A rapid, flexible method for incorporating controlled antibiotic release into porous polymethylmethacrylate space maintainers for craniofacial reconstruction
P. M.
Mountziaris
ab,
S. R.
Shah
a,
J.
Lam
a,
G. N.
Bennett
c and
A. G.
Mikos
*a
aDepartment of Bioengineering, Rice University, Houston, Texas, USA. E-mail: mountzp@mail.amc.edu; srs8@rice.edu; johnny.lam@rice.edu; mikos@rice.edu; Fax: +1-713-348-4244; Tel: +1-713-348-4204
bDivision of Plastic Surgery, Albany Medical Center, Albany, NY, USA
cDepartment of BioSciences, Rice University, Houston, Texas, USA. E-mail: gbennett@rice.edu
First published on 4th September 2015
Abstract
Severe injuries in the craniofacial complex, resulting from trauma or pathology, present several challenges to functional and aesthetic reconstruction. The anatomy and position of the craniofacial region make it vulnerable to injury and subsequent local infection due to external bacteria as well as those from neighbouring structures like the sinuses, nasal passages, and mouth. Porous polymethylmethacrylate (PMMA) “space maintainers” have proven useful in staged craniofacial reconstruction by promoting healing of overlying soft tissue prior to reconstruction of craniofacial bones. We describe herein a method by which the porosity of a prefabricated porous PMMA space maintainer, generated by porogen leaching, can be loaded with a thermogelling copolymer-based drug delivery system. Porogen leaching, space maintainer prewetting, and thermogel loading all significantly affected the loading of a model antibiotic, colistin. Weeks-long release of antibiotic at clinically relevant levels was achieved with several formulations. In vitro assays confirmed that the released colistin maintained its antibiotic activity against several bacterial targets. Our results suggest that this method is a valuable tool in the development of novel therapeutic approaches for the treatment of severe complex, infected craniofacial injuries.
Introduction
The craniofacial region is particularly vulnerable to injury and subsequent wound infection due to its anatomy and position. Substantial bone and soft tissue loss can result from severe trauma or tumor resection. Reconstruction of these defects often requires a multidisciplinary approach with a series of staged procedures.1–3 Emerging craniofacial tissue engineering strategies that combine biomaterials, autologous cells, and/or signaling factors offer a promising alternative to the prosthetics and other surgical reconstructive approaches currently in use.4,5 However, wound bed infection due to contamination at the time of initial trauma, repeated surgical interventions, and/or bacterial overgrowth in devascularized and devitalized damaged tissues, remains a major barrier to effective reconstruction and implementation of tissue engineering approaches. Infection is of particular concern in craniofacial reconstruction, where tissue defects are often exposed to bacteria not only from the external environment, but also from neighboring structures including the sinuses, nasal passages, and the mouth.6,7 For instance, wound infection rates approaching 100% have been reported following gunshot injuries to the face.8,9Several groups, including ours, have previously reported on the optimization of polymethylmethacrylate (PMMA) temporary implants, or “space maintainers,” for complex craniofacial reconstruction.10–19 Space maintainers ideally serve a dual purpose, enabling healing of the soft tissue envelope overlying the bony injury, while preventing wound contracture in order to preserve the hard tissue defect site. Soft tissue healing and maintenance of defect geometry facilitate later reconstruction and may allow time for the expansion of autologous cells to enable generation of a custom-designed tissue engineered construct.10,19 When compared to solid polymeric space maintainers, porous implants have shown superior outcomes in terms of healing of the overlying soft tissue cuff.9,10,18 However, the use of porous implants presents a challenge because the pores can harbor bacteria, resulting in a higher available surface area for biofilm formation and subsequent wound infection.15,20,21
In recent years, the search for improved craniofacial reconstructive strategies to manage local infection and promote tissue regeneration has been further motivated by combat operations, where the prevalent use of improvised explosive devices has resulted in a high frequency of severe craniofacial injuries.22–25 Unfortunately, many soldiers have returned with combat wound infections and even osteomyelitis, often with multi-drug resistant Acinetobacter baumannii species.26,27 One of the last-resort antibiotics for these infections, colistin, is limited by its poor penetration into bone, requiring prolonged therapy that carries a significant risk of kidney and nerve damage due to colistin's known nephro- and neurotoxic side effects.12,28 Placement of a porous space maintainer into such a contaminated wound would further complicate therapy by providing an additional barrier to diffusion.
To address this challenge, several strategies for the fabrication of antibiotic-loaded porous space maintainers have been described to enable local delivery of various antibiotics, including colistin.12,13,15,29 Our group has described several techniques for incorporating drug delivery systems into porous PMMA space maintainers to enable precise spatial and temporal control of antibiotic release.12,13,15 However, translation of these designs into clinical products remains quite challenging due to their numerous components and overall complexity. With that in mind, the goal of this study was to develop an antibiotic delivery system based on porous space maintainers that can be assembled at the point of care (e.g., within the operating room) and deliver antibiotics at meaningful concentrations, i.e., exceeding the minimum inhibitory concentration (MIC) for common pathogens, for a period of a week or more, as would be required in the treatment of infected craniofacial bone defects while awaiting soft tissue healing.
Previous design of antibiotic-releasing space maintainers utilized antibiotic-loaded biodegradable microspheres incorporated directly into the solid phase of the porous space maintainer during fabrication.12,13,15 The current design, intended for point of care loading of antibiotics, is based on previously described non-drug-loaded porous PMMA-based space maintainers, in which 30 wt% of a 9% carboxymethylcellulose (CMC) hydrogel was used as a porogen within the bulk material.10 This formulation was shown to optimize in vivo closure of intraoral soft tissue defects while inducing a favorable tissue response with minimal inflammatory reaction at the implant-tissue interface.10
The goal of this study is to develop a simple and convenient method to load prefabricated porous space maintainers with a variety of antibiotics. A thermogelling copolymer was selected as the antibiotic carrier with the intention that it could penetrate the pores of the space maintainer in its liquid state and subsequently undergo a transition at body temperature to form a gel that serves as a depot for drug delivery. The thermogelling copolymer formulations selected consists of poly(DL-lactic-co-glycolic acid) (PLGA) and poly(ethylene glycol) (PEG), since this type of PLGA-PEG-PLGA block copolymer is also currently being studied for controlled release of chemotherapeutics.30,31
Although drug release from both unmodified thermogels32–34 as well as from solid PMMA35 typically occurs on the scale of hours to days, clinically relevant weeks-long release from thermogel-loaded porous PMMA space maintainers could be achieved by optimizing parameters such as the lactic acid to glycolic acid ratio (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G) of the PLGA block, thermogel loading method, and prewetting of the PMMA space maintainer. Colistin was selected as a model antibiotic due to its relevance in the treatment of severe complex, infected craniofacial injuries. The objective of this work was to develop a method for incorporating an antibiotic drug delivery system into prefabricated porous space maintainers to provide antibiotic release over the course of several weeks, as might be necessary to treat infected craniofacial bone defects while awaiting soft tissue envelope healing; we additionally aimed to deliver the antibiotic at meaningful concentrations, which we defined as a concentration exceeding the minimum inhibitory concentration of bacteria commonly infecting these wounds, including Acinetobacter baumannii. We hypothesize that in vitro colistin release can be modulated by varying scaffold prewetting, porogen leaching, thermogel L
G) of the PLGA block, thermogel loading method, and prewetting of the PMMA space maintainer. Colistin was selected as a model antibiotic due to its relevance in the treatment of severe complex, infected craniofacial injuries. The objective of this work was to develop a method for incorporating an antibiotic drug delivery system into prefabricated porous space maintainers to provide antibiotic release over the course of several weeks, as might be necessary to treat infected craniofacial bone defects while awaiting soft tissue envelope healing; we additionally aimed to deliver the antibiotic at meaningful concentrations, which we defined as a concentration exceeding the minimum inhibitory concentration of bacteria commonly infecting these wounds, including Acinetobacter baumannii. We hypothesize that in vitro colistin release can be modulated by varying scaffold prewetting, porogen leaching, thermogel L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio, as well as the thermogel loading method, without disrupting colistin's in vitro anti-bacterial activity.
G ratio, as well as the thermogel loading method, without disrupting colistin's in vitro anti-bacterial activity.
Materials and methods
Experimental design
The study groups are summarized in Table 1. All 12 groups consisted of porous PMMA space maintainers fabricated according to established methods9 using a 9% w/w carboxymethylcellulose (CMC) hydrogel as a porogen, which was mixed at 30 w/w% with the contents of a clinical-grade kit for methylmethacrylate (MMA) polymerization, as detailed below. After fabrication and curing, CMC was leached from the space maintainer pores, and various methods were subsequently used to fill the pores with colistin. All methods utilized 5% w/v aqueous solutions of colistin, some of which also contained thermogelling polymers dissolved at 25% w/v. A factorial design was used to evaluate the effect of 2 porous space maintainer treatments (“prewet” vs. “no prewet”), thermogelling polymer type (“L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1
G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1”, “L
1”, “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3
G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1”, and “No gel”), and thermogel loading method (“pregel” vs. “dip”). Two additional control groups were included consisting of “prewet” and “no prewet” scaffolds which did not undergo leaching of CMC prior to antibiotic loading (“No gel, +CMC”). A schematic of the “pregel” and “dip” loading methods is depicted in Fig. 1.
1”, and “No gel”), and thermogel loading method (“pregel” vs. “dip”). Two additional control groups were included consisting of “prewet” and “no prewet” scaffolds which did not undergo leaching of CMC prior to antibiotic loading (“No gel, +CMC”). A schematic of the “pregel” and “dip” loading methods is depicted in Fig. 1.
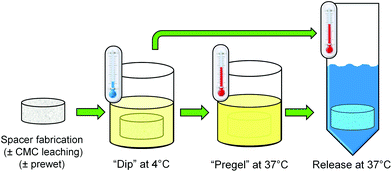 | ||
| Fig. 1 A schematic depiction of the “pregel” and “dip” methods used to load prefabricated PMMA space maintainers with the various thermogel formulations described in Table 1. | ||
| Group name | CMC leached | Thermogelling polymer present | Prewet | Dip (−) vs. Pregel (+) |
|---|---|---|---|---|
| No gel | + | − | − | − |
| + | − | + | − | |
| No gel, +CMC | − | − | − | − |
| − | − | + | − | |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
+ | + | − | − |
| + | + | + | ||
| + | + | + | − | |
| + | + | + | ||
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
+ | + | − | − |
| + | + | + | ||
| + | + | + | − | |
| + | + | + |
Preparation of porous PMMA space maintainers
Porous PMMA space maintainers were fabricated according to established methods10 using clinical-grade and United States Pharmocopeia (USP)-grade materials. A CMC hydrogel was prepared by dissolving 9% w/w low viscosity CMC (Type 7LFPH, Ashland Inc., Covington, KY) in ultrapure (Type I) water (Millipore Super-Q, Billerica, MA). A single hydrogel batch was used for all experiments described herein. This hydrogel served as a porogen to generate porous PMMA constructs using a clinical-grade bone cement kit (SmartSet High Viscosity, Depuy Orthopaedics, Warsaw IN), containing a powder phase of MMA/methyl acrylate copolymer, benzoyl peroxide, and zirconium dioxide, and a liquid phase consisting of MMA, N-N-dimethyl-p-toluidine, and hydroquinone. To fabricate porous space maintainers containing 30% w/w CMC hydrogel in PMMA, CMC hydrogel was thoroughly blended with the powder phase, the liquid phase was added, and thoroughly mixed for 90 s to achieve a dough-like consistency. This mixture was packed into custom-fabricated cylindrical Teflon® (Dupont, Wilmington, DE) molds, 10 mm diameter by 6 mm height, and allowed to harden at room temperature for 30 min.The resulting specimens were randomly assigned to each of the study groups. For all constructs except those in +CMC groups, each space maintainer was placed in a 200-fold volumetric excess of ultrapure water and gently agitated to leach out the CMC. Ultrapure water was aspirated and replaced at 12 h intervals. After 48 h, all constructs were vacuum-dried for 24 h. The dry weight of each space maintainer was determined immediately following fabrication as well as after vacuum drying to determine the wt% of CMC and/or water removed. One day prior to beginning antibiotic release, specimens assigned to “prewet” groups were placed in a 200-fold volumetric excess of 50% v/v ethanol in ultrapure water, and incubated for 12 h at room temperature with gentle agitation, followed by 2 h in sterile phosphate-buffered saline (PBS, pH 7.4).
Thermogelling polymer preparation
Triblock copolymers of PLGA-PEG-PLGA capable of thermogelling and suitable for in vivo use were obtained in two different formulations (AK12 and AK24, Akina Polymers, West Lafayette, IN), which differed in terms of the lactic- to glycolic-acid (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G) ratios of the PLGA blocks. According to data provided by the manufacturer, the L
G) ratios of the PLGA blocks. According to data provided by the manufacturer, the L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratios of the specific copolymer lots used herein were 16
G ratios of the specific copolymer lots used herein were 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 and 19
21 and 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, respectively, and are approximated as 1
7, respectively, and are approximated as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 throughout the text. These thermogelling polymers were dissolved at 25% w/v in sterile PBS (pH 7.4) with vigorous agitation at 4 °C for 72 h according to the manufacturer's instructions and used immediately for antibiotic-loading of space maintainers.
1 throughout the text. These thermogelling polymers were dissolved at 25% w/v in sterile PBS (pH 7.4) with vigorous agitation at 4 °C for 72 h according to the manufacturer's instructions and used immediately for antibiotic-loading of space maintainers.
Antibiotic loading and release
Colistin sulfate salt (C4461, Sigma-Aldrich, St. Louis, MO) was dissolved at 5% w/v in sterile PBS, as well as at 5% w/v in each of the two thermogelling polymer solutions. Space maintainers assigned to thermogel-free groups were each submerged in colistin/PBS solution at room temperature with gentle agitation for 10 min. Space maintainers assigned to thermogel groups were submerged in the appropriate colistin/thermogel solution with vigorous agitation at 4 °C. After 10 min, those assigned to “pregel” groups were transferred to a 37 °C incubator for 10 min (remaining in the thermogel/colistin solution), while those in “dip” groups were immediately removed from the solution. Following incubation in the respective antibiotic solutions, the weight of each space maintainer was obtained, and then 5 ml of prewarmed, sterile PBS were then added to each space maintainer. Samples were incubated at 37 °C with mild agitation. At predetermined time points (12 h and days 1, 2, 3, 4, 5, 7, 10, 14, 21, 28), the entire supernatant was removed and replaced with fresh sterile PBS.Colistin concentration in the release media was determined via high-performance liquid chromatography (HPLC), according to established methods.12,13 Samples were passed through 0.2 μm filters and then analyzed using a previously described HPLC system consisting of an XTerra® RP 18 column (250 cm × 4.6 μm, Waters, Milford, MA) at 45 °C mounted within a Waters 2695 separation module and attached to a 2996 photodiode array detector (Waters). The mobile phase had a flow rate of 0.5 ml min−1 and consisted of HPLC-grade acetonitrile with 0.1% v/v trifluoroacetic acid (Sigma) and ultrapure water with 0.1% v/v trifluoroacetic acid, with a linear gradient of 10–65% v/v acetonitrile in water over 20 min. Absorbance was monitored at 214 nm. Standard solutions of colistin ranging from 5–1000 μg ml−1 in sterile PBS (pH 7.4) were used to generate calibration curves correlating colistin concentration to the combined peak areas of colistin A and colistin B, which were eluted at 13.2 min and 13.9 min, respectively. Daily release was approximated by dividing the absolute amount of colistin in the release media at a particular timepoint by the number of 24 h periods that had elapsed since the previous time point. Cumulative release represents a percentage of the total amount of colistin released over time.
Bacterial susceptibility
The antibiotic activity of colistin in the release media was analyzed using Acinetobacter baumannii, as previously described,15 as well as a second bacterial species, Escherichia coli (ATCC 25922, American Type Culture Collection, Manassas, VA), according to International Standard ISO 20776-1. A. baumannii (isolate #170) was provided by Brooke Army Medical Center (San Antonio, TX) and originated from a culture specimen of a deep wound of a soldier injured in Operation Iraqi Freedom. The 12 h time point was selected as colistin concentrations were sufficiently high for all groups to generate the entire range of required working solutions outlined in ISO 20776-1. For each sample, an aliquot of the release media was sterile-filtered and serially diluted using sterile ultrapure water followed by additional dilution with Mueller Hinton broth (Sigma) to generate 50 μl aliquots with concentrations ranging from 0–32 μg ml−1. Two identical sets of sterile microwell plates were prepared, one for each bacterial strain. Each plate contained experimental samples as well as standard dilutions of fresh colistin. For one set of plates, a standard 0.5 McFarland suspension of A. baumannii cultured in Mueller Hinton broth was diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 with broth and 50 μl were added to each well. The same procedure was repeated for the second set of plates using E. coli. Experiments were performed in triplicate. The lowest concentration well without growth after 18 h at 37 °C was denoted the minimum inhibitory concentration (MIC).
100 with broth and 50 μl were added to each well. The same procedure was repeated for the second set of plates using E. coli. Experiments were performed in triplicate. The lowest concentration well without growth after 18 h at 37 °C was denoted the minimum inhibitory concentration (MIC).
Statistics
All values are expressed as mean ± standard deviation for n = 4 specimens per group, except for the values in Table 2 which represent mean ± standard deviation for n = 10 per group and the data points in Fig. 5, each of which represents the mean ± standard deviation for n = 8 specimens per variable. Space maintainer weights, burst release values, and bacterial susceptibility results were each analyzed using analysis of variance (p < 0.05), followed by Bonferroni post hoc analysis (p < 0.05) for multiple comparisons. Theoretical and actual colistin loading values for the various space maintainer groups were compared using two-way analysis of variance (p < 0.05), followed by Bonferroni post hoc analysis (p < 0.05) for multiple comparisons. Release data were analyzed using a repeated measures analysis of variance (p < 0.05) followed by Bonferroni post hoc analysis (p < 0.05). The effect of two independent factors (prewetting and CMC leaching, or prewetting and thermogel loading method) on total colistin loading was evaluated using two-way analysis of variance (p < 0.05).| Batch | Initial weight (mg) | Post-leach dry weight (mg) | Post-leach dry weight (% initial weight) |
|---|---|---|---|
| No significant differences amongst any values within each column (p > 0.05). | |||
| 1 | 560 ± 20 | 440 ± 19 | 78 ± 3 |
| 2 | 560 ± 15 | 420 ± 23 | 75 ± 4 |
| 3 | 570 ± 18 | 410 ± 13 | 72 ± 3 |
| 4 | 570 ± 12 | 430 ± 24 | 75 ± 4 |
| 5 | 580 ± 11 | 430 ± 10 | 74 ± 2 |
Results
PMMA space maintainer characteristics
PMMA space maintainers for this study were prepared in five batches and then randomly assigned to the groups depicted in Table 1. When space maintainers from each batch were compared, there were no differences in average weight (p > 0.05) either before or after CMC leaching, as shown in Table 2. Similarly, there were no differences amongst the twelve experimental groups in average space maintainer weight (p > 0.05) either before or after CMC leaching (Table 3). Within each group, initial and dry weights differed significantly (p < 0.05).| Group | Prewet | Initial weight (mg) | Post-leach dry weight (% initial weight) |
|---|---|---|---|
| No significant differences amongst any values within each column (p > 0.05). | |||
| No gel | − | 570 ± 11 | 76 ± 4 |
| No gel, +CMC | − | 580 ± 7 | 76 ± 2 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel 1, pregel |
− | 560 ± 18 | 74 ± 2 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip 1, dip |
− | 560 ± 9 | 73 ± 4 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel 1, pregel |
− | 570 ± 27 | 75 ± 5 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip 1, dip |
− | 570 ± 19 | 75 ± 5 |
| No gel | + | 560 ± 13 | 75 ± 5 |
| No gel, +CMC | + | 590 ± 14 | 75 ± 5 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel 1, pregel |
+ | 570 ± 7 | 74 ± 2 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip 1, dip |
+ | 560 ± 8 | 75 ± 5 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel 1, pregel |
+ | 580 ± 13 | 74 ± 2 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip 1, dip |
+ | 560 ± 9 | 74 ± 2 |
Antibiotic loading
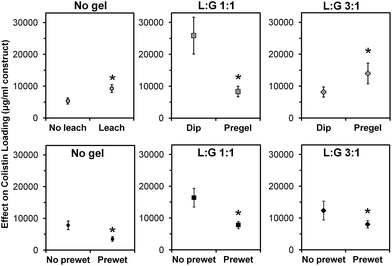
On Day 0 of the release study, the applicable space maintainer groups were prewet and then all groups were loaded with colistin according to the experimental design (Table 1, Fig. 1). Following loading, the average weight for each group increased significantly (p < 0.05) compared to the dry weight shown in Table 3, except for “No gel, +CMC (+prewet),” which still showed an increasing trend (p > 0.05) from a dry weight of 75 ± 5% to 81 ± 2% initial space maintainer weight on Day 0. Fig. 2 summarizes the weights for all groups on Day 0 of the release study as a percentage of the initial weight of each space maintainer. For all pairs of treatment groups except “No gel, +CMC,” the +prewet version weighed significantly more than the non-prewet group (p < 0.05). “No gel, +CMC” (+prewet and no prewet) had the lowest weight on Day 0, while “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3
G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel” had the highest, with the +prewet group significantly differing from all others (p < 0.05).
1, pregel” had the highest, with the +prewet group significantly differing from all others (p < 0.05).
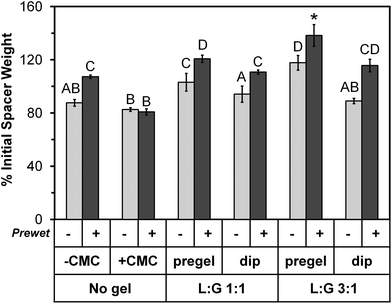 | ||
Fig. 2 Day 0 space maintainer weight after colistin loading and immediately prior to placement in release medium, expressed as percentage of initial space maintainer weight shown in Table 3. The 12 groups are identified along the bottom of the image using the same notations depicted in the study design (Table 1). Groups marked with the same letter (A–D) did not significantly differ from each other (p > 0.05), but differ from all other groups (p < 0.05). The “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel (+prewet)” group is marked with a “*” to indicate that it significantly differs from all other groups (p < 0.05). Each column represents the mean ± standard deviation for n = 4 space maintainers per group. Each space maintainer's weight was expressed as a percentage of its corresponding initial weight prior to calculation of the mean. 1, pregel (+prewet)” group is marked with a “*” to indicate that it significantly differs from all other groups (p < 0.05). Each column represents the mean ± standard deviation for n = 4 space maintainers per group. Each space maintainer's weight was expressed as a percentage of its corresponding initial weight prior to calculation of the mean. | ||
In addition to the weight increase due to prewetting, for each thermogel type, “pregel” groups weighed more than corresponding (+prewet or no prewet) “dip” groups (p < 0.05). However, this did not correlate with increased total colistin loading, as shown in Fig. 3. The total amount of colistin released from each space maintainer, measured over time via HPLC until the release reached a consistent value (“Actual colistin”) is compared to “Theoretical colistin” values derived from the weight gain of each dried space maintainer (Day 0 – dry), taking into account the weight percent of colistin in each loading solution as well as the weight of the thermogel, if applicable. For all +prewet groups except “No gel, +CMC (+prewet)” and “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1
G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip (+prewet),” the theoretical colistin value significantly (p > 0.05) overestimated the actual measured value. In contrast, for all non-prewet groups, except “L
1, dip (+prewet),” the theoretical colistin value significantly (p > 0.05) overestimated the actual measured value. In contrast, for all non-prewet groups, except “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3
G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip (no prewet),” the theoretical total colistin loading was an adequate estimate of the true value as it did not differ significantly (p > 0.05) from the actual value.
1, dip (no prewet),” the theoretical total colistin loading was an adequate estimate of the true value as it did not differ significantly (p > 0.05) from the actual value.
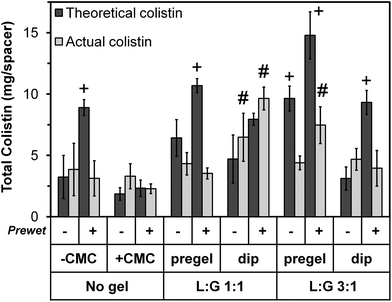 | ||
| Fig. 3 Total colistin loaded into each space maintainer on Day 0. “Actual colistin” values represent the total amount released from each space maintainer, measured until the release reached a consistent zero value. “Theoretical colistin” values were derived from the weight gain of each dried space maintainer upon colistin loading, taking into account the weight percent of colistin in each loading solution as well as the presence of thermogel, if applicable. The 12 groups are identified along the bottom of the image using the same notations depicted in the study design (Table 1). Theoretical values with “+” differed significantly from the corresponding actual value (p < 0.05). Actual values with “#” significantly differed from all other groups (p < 0.05) except those marked with the same notation. Each column represents the mean ± standard deviation for n = 4 space maintainers per group. | ||
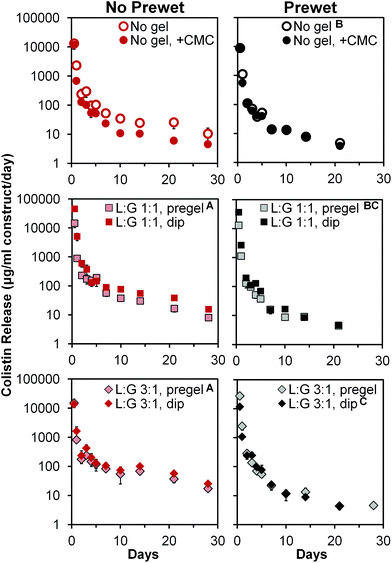
Antibiotic release
Colistin release profiles from all 12 groups are shown in Fig. 4. All groups exhibited a notable 24 h burst release followed by daily release of non-zero amounts of colistin (note per day normalization in y-axis). Burst release values measured at 12 h ranged from 4200 ± 750 to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ± 1800 μg colistin per ml construct volume, for “No gel, +CMC (+prewet)” and “L
800 ± 1800 μg colistin per ml construct volume, for “No gel, +CMC (+prewet)” and “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1
G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip (no prewet),” respectively. When taken as a percentage of the total colistin released from each construct, 12 h burst release ranged from 69 ± 11% to 90 ± 3%, with non-prewet groups generally having significantly lower (p < 0.05) burst release values than their corresponding +prewet counterparts, as demonstrated in Table 4. Following this initial burst release, non-zero colistin release was noted for all groups, with the daily amount released generally declining with time (Fig. 4). Several of the non-prewet groups showed a small spike in daily release around days 14–21, with subsequent decline in release, which remained at non-zero levels by day 28. In contrast, for all prewet groups except “L
1, dip (no prewet),” respectively. When taken as a percentage of the total colistin released from each construct, 12 h burst release ranged from 69 ± 11% to 90 ± 3%, with non-prewet groups generally having significantly lower (p < 0.05) burst release values than their corresponding +prewet counterparts, as demonstrated in Table 4. Following this initial burst release, non-zero colistin release was noted for all groups, with the daily amount released generally declining with time (Fig. 4). Several of the non-prewet groups showed a small spike in daily release around days 14–21, with subsequent decline in release, which remained at non-zero levels by day 28. In contrast, for all prewet groups except “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3
G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel (+prewet),” colistin release had ceased by day 28. Almost all groups had significantly different (p < 0.05) release profiles, except for the two non-prewet pregel groups, whose release did not differ significantly, but differed from all other groups. In addition, “LG 1
1, pregel (+prewet),” colistin release had ceased by day 28. Almost all groups had significantly different (p < 0.05) release profiles, except for the two non-prewet pregel groups, whose release did not differ significantly, but differed from all other groups. In addition, “LG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel (+prewet)” did not significantly differ from either “No gel (+prewet)” or “LG 3
1, pregel (+prewet)” did not significantly differ from either “No gel (+prewet)” or “LG 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip (+prewet),” though the latter two release profiles significantly differed from each other (p < 0.05). At day 28, none of the prewet groups had a daily release of colistin greater than the MIC of A. baumannii (8 μg ml−1, Table 5); in the non-prewet groups, all groups with the exception of No gel, +CMC, and LG 1
1, dip (+prewet),” though the latter two release profiles significantly differed from each other (p < 0.05). At day 28, none of the prewet groups had a daily release of colistin greater than the MIC of A. baumannii (8 μg ml−1, Table 5); in the non-prewet groups, all groups with the exception of No gel, +CMC, and LG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pregel had daily release in excess of the MIC of A. baumannii.
1 pregel had daily release in excess of the MIC of A. baumannii.
| Group | No prewet (%) | Prewet (%) |
|---|---|---|
| *Significantly differs from corresponding “no prewet” value (p < 0.05). **Significantly differs from all other values except “No gel, no prewet” (p < 0.05). | ||
| No gel | 73 ± 7 | 86 ± 2* |
| No gel, +CMC | 84 ± 4 | 86 ± 1 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel 1, pregel |
78 ± 8 | 87 ± 3* |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip 1, dip |
83 ± 2 | 90 ± 3* |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel 1, pregel |
81 ± 6 | 87 ± 3 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip 1, dip |
69 ± 11** | 85 ± 5* |
| Group | Prewet | MIC for A. baumannii (μg ml−1) | MIC for E. coli (μg ml−1) |
|---|---|---|---|
| *Significantly differs from corresponding colistin standard (p < 0.05). | |||
| Colistin standard | n/a | 8 ± 0 | 4 ± 0 |
| No gel | − | 8 ± 0 | 6 ± 2 |
| No gel, +CMC | − | 8 ± 0 | 8 ± 0* |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel 1, pregel |
− | 8 ± 0 | 8 ± 0* |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip 1, dip |
− | 8 ± 0 | 8 ± 0* |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel 1, pregel |
− | 8 ± 0 | 5 ± 2 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip 1, dip |
− | 4 ± 2* | 8 ± 0* |
| No gel | + | 8 ± 0 | 8 ± 0* |
| No gel, +CMC | + | 8 ± 0 | 8 ± 0* |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel 1, pregel |
+ | 8 ± 0 | 5 ± 2 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1 G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip 1, dip |
+ | 8 ± 0 | 4 ± 0 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pregel 1, pregel |
+ | 8 ± 0 | 5 ± 2 |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3 G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip 1, dip |
+ | 8 ± 0 | 5 ± 2 |
Main effects analysis (Fig. 5) of the study groups indicated that leaching of CMC, prewetting, and thermogel loading method all significantly affected colistin loading (p < 0.05). Leaching of CMC, which was done for all space maintainers except those in the two “No gel, +CMC” groups, nearly doubled the total amount of colistin loaded (p < 0.05). Prewetting significantly reduced colistin loading (p < 0.05) in both the control and thermogel-loaded constructs. Pregelling of the two thermogel formulations had opposite effects, significantly decreasing colistin loading of “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1
G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1” constructs, while significantly increasing colistin loading of “L
1” constructs, while significantly increasing colistin loading of “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3
G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1” constructs (p < 0.05).
1” constructs (p < 0.05).
Bacterial susceptibility
Colistin released from the various space maintainer formulations had a consistent effect on A. baumannii, as shown in Table 5. Fresh colistin, used as a standard, had a MIC of 8 ± 0 μg ml−1. All other groups also had a MIC of 8 ± 0 μg ml−1 for A. baumannii, except for “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3
G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dip (no prewet),” which had a significantly lower MIC of 4 ± 2 μg ml−1 (p < 0.05). E. coli showed a more variable susceptibility to colistin from the various samples (Table 5). Fresh colistin standard resulted in a MIC of 4 ± 0 μg ml−1. Most of the study formulations resulted in slightly higher MIC values for E. coli, which significantly differed from the standard in the case of six groups: “No gel, +CMC” (both +prewet and no prewet); “No gel” (+prewet); “L
1 dip (no prewet),” which had a significantly lower MIC of 4 ± 2 μg ml−1 (p < 0.05). E. coli showed a more variable susceptibility to colistin from the various samples (Table 5). Fresh colistin standard resulted in a MIC of 4 ± 0 μg ml−1. Most of the study formulations resulted in slightly higher MIC values for E. coli, which significantly differed from the standard in the case of six groups: “No gel, +CMC” (both +prewet and no prewet); “No gel” (+prewet); “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 1
G 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1” (no prewet, both pregel and dip groups); l and “L
1” (no prewet, both pregel and dip groups); l and “L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3
G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dip” (no prewet), all of which had a MIC of 8 ± 0 μg ml−1.
1, dip” (no prewet), all of which had a MIC of 8 ± 0 μg ml−1.
Discussion
PMMA space maintainers are frequently used to stent soft tissue in infected wounds, allowing time for the soft tissue to heal, while preserving a “pocket” for future bone reconstruction.35 The advent of advanced reconstructive techniques, including vascularized free tissue transfer, has decreased the frequency of space maintainer use in civilian craniofacial reconstruction.1,2,5,7,8,10 However, recent conflicts around the world have resulted in a growing number of patients with devastating blast injuries complicated by heavy microbial contamination, often with multi-drug resistant Acinetobacter baumannii.22–27 It is often impossible to follow the ideal civilian surgical reconstruction timeline in these patients, in which vascularized free tissue transfer would occur within 3–7 days, due to challenges including delayed evacuation and limited resources at nearby hospitals.1,25 The method described herein, in which antibiotic-laden PMMA space maintainers can be readily assembled with a variety of antibiotics and provide weeks-long release, presents an important advance in the care of these wounded soldiers. This method can also be extrapolated to the care of civilian patients, for instance, in those with infected total hip replacements, where it is commonplace to perform staged reconstruction that includes antibiotic-loaded PMMA space maintainers.35,36Porous space maintainers were selected in anticipation of future surgical implantation, as they have shown superior outcomes compared to non-porous PMMA implants in terms of clinical and in vivo healing of the overlying soft tissue cuff.10,11,19 All groups consisted of porous PMMA space maintainers fabricated according to established methods using a 9% w/w CMC hydrogel as a porogen, mixed at 30 w/w% with a clinical-grade kit for MMA polymerization, which has been previously shown to result in spacers with 16.9 ± 4.1% porosity and 39.7 ± 9.4% interconnectivity (at a 40 μm minimum connection size) as measured by microcomputed tomography (μ-CT).10 However, porosity presents a challenge as it creates a higher surface area for bacterial contamination.15,20,21 Our method takes advantage of the proven benefits of prefabricated porous PMMA space maintainers, and diminishes the risk of later infection by filling the pores with a controlled release system for antibiotic delivery. This presents a significant advantage over the current standard of antibiotic-loaded solid PMMA space maintainers, in which antibiotic is encapsulated within the PMMA phase during polymerization.35 Although technically simple, numerous studies have shown that only the antibiotic near the space maintainer surface is released, within hours to days, while >90% remains permanently entrapped within the solid PMMA cement and unavailable for antimicrobial treatment.35–37 Several recent studies have described porous PMMA space maintainers in which antibiotic-loaded degradable PLGA or gelatin microparticles are incorporated into porous PMMA; the microparticles degrade over time, generating further pores within the space maintainer and resulting in weeks-long clinically relevant antibiotic release.12,13,15 In these previous studies, the antibiotics or the antibiotic-loaded microparticles were loaded at the time of space maintainer fabrication. However, the flexibility of the porous space maintainer could be further expanded by the development of a system based on an infiltrating thermogelling polymer such as PLGA-PEG-PLGA into a prefabricated construct.
In this study, colistin was used as a model antibiotic, and the effects of several independent factors, including scaffold prewetting, porogen leaching, and thermogel loading method, were examined. Prewetting was investigated as a possible means to increase the infiltration of thermogelling liquid into the pores of the space maintainer by increasing the hydrophilicity of the bulk material, while simultaneously removing leachable methacrylate from the construct, improving the biocompatibility of the space maintainer.18 While a moderate theoretical loading increase was projected in prewet samples, the prewetting appears to negatively impact loading of antibiotic. This indicates that potential affinity disparities between the thermogel, water, antibiotic, and bulk materials could have resulted in infiltration of more water without thermogel-bound antibiotic or increased diffusion of antibiotic from the construct during gelation. The molar ratio of lactic acid to glycolic acid also affects the retention of antibiotics,38 and it appears that colistin may be retained by affinity to the increased hydrophobicity of the L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G 3
G 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition, which contains a higher proportion of hydrophobic lactic acid units.
1 composition, which contains a higher proportion of hydrophobic lactic acid units.
Pre-leaching CMC from constructs significantly increased antibiotic loading, as shown in Fig. 5, likely by providing more physical space for the thermogel to occupy within the pores of the space maintainer. Fig. 4 demonstrates that leaching also significantly affected the release profile, particularly for the non-prewet spacers (“No gel” vs. “No gel, +CMC”). Although significant, the +prewet groups showed a less obvious difference in release profile, which may stem from leaching of some of the CMC from the “No gel, +CMC (+prewet)” spacers during the prewet process (Fig. 4).
Pre-leaching CMC from constructs improves antibiotic loading by providing more physical space for the thermogel to occupy within the pores of the space maintainer.
The release kinetics suggest an initial diffusion-controlled release of colistin, followed by thermogel degradation-controlled release after 14 days up to 28 days, consistent with previous studies of drug release from PLGA-PEG-PLGA.38 Shi et al. fabricated porous space maintainers using colistin-swollen gelatin microparticles as a porogen and found that the drug released with Fickian diffusion kinetics over 10–14 days.13 Similarly, in a study by Spicer et al. of colistin-loaded porous space maintainers fabricated using gelatin as a porogen, colistin incorporated directly into the gelatin released over 7 days with diffusion-controlled release kinetics.15 In the same study, colistin-loaded PLGA microparticles were shown to release drug from porous space maintainers for up to 8 weeks with initial diffusion-controlled release followed by microparticle degradation-controlled release, similar to the kinetics seen with PLGA-PEG-PLGA thermogel but on a longer timescale.15 Colistin is a large, positively charged peptide antibiotic, and as such, physicochemical interactions with the PLGA matrix leads to an early burst release followed by degradation-controlled release.39 The 28 day release observed with PLGA-PEG-PLGA compared to the 8 week release observed with PLGA microparticles may be a result of the incorporation of hydrophilic PEG, reducing the affinity between drug and material.15,38 While the addition of PEG decreases the duration of release, the use of a triblock copolymer allows for thermogelation within the pores of a prefabricated porous space maintainers, resulting in greater flexibility to choose a variety of drugs at the time of implantation. PLGA-PEG-PLGA has also been evaluated with other drugs, and it has been demonstrated that the release kinetics are affected by the type of drug being released. Qiao et al. demonstrated that when 5-fluorouracil, a hydrophilic drug, is loaded into a PLGA-PEG-PLGA thermogel, release appears to be entirely diffusion-mediated; in contrast, incorporation of the hydrophobic drug indomethacin results in biphasic release characterized by early diffusion and late degradation-controlled release, similar to kinetics seen with colistin in both pure PLGA and in the triblock copolymer.38,39 Kim et al. loaded the protein drug insulin into PLGA-PEG-PLGA both with and without zinc and showed that in vitro release kinetics are likely influenced by the hydrophobicity of insulin, which causes it to partition preferentially toward the hydrophobic domains of the polymer micelles.40 A follow-up study by Choi et al. also using insulin shows a release profile that is similar to that of indomethacin and colistin, highlighting that drug hydrophobicity and partitioning are important parameters that govern release kinetics from PLGA-PEG-PLGA copolymers.41
Further studies should include the utilization of alternative antibiotics of varying partition coefficient, charge, and/or molecular weight, which could offer insight into the effects of antibiotic characteristics on interactions with the thermogel and scaffold. The in vivo efficacy of these systems will also be studied. This work could also be expanded to investigate the thermogel as a carrier for drug-loaded microparticles or nanoparticles, which may impart distinct release kinetics desirable for long-term infection prevention.
Conclusions
This study investigated PLGA-PEG-PLGA thermogelling copolymer as an antibiotic carrier for the eventual application of preventing and treating infections that may occur in bone defects containing prefabricated implantable porous space maintainers. The effects of porogen leaching, space maintainer prewetting, and loading method on drug loading and release kinetics were assessed using colistin as a model drug. In order to improve the loading of drug into the space maintainer, space maintainers should be pre-leached of CMC before attempting to load the thermogel. Pregelling the thermogel before implantation can result in decreased drug delivery, though it appears that increasing the L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio can improve colistin loading, which may be due to hydrophobic interactions. Prewetting should be avoided, as this decreases the loading of drug. The release kinetics are characterized by diffusion early, and after day 14, thermogel degradation appears to mediate release until day 28. The results from this study indicate that infiltration of a thermogelling PLGA-PEG-PLGA copolymer into the porosity of a prefabricated space maintainer is a simple and effective way to achieve controlled release of antibiotics from implantable space maintainers while capitalizing on the flexibility to choose a variety or combination of antibiotics at the time of implantation.
G ratio can improve colistin loading, which may be due to hydrophobic interactions. Prewetting should be avoided, as this decreases the loading of drug. The release kinetics are characterized by diffusion early, and after day 14, thermogel degradation appears to mediate release until day 28. The results from this study indicate that infiltration of a thermogelling PLGA-PEG-PLGA copolymer into the porosity of a prefabricated space maintainer is a simple and effective way to achieve controlled release of antibiotics from implantable space maintainers while capitalizing on the flexibility to choose a variety or combination of antibiotics at the time of implantation.
Disclosure statement
The authors have no competing financial interests, nor any other conflict of interest.Abbreviations
| CMC | Carboxymethylcellulose |
| FDA | United States food and drug administration |
| HPLC | High-performance liquid chromatography |
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G G | Lactic acid to glycolic acid ratio |
| MIC | Minimum inhibitory concentration |
| MMA | Methylmethacrylate |
| PBS | Phosphate-buffered saline |
| PLGA | Poly(DL-lactic-co-glycolic acid) |
| PEG | Poly(ethylene glycol) |
| PMMA | Polymethylmethacrylate |
| USP | United States pharmocopeia |
Acknowledgements
This work was supported by the Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort, under Award No. W81XWH-14-2-0004. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.We would like to acknowledge the support of Drs F. Kurtis Kasper, Mark E. Wong, and Anthony Ratcliffe in the development of this work. We would also like to acknowledge Drs Clinton K. Murray and Katrin Mende of the U.S. Army Institute of Surgical Research for supplying the clinical isolate of Acinetobacter baumannii. SRS would like to acknowledge support from the Baylor College of Medicine Medical Scientist Training Program and from a Ruth L. Kirschstein Fellowship from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (F30AR067606).
References
- S. R. Shackford, J. E. Kahl, R. Y. Calvo, R. A. Kozar, C. E. Haugen and K. E. Kaups, et al. , J. Traum. Acute Care Surg., 2014, 76, 347–352 CrossRef PubMed.
- J. J. Pribaz, D. D. Weiss, J. B. Mulliken and E. Eriksson, Plast. Reconstr. Surg., 1999, 104, 357–365 CAS.
- A. M. Henslee, P. P. Spicer, S. R. Shah, A. M. Tatara, F. K. Kasper, A. G. Mikos and M. E. Wong, Oral and Maxillofac. Surg. Clin. North Am., 2014, 26, 143–149 CrossRef PubMed.
- J. J. Kim and G. R. D. Evans, Clin. Plast. Surg., 2012, 39, 359–376 CrossRef PubMed.
- S. M. Susarl, E. Swanson and C. R. Gordon, Ann. Plast. Surg., 2011, 6, 655–661 CrossRef PubMed.
- K. Petersen, M. H. Colyer, D. K. Hayes, R. G. Hale and R. B. Bell, PoC-RIG Panel, J. Trauma, 2011, 71, S264–S2S9 CrossRef CAS PubMed.
- N. R. Dean, S. M. McKinney, M. K. Wax, P. J. Louis and E. L. Rosenthal, Craniomaxillofac. Trauma Reconstr., 2011, 4, 25 CrossRef PubMed.
- T. Kihtir, R. R. Ivatury, R. J. Simon, Z. Nassoura and S. Leban, J. Trauma, 1993, 35, 569–577 CrossRef CAS.
- E. Suominen and E. Tukiainen, Clin. Plast. Surg., 2001, 28, 323–337 CAS.
- J. D. Kretlow, M. Shi, S. Young, P. P. Spicer, N. Demian and J. A. Jansen, et al. , Tissue Eng., Part C, 2010, 16, 1427–1438 CrossRef CAS PubMed.
- C. Nguyen, S. Young, J. D. Kretlow, A. G. Mikos and M. Wong, J. Oral Maxillofac. Surg., 2011, 69, 11–18 CrossRef PubMed.
- M. Shi, J. D. Kretlow, A. Nguyen, S. Young, L. Scott Baggett and M. E. Wong, et al. , Biomaterials, 2010, 31, 4146–4156 CrossRef CAS PubMed.
- M. Shi, J. K. Kretlow, P. P. Spicer, Y. Tabata, N. Demian and M. E. Wong, et al. , J. Controlled Release, 2011, 152, 196–205 CrossRef CAS PubMed.
- P. P. Spicer, J. D. Kretlow, A. M. Henslee, M. Shi, S. Young and N. Demian, et al. , J. Biomed. Mater. Res., Part A, 2012, 100A, 827–833 CrossRef CAS PubMed.
- P. P. Spicer, S. R. Shah, A. M. Henslee, B. M. Watson, L. A. Kinard and J. D. Kretlow, et al. , Acta Biomater., 2013, 9, 8832–8839 CrossRef CAS PubMed.
- N. M. Goodger, J. Wang, G. W. Smagalski and B. Hepworth, J. Oral Maxillofac. Surg., 2005, 63, 1048–1051 CrossRef CAS PubMed.
- B. B. Chisholm, D. Lew and K. Sadasivan, J. Oral Maxillofac. Surg., 1993, 51, 444–449 CrossRef CAS.
- L. Wang, D. M. Yoon, P. P. Spicer, A. M. Henslee, D. W. Scott and M. E. Wong, et al. , J. Biomed. Mater. Res., Part B, 2013, 101B, 813–825 CrossRef CAS PubMed.
- A. M. Henslee, P. P. Spicer, S. R. Shah, A. M. Tatara, F. K. Kasper and A. G. Mikos, et al. , Oral Maxillofac. Surg. Clin. North Am., 2014, 26, 143–149 CrossRef PubMed.
- M. B. Nair, J. D. Kretlow, A. G. Mikos and F. K. Kasper, Curr. Opin. Biotechnol., 2011, 22, 721–725 CrossRef CAS PubMed.
- M. L. Bruens, H. Pieterman, J. R. de Wijn and J. M. Vaandrager, J. Craniofac. Surg., 2003, 14, 63–68 CrossRef.
- T. A. Lew, J. A. Walker, J. C. Wenke, L. H. Blackbourne and R. G. Hale, J. Oral Maxillofac. Surg., 2010, 68, 3–7 CrossRef PubMed.
- S. L. Eskridge, C. A. Macera, M. R. Galarneau, T. L. Holbrook, S. I. Woodruff and A. J. MacGregor, et al. , Injury, 2012, 43, 1678–1682 CrossRef PubMed.
- D. Tong and R. Beirne, Mil. Med., 2013, 178, 421–426 CrossRef PubMed.
- I. L. Valerio, J. Sabino, G. S. Mundinger and A. Kumar, Ann. Plast. Surg., 2014, 72, S38–S45 CrossRef CAS PubMed.
- P. Scott, G. Deye, A. Srinivasan, C. Murray, K. Moran and E. Hulten, et al. , Clin. Infect. Dis., 2007, 44, 1577–1584 CrossRef CAS PubMed.
- D. R. Hospenthal, H. K. Crouch, J. F. English, F. Leach, J. Pool and N. G. Conger, et al. , J. Trauma, 2011, 71, S52–SS7 CrossRef PubMed.
- A. C. Gales, R. N. Jones and H. S. Sader, J. Antimicrob. Chemother., 2011, 66, 2070–2074 CrossRef CAS PubMed.
- A. M. Henslee, S. R. Shah, M. E. Wong, A. G. Mikos and F. K. Kasper, J. Biomed. Mater. Res., Part A, 2014, 103, 1485–1497 CrossRef PubMed.
- N. L. Elstad and K. D. Fowers, Adv. Drug Delivery Rev., 2009, 61, 785–794 CrossRef CAS PubMed.
- L. Yu, T. Ci, S. Zhou, W. Zeng and J. Ding, Biomater. Sci., 2013, 1, 411–420 RSC.
- S. Choi, M. Baudys and S. Kim, Pharm. Res., 2004, 21, 827–831 CrossRef CAS.
- S. Sato, M. J. V. Fonseca, J. O. D. Ciampo, J. R. Jabor and V. Pedrazzi, Braz. Oral Res., 2008, 22, 145–150 CrossRef PubMed.
- W. Zhu, T. Masaki, Y. H. Bae, R. Rathi, A. K. Cheung and S. E. Kern, J. Biomed. Mater. Res., Part B, 2006, 77B, 135–143 CrossRef CAS PubMed.
- A. Bistolfi, G. Massazza, E. Verné, A. Massè, D. Deledda and S. Ferraris, et al. , ISRN Orthopedics, 2011, 2011, 1–8 CrossRef PubMed.
- K. Anagnostakos, P. Wilmes, E. Schmitt and J. Kelm, Acta Orthop., 2009, 80, 193–197 CrossRef PubMed.
- E. Bertazzoni Minelli, A. Benini, E. Samaila, M. Bondi and B. Magnan, J. Chemother., 2015, 27, 17–24 CrossRef CAS PubMed.
- M. Qiao, D. Chen, X. Ma and Y. Liu, Int. J. Pharm., 2005, 294, 103–112 CrossRef CAS PubMed.
- S. R. Shah, A. M. Henslee, P. P. Spicer, S. Yokota, S. Petrichenko, S. Allahabadi, G. N. Bennett, M. E. Wong, F. K. Kasper and A. G. Mikos, Pharm. Res., 2014, 31, 3379–3389 CrossRef CAS PubMed.
- J. K. Kim, S. Choi, J. J. Koh, M. Lee, K. S. Ko and S. W. Kim, Pharm. Res., 2001, 18, 548–550 CrossRef.
- S. Choi and S. W. Kim, Pharm. Res., 2003, 20, 2008–2010 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |