2011, 76, 6067 CrossRef CAS PubMed.
- L. Ni, W. Chang, H.-L. Hou, Z.-J. Li and X. Gao, Org. Biomol. Chem., 2011, 9, 6646 CAS.
- S. G. Frankiss, J. Phys. Chem., 1967, 71, 3418 CrossRef CAS.
- T. Q. Nguyen, F. Qu, X. Huang and A. F. Janzen, Can. J. Chem., 1992, 70, 2089 CrossRef.
- W. Makulski, J. Mol. Struct., 2013, 1036, 168 CrossRef CAS.
- K. George, A. L. Hector, W. Levason, G. Reid, G. Sanderson, M. Webster and W. Zhang, Dalton Trans., 2011, 40, 1584 RSC.
- W. Levason, D. Pugh and G. Reid, Inorg. Chem., 2013, 52, 5185 CrossRef CAS PubMed.
- V. O. Gelmboldt, E. V. Ganin, M. M. Botoshansky, V. Y. Anisimov, O. V. Prodan, V. C. Kravtsov and M. S. Fonari, J. Fluorine Chem., 2014, 160, 57 CrossRef CAS.
- R. D. Chambers, S. R. Korn and G. Sandford, J. Fluorine Chem., 1994, 69, 103 CrossRef CAS.
- M. Keshavzarz-K, B. Knight, G. Srdanov and F. Wudl, J. Am. Chem. Soc., 1995, 117, 11371 CrossRef.
- E. Champeil, C. Crean, C. Larraya, G. Pescitelli, G. Proni and L. Ghosez, Tetrahedron, 2008, 64, 10319 CrossRef CAS.
- P. J. Fagan, P. J. Krusic, D. H. Evans, S. A. Lerke and E. Johnston, J. Am. Chem. Soc., 1992, 114, 9697 CrossRef CAS.
- R. A. Bunce, E. J. Lee and M. T. Grant, J. Heterocycl. Chem., 2011, 48, 620 CrossRef CAS.
- R. A. Bunce, T. Nego, N. Sonobe and L. M. Slaughter, J. Heterocycl. Chem., 2009, 45, 551 CrossRef.
- N. S. Goulioukina, A. Y. Mitrofanov and I. P. Beletskaya, J. Fluorine Chem., 2012, 136, 26 CrossRef CAS.
- Y. Xiong, J. Wu, S. Xiao and S. Cao, Chin. J. Chem., 2012, 30, 2747 CrossRef CAS.
- A. Pažitný, T. Solčán and D. Végh, J. Fluorine Chem., 2009, 130, 267 CrossRef.
- R. P. Houghton and M. Voyle, J. Organomet. Chem., 1983, 259, 183 CrossRef CAS.
- R. P. Hughes and D. C. Lindner, Organometallics, 1996, 15, 5678 CrossRef CAS.
- R. P. Hughes, D. C. Lindner, L. M. Liable-Sands and A. L. Rheingold, Organometallics, 2001, 20, 363 CrossRef CAS.
- J. Meisenheimer, Justus Liebigs Ann. Chem., 1902, 323, 205 CrossRef CAS.
- L. Forlani, C. Boga, A. Mazzanti and N. Zanna, Eur. J. Org. Chem., 2012, 1123 CrossRef CAS.
- R. A. Manderville, J. M. Dust and E. Buncel, J. Phys. Org. Chem., 1996, 9, 515 CrossRef CAS.
- J. Miller, Aromatic Nucleophilic Substitution, Elsevier, Amsterdam, 1968 Search PubMed.
- L. C. Chan, B. G. Cox, I. C. Jones and S. Tomasi, J. Phys. Org. Chem., 2011, 24, 751 CAS.
- N. Chéron, L. El Kaïm, L. Gramaud and P. Fleurat-Lessard, Chem.–Eur. J., 2011, 17, 14929 CrossRef PubMed.
- A. Nova, R. Mas-Ballesté and A. Lledós, Organometallics, 2012, 31, 1245 CrossRef CAS.
- S. Park and S. Lee, Bull. Korean Chem. Soc., 2010, 31, 2571 CrossRef CAS.
- Z. Wu and R. Glaser, J. Am. Chem. Soc., 2004, 126, 10632 CrossRef CAS PubMed.
- A. H. Renfrew, J. A. Taylor, J. M. J. Whitmore and A. Williams, J. Chem. Soc., Perkin Trans. 2, 1993, 1703 RSC.
- M. N. Glukhovtsev, R. D. Bach and S. J. Laiter, J. Org. Chem., 1997, 62, 4036 CrossRef CAS.
- I. Fernández, G. Frenking and E. Uggerud, J. Org. Chem., 2010, 75, 2971 CrossRef PubMed.
- A. Hirsch, in Fullerenes and Related Structures, ed. A. Hirsch, Springer, Berlin, 1999, p. 1 Search PubMed.
- T. Kitagawa and K. Takeuchi, Bull. Chem. Soc. Jpn., 2001, 74, 785 CrossRef CAS.
- P. de la Cruz, A. de la Hoz, F. Langa, N. Martín, M. C. Pérez and L. Sánchez, Eur. J. Org. Chem., 1999, 3433 CrossRef CAS.
- R. C. Haddon, Science, 1993, 261, 1545 CAS.
- K. Choho, G. Van Lier, G. Van De Woude and P. Geerlings, J. Chem. Soc., Perkin Trans. 2, 1996, 1723 RSC.
- G. Van Lier, B. Safi and P. Geerlings, J. Chem. Soc., Perkin Trans. 2, 1998, 349 RSC.
- M. C. Amat, G. Van Lier, M. Solà, M. Duran and P. Geerlings, J. Org. Chem., 2004, 69, 2374 CrossRef CAS PubMed.
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1 RSC.
- G. Paternò, A. J. Warren, J. Spencer, G. Evans, V. García Sakai, J. Blumberger and F. Cacialli, J. Mater. Chem., 2013, 1, 5619 Search PubMed.
- M. Casalegno, S. Zanardi, F. Frigerio, R. Po, C. Carbonera, G. Marra, T. Nicolini, G. Raos and S. V. Meille, Chem. Commun., 2013, 49, 4525 RSC.
- R. C. I. MacKenzie, J. M. Frost and J. Nelson, J. Chem. Phys., 2010, 132, 064904 CrossRef PubMed.
- D. L. Cheung and A. Troisi, J. Phys. Chem. C, 2010, 114, 20479 CAS.
- J. H. Choi, T. Honda, S. Seki and S. Fukuzumi, Chem. Commun., 2011, 47, 11213 RSC.
- H. Oberhofer and J. Blumberger, Phys. Chem. Chem. Phys., 2012, 14, 13846 RSC.
- A. M. Nardes, A. J. Ferguson, J. B. Whitaker, B. W. Larson, R. E. Larsen, K. Maturová, P. A. Graf, O. V. Boltalina, S. H. Strauss and N. Kopidakis, Adv. Funct. Mater., 2012, 22, 4115 CrossRef CAS.
- F. Gajdos, H. Oberhofer, M. Dupuis and J. Blumberger, J. Phys. Chem. Lett., 2013, 4, 1012 CrossRef CAS PubMed.
- M. T. Rispens, A. Meetsma, R. Rittberger, C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Chem. Commun., 2003, 2116 RSC.
- N. R. Tummala, S. Mehraeen, Y.-T. Fu, C. Risko and J.-L. Brédas, Adv. Funct. Mater., 2013, 23, 5800 CrossRef CAS.
- C. J. Tassone, A. L. Ayzner, R. D. Kennedy, M. Halim, M. So, Y. Rubin, S. H. Tolbert and B. J. Schwartz, J. Phys. Chem. C, 2011, 115, 22564 Search PubMed.
- R. D. Kennedy, M. Halim, S. I. Khan, B. J. Schwartz, S. H. Tolbert and Y. Rubin, Chem.–Eur. J., 2012, 18, 7418 CrossRef CAS PubMed.
- T. J. Savenije, A. J. Ferguson, N. Kopidakis and G. Rumbles, J. Phys. Chem. C, 2013, 117, 24085 CAS.
- A. J. Ferguson, N. Kopidakis, S. E. Shaheen and G. Rumbles, J. Phys. Chem. C, 2011, 115, 23134 CAS.
- W. J. Grzegorczyk, T. J. Savenije, T. E. Dykstra, J. Piris, J. M. Schins and L. D. A. Siebbeles, J. Phys. Chem. C, 2010, 114, 5182 CAS.
- P.-L. T. Boudreault, A. Najari and M. Leclerc, Chem. Mater., 2011, 23, 456 CrossRef CAS.
- B. W. Larson, J. B. Whitaker, A. A. Popov, N. Kopidakis, G. Rumbles, O. V. Boltalina and S. H. Strauss, Chem. Mater., 2014, 26, 2361 CrossRef CAS.
- F. Padinger, R. S. Rittberger and N. S. Sariciftci, Adv. Funct. Mater., 2003, 13, 85 CrossRef CAS.
- E. Verploegen, R. Mondal, C. J. Bettinger, S. Sok, M. F. Toney and Z. Bao, Adv. Funct. Mater., 2010, 20, 3519 CrossRef CAS.
- W.-H. Tseng, J.-Y. Wang, M.-H. Chen, C.-Y. Wang, H. Lo and C.-I. Wu, J. Photonics Energy, 2012, 2, 021009 CrossRef.
- V. S. Reddy, S. Karak, S. K. Ray and A. Dhar, J. Phys. D: Appl. Phys., 2009, 42, 145103 CrossRef.
- A. Kumar, G. Li, Z. Hong and Y. Yang, Nanotechnology, 2009, 20, 165202 CrossRef PubMed.
- D. F. Shriver and M. A. Drezdzon, The Manipulation of Air-Sensitive Compounds, Wiley-Interscience, New York, 2nd edn, 1986 Search PubMed.
- G. M. Sheldrick, TWINABS: Bruker AXS Scaling for Twinned Crystals (v. 2012/1), Bruker AXS, Madison, WI, 2003 Search PubMed.
- G. M. Sheldrick, Crystallography Program APEX2 (v. 5-0), Bruker AXS, Madison, WI, 2014 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., 2008, A64, 112 CrossRef PubMed.
- G. M. Sheldrick, Crystallography Software Package SHELXTL (v. 6.14 UNIX), Bruker AXS, Madison, WI, 2001 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339 CrossRef CAS.
- D. N. Laikov, Chem. Phys. Lett., 1997, 281, 151 CrossRef CAS.
- D. N. Laikov and Y. A. Ustynyuk, Russ. Chem. Bull., 2005, 54, 820 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- N. C. Handy and A. J. Cohen, Mol. Phys., 2001, 99, 403 CrossRef CAS.
- A. A. Granovsky, Firefly (v. 8.0.0), 2013 Search PubMed.
- M. Swart, M. Solà and F. M. Bickelhaupt, J. Comput. Chem., 2007, 28, 1551 CrossRef CAS PubMed.
- A. P. Bento, M. Solà and F. M. Bickelhaupt, J. Chem. Theory Comput., 2008, 4, 929 CrossRef CAS.
- M. Swart, M. Sola and F. M. Bickelhaupt, J. Chem. Phys., 2009, 131, 094103 CrossRef PubMed.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures and tables described in the text. CSD-428507. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4sc02970d |
| This journal is © The Royal Society of Chemistry 2015 |