Is the combination of nanofiltration membranes and AOPs for removing microcontaminants cost effective in real municipal wastewater effluents?
S.
Miralles-Cuevas
c,
I.
Oller
ab,
A.
Agüera
b,
J. A. Sánchez
Pérez
b,
Ricardo
Sánchez-Moreno
ab and
S.
Malato
*ab
aPlataforma Solar de Almería-CIEMAT, Ctra Senés km 4, 04200 Tabernas (Almería), Spain. E-mail: sixto.malato@psa.es; Fax: +34950365015; Tel: +34950387940
bCIESOL, Joint Centre of the University of Almería-CIEMAT, 04120 Almería, Spain
cLaboratorio de Investigaciones Medioambientales en Zonas Áridas, LIMZA, EUDIM, University of Tarapacá, Avda. General Velásquez 1775, Arica, Chile
First published on 21st March 2016
Abstract
The purpose of this work was the economic assessment of different municipal wastewater treatment plant (MWTP) effluent treatment operating strategies based on the combination of nanofiltration (NF) and EDDS-assisted solar photo-Fenton at neutral pH or ozonation at natural pH. The study focused on microcontaminant (MC) removal as evaluated by LC-Qtrap-MS. Direct treatment of MCs in MWTP effluents was compared with their treatment in an NF rejection stream. Costs were estimated based on a flow rate of 1000 m3 per day (365 operating days per year) and over 90% degradation of 35 different MCs. The highest operating costs were related to EDDS and the hydrogen peroxide for photo-Fenton, pumping for NF and O3 generation for ozonation. It was concluded that both photo-Fenton and ozonation applied to NF rejection lowered treatment costs compared to MWTP effluent treatment. Ozonation was the most economical in all cases.
Water impactMembrane processes are one of the most widely used strategies for improving quality of municipal wastewater treatment plant effluents. However, membrane rejection must be adequately treated for removal of microcontaminants. This work focuses on economic assessment of combining solar photo-Fenton or ozonation with nanofiltration to treat these effluents. The procedure for calculating costs should be carefully applied case by case. |
1. Introduction
Different sources of contamination, such as leakage from sewer networks and septic tanks, fertilizers used in farming, intentional or inadvertent waste disposal, and discharge of industrial, and urban and hospital wastewater effluents, are increasing the contamination load on municipal wastewater treatment plants (MWTP). This contamination is mainly provoked by microcontaminants (MCs). MCs are not necessarily new compounds. They may have been present in the environment for a long time, but their presence and implication for the environment's integrity have only recently been recognized.1,2 Advances in analytical techniques have resulted in the detection of very low concentrations of MCs in water.3–5Membrane processes are one of the most widely used strategies for improving the quality of MWTP effluents. However, the membrane rejection stream must be managed adequately, so integrated water treatment systems should be implemented in treatment plants for effective membrane rejection management, including appropriate treatment of MCs. Such combined systems could involve hybrid advanced oxidation processes (AOPs) and membranes such as membrane bioreactors/reverse osmosis or nanofiltration (NF), NF/Fenton reaction, NF/photo-Fenton, NF/ozonation, NF/electrochemical oxidation, etc. NF is a complex process dependent on microhydrodynamics and interfacial events occurring on the membrane surface and in nanopores. Rejection from NF membranes may be attributed to a combination of steric, Donnan, dielectric and transport effects.6–8 In addition, the NF system is able to generate large amounts of high-quality water, accompanied by an excellent MC removal capacity. Lower prices of membranes, lower energy consumptions and enhanced membrane lifetime are the main reasons for the worldwide acceptance and popularity of NF.9 NF is applied for treatment of ground water, surface water and wastewater reclamation. Apart from the conventional application of removing divalent salts and conventional organic contaminants, recent studies have shown that NF has advantages over reverse osmosis for many new interesting applications such as removal of arsenic (As)10,11 and MCs such as pharmaceutical active compounds12,13 and hormones.6,14
Recent studies uphold AOPs and their combinations as the most promising and highly competitive innovative water and wastewater treatment methods for the removal of biorecalcitrant compounds.15–17 The degradation of MCs is easily accomplished by nonselective attack of the highly oxidative hydroxyl radicals. AOPs are not intended to replace conventional systems, but to supplement existing systems to produce a better-quality effluent.17 Nevertheless, a few studies can be found in the literature dealing with the total treatment cost of AOPs combined with membrane technologies, compared to direct treatment only by AOPs. Other authors18 reported treatment of an industrial wastewater (containing dyes) by using NF/reverse osmosis membranes in the pretreatment before the catalytic reactor, and estimated that the total specific costs of industrial NF processes in usefully adjusted and designed plants were from US$1 to 6 per m3 of treated effluent. Madsen et al. investigated how the energy consumption of electrochemical oxidation could be lowered by combining the process with membrane filtration in a setup where electrochemical oxidation was applied to the membrane retentate stream using natural groundwater spiked with the 2,6-dichlorobenzamide pesticide residue.19 They also showed that the use of reverse osmosis membranes with 90% recovery combined with electrochemical oxidation required 95% less energy consumption (0.96 kW h m−3) than the stand-alone electrochemical oxidation treatment (18.5 kW h m−3). On the other hand, there are only a few studies on the total cost (TC) of AOPs for treatment of real municipal wastewater treatment effluents. Prieto-Rodríguez et al. compared solar photo-Fenton, ozonation and photocatalysis with TiO2 for the treatment of municipal wastewater treatment plant (MWTP) effluents in which 66 MCs were detected.20 The total cost of solar photo-Fenton and ozonation for 90% elimination of MCs was 0.19€ per m3 and 0.45€ per m3, respectively, based on a design flow of 5000 m3 per day. Yoon et al. reported the total cost of ozonation as a post-treatment for color removal in wastewater from swine in membrane filtration systems comparing ultrafiltration and NF.21 Design factors used for calculating the total cost were 20 m3 per day treatment capacity and 20 h per day operation. The TC was 1.49€ per m3 for ultra-filtered water and 0.60€ per m3 for NF-filtered water.
The purpose of this work was the economic assessment of different operating strategies based on the combination of nanofiltration and advanced oxidation processes (AOPs) such as solar photo-Fenton and ozonation to treat MWTP effluents. The study focused on the removal of MCs detected in MWTP effluents by LC-Qtrap-MS. The cost of NF rejection stream treatment by ozonation or EDDS ((S,S)-ethylenediamine-N,N′-disuccinic acid)-assisted solar photo-Fenton at neutral pH was compared to the cost of direct treatment of MWTP effluents by the same AOPs. The Fenton process is one of the most well-known advanced oxidation processes (AOPs), and its oxidative action is due to the formation of reactive oxygen species (mainly HO˙) following the oxidation of ferrous iron by hydrogen peroxide. Ferrous iron, in the absence of any other complexing agent, has the tendency to rapidly become oxidized and form different aqua–Fe(III) species which exhibit significant photoactivity in the UV-visible part of the solar spectrum and allow Fe2+ regeneration when illuminated. The regenerated Fe2+ reacts again with H2O2, perpetuating the formation of HO˙ in the process known as photo-Fenton. At pH higher than 3, however, insoluble ferric complexes begin to form that tend to rapidly precipitate. To avoid this limitation, the use of iron-chelating agents is being considered. The resulting iron complexes maintain their solubility at a wide pH range and can be photochemically reactive that assures the turnover of Fe2+. EDDS complexes with iron are an attractive alternative due to their reported biodegradability and lack of toxicity.
2. Methods
2.1. Reagents and chemicals
All MC standards were analytical grade (>99%) purchased from Sigma-Aldrich (Steinheim, Germany). Samples from experiments were prepared by appropriate dilution of the stock solutions in acetonitrile/water, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (v/v). Fe(III) from Fe2(SO4)3·H2O 75% used for the photo-Fenton experiment was supplied by Sigma Aldrich. EDDS solution in water (35% w/v) was supplied by Panreac. Reagent-grade hydrogen peroxide (30% w/v) and sulfuric acid (98%) were also from Sigma-Aldrich. Commercial cartridges packed with Oasis™ HLB (200 mg, 6 cm3) were purchased from Waters (Mildford, MA, USA). N2 for removal of residual dissolved O3 present in samples to stop the reaction was provided by Carburos Metálicos.
90 (v/v). Fe(III) from Fe2(SO4)3·H2O 75% used for the photo-Fenton experiment was supplied by Sigma Aldrich. EDDS solution in water (35% w/v) was supplied by Panreac. Reagent-grade hydrogen peroxide (30% w/v) and sulfuric acid (98%) were also from Sigma-Aldrich. Commercial cartridges packed with Oasis™ HLB (200 mg, 6 cm3) were purchased from Waters (Mildford, MA, USA). N2 for removal of residual dissolved O3 present in samples to stop the reaction was provided by Carburos Metálicos.
2.2. Analytical measurements
Dissolved organic carbon (DOC) and total inorganic carbon (TIC) were measured using a Shimadzu TOC-VCSN analyzer (Kyoto, Japan). Anion concentrations were determined with a Metrohm 872 Extension Module 1 and 2 ion chromatograph (IC) system configured for gradient analysis. Cation and ammonium concentrations were determined using a Metrohm 850 Professional IC configured for isocratic analysis. Water conductivity was analyzed using a CRISON GLP3u conductivity meter. Turbidity was measured using a Hach 2100 N turbidimeter. Chemical oxygen demand (COD) was determined using Merck kits in a 10 to 50 mg L−1 range using a NOVA-30 Spectroquant photometer. The H2O2 concentration was determined by spectrophotometry using titanium(IV) oxysulfate following DIN 38402H15. The total iron concentration (aqueous solution containing iron was filtered with a 0.45 μm diameter PTFE syringe-driven filter) was measured using the 1,10-phenanthroline method following ISO 6332.The analytical method used for the detection and quantification of the target MCs in the MWTP effluent used in this study was carried out using a hybrid quadrupole/linear ion trap mass spectrometer system (5500 QTRAP®, AB Sciex Instruments, Foster City, CA, USA) under multiple reaction monitoring (MRM) conditions. The instrument was equipped with an electrospray ionization (ESI) source operating in positive mode. The analytes were separated using an HPLC series 1200 (Agilent Technologies, Wilmington, DE, USA) equipped with a reverse-phase C-18 analytical column (Zorbax eclipse plus SB, Agilent Technologies, 5 μm 150 × 4.6 mm). The LC conditions for the analysis were 10% ACN : 90% MilliQ-water (0.1% formic acid) at t = 0 min to 100% ACN in t = 40 min and held for 10 min at a flow rate of 400 μL min−1. The injection volume was 5 μL.
The mass spectrometer was optimized to identify 75 MCs, which were quantified by matrix matched calibration. Calibration curves included analyte concentrations of 0.01, 0.05, 0.1, 0.5 and 1 μg L−1. The MS–MS parameters (declustering potential, DP; collision energy, CE; and cell exit potential, CXP) were optimized for maximum sensitivity by direct infusion of the standards of each compound into the MS. Two MRM transitions were selected for each compound, one for quantification and the second for confirmation. Tandem MS analyses were performed in the MRM-MS with unit resolution set to low for Q1 and Q3. The ESI source operating conditions were the following: an ion spray voltage of 5000 V, a probe temperature of 500 °C, and nebulizer gas pressures of (GS1 and GS2) 50 psi and 40 psi, respectively. Nitrogen was used as the nebulizer and collision gas. The instrument detection limits (IDLs) were in the 0.04–3.6 pg range, and the amounts injected as absolute, method detection limits (MDLs) were between 1 and 150 ng L−1 and the method quantification limits (MQLs) were between 2 and 450 ng L−1, depending on the target contaminant.
2.3. Pilot plant setups
The NF system consisted of two FILMTEC NF90-2540 membranes, operated in parallel with a total surface area of 5.2 m2 as described in detail elsewhere.22 It should be highlighted that it was operated in batch mode, that is, the concentrate stream was returned to the feed tank, while the permeate stream was purged. The initial 1 m3 volume was concentrated to 0.25 m3 with a Volumetric Concentration Factor (VCF, eqn (1)) of 4.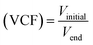 | (1) |
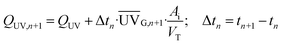 | (2) |
 (W m−2) is the average solar ultraviolet radiation (λ < 400 nm) measured between tn+1 and tn, and Ai (3 m2) is the irradiated surface. VT is the total volume of the water loaded in the pilot plant (35 L) and Vi is the total irradiated volume (22 L).
(W m−2) is the average solar ultraviolet radiation (λ < 400 nm) measured between tn+1 and tn, and Ai (3 m2) is the irradiated surface. VT is the total volume of the water loaded in the pilot plant (35 L) and Vi is the total irradiated volume (22 L).
The ozonation system is an Anseros PAP-pilot plant (Anseros Klaus Nonnenmacher GmbH, Germany) for batch operation. The process tank is a 10-L Pyrex cylindrical vessel provided with a cap containing inlets for gas feeding, and outlets for sampling and venting. The ozonation system is also equipped with a magnetic stirrer for appropriate homogenization, two non-dispersive UV analyzers (Ozomat GM-6000-OEM) for measuring the inlet and outlet ozone gas concentrations, a flow meter for inlet air regulation, two oxygen generators (Anseros SEP100) and an ozone generator (Anseros COM-AD02). The oxygen generator supplies a maximum air flow of 6 L min−1 with an oxygen concentration of up to 90%. The highly concentrated oxygen stream is fed into the ozone generator, which produces O3 in high-voltage discharge conduits. The ozone generator produces a constant 24 g of O3 m−3 flow. A thermal ozone destructor is connected to the reactor exhaust preventing its release into the atmosphere.
2.4. MWTP effluent pretreatment
All the experiments were conducted with 1 m3 samples of real wastewater effluent collected at different times of the year from a MWTP in El Ejido (Almería, Spain). This MWTP was designed for a population of 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 and has an inlet flow of 12
300 and has an inlet flow of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 m3 per day. The starting concentrations of MCs in the effluent were slightly different depending on the day they were collected (47–53 μg L−1) due to the inherent variability of real MWTP effluents. However, the basic composition of the effluent did not vary substantially (Table 1). Wastewater was collected downstream of the MWTP secondary biological treatment, stored at 5 °C and used as received within the next 10 days. The effluent was pretreated before NF by conventional filtration (through a 75 μm sand filter and two microfilters, 25 and 5 μm, respectively), from which mainly suspended solids (62–88% turbidity reduction) were removed.
500 m3 per day. The starting concentrations of MCs in the effluent were slightly different depending on the day they were collected (47–53 μg L−1) due to the inherent variability of real MWTP effluents. However, the basic composition of the effluent did not vary substantially (Table 1). Wastewater was collected downstream of the MWTP secondary biological treatment, stored at 5 °C and used as received within the next 10 days. The effluent was pretreated before NF by conventional filtration (through a 75 μm sand filter and two microfilters, 25 and 5 μm, respectively), from which mainly suspended solids (62–88% turbidity reduction) were removed.
| Parameter | Direct effluent (D) (mg L−1) | Concentrate effluent (C) (mg L−1) |
|---|---|---|
| Na+ | 150–200 | 640–700 |
| K+ | 15–20 | 50–70 |
| Mg2+ | 40–60 | 75–210 |
| Ca2+ | 70–150 | 120–150 |
| SO42− | 150–300 | 500–900 |
| NH4+ | 20–40 | 70–150 |
| Cl− | 300–400 | 1000–1200 |
| HCO3− | 400–500 | 800–1500 |
| TN | 40–50 | 75–120 |
| TIC | 100–120 | 300–360 |
| COD | 50–70 | 120–180 |
| DOC | 10–30 | 40–60 |
| Turbidity (N.T.U.) | 3.0–5.0 | 10–12 |
| Conductivity (mS cm−1) | 2.0–2.2 | 6.0–6.5 |
| pH | 7.0–7.5 | 7.5–8.0 |
2.5. Experimental procedures
After the MWTP effluent had been pretreated, it was concentrated from 1 to 0.25 m3 by NF. The starting upstream membrane system operating conditions were an inlet flow rate of 100 L h−1 at a pressure of 3 bar. The concentrate and permeate flow rates were set at 50 L h−1, maintaining a transmembrane pressure not higher than 5 bar. The concentrate stream was returned to the feed tank while the “clean” permeate was purged.1. Bicarbonates were stripped after addition of concentrated sulfuric acid.
2. 1.5 mM of H2O2 was added and homogenized for 10 min (this concentration was the same in all the experiments adding more H2O2 as needed, so there was always enough in the system).
3. Fe(III)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDDS (1
EDDS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was added and homogenized for 10 min before the CPC reactor was uncovered. 0.1 mM Fe(III) was used for D and 0.2 mM Fe(III) for C, as the MCs in C could not be degraded as required by using 0.1 mM of Fe(III) as in previous studies.24
2) was added and homogenized for 10 min before the CPC reactor was uncovered. 0.1 mM Fe(III) was used for D and 0.2 mM Fe(III) for C, as the MCs in C could not be degraded as required by using 0.1 mM of Fe(III) as in previous studies.24
4. DOC, iron and H2O2 concentrations were measured immediately after sampling. Excess H2O2 was eliminated by adding bovine liver catalase.
5. Samples were preconcentrated 100 times by automated solid-phase extraction following a previously described protocol24 prior to analyses by LC-Qtrap-MS.
MWTP effluent was used as received. The ozone generator was turned on at 30% power with a constant production of 24–28 g of O3 m−3. The samples were collected every 1–2 min and residual ozone was removed with N2 to stop the reaction. DOC was measured immediately after sampling and then the samples were preconcentrated 100 times by automated solid phase extraction. Ozone gas was measured at the system inlet (CO3,i, g of N m−3) and outlet (CO3,o, g of N m−3) and consumption corresponding to each sample (O3cons,n g L−1) was calculated by using eqn (3) taking the inlet air flow rate (Qa N m3 h−1) into account and ozone consumption in the previous sample:
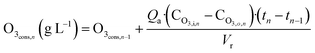 | (3) |
2.6. Economic assessment
Costs were estimated based on a 1000 m3 per day flow rate (365 operating days per year) corresponding to 3.65 × 105 m3 per year. Chemical costs included in the calculation were (industrial-grade prices): 0.45€ per L for H2O2 solution 33% (w/v), 0.71€ per kg for Fe2(SO4)3·H2O, 0.10€ per L for H2SO4 (98% w/v), 0.1€ per kW h for electricity and 3.5€ per L for EDDS (35% w/v). According to the data provided by the ozonation system manufacturer, 23.1€ is the cost per kg of O3 produced (electricity 20 W and air flow 100 L h−1).Amortization (AC) was calculated by using eqn (4) as a function of the interest (i) on investment (6% for ozone and photo-reactor and 7% for membrane system, based on amortization coefficients applied in Spain [http://www.individual.efl.es/ActumPublic/ActumG/actgrat-listado.jsp Memento Fiscal]) and considering a 20 year plant lifetime. Operating costs included are personnel, maintenance, energy and reagents evaluated for each case. IC stands for investment cost.
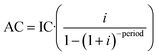 | (4) |
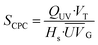 | (5) |
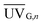 (W m−2) is the average annual local global solar UV irradiation. VT is the total annual volume of water to be treated and Hs is the annual hours of operation. The QUV required for 90% degradation of MCs was used in this study.
(W m−2) is the average annual local global solar UV irradiation. VT is the total annual volume of water to be treated and Hs is the annual hours of operation. The QUV required for 90% degradation of MCs was used in this study.
Table 2 shows the CPC investment costs for different solar collector sizes. Costs are based on previous studies performed in the EU CADOX Project.25 Costs related to land, taxes, or incentive fees (often applied in environmental projects) were not taken into account as they would be closely related to plant location.
| Collector surface CPC (m2) | 100 | 500 | 1000 |
| Control and instrumentation (€) | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
| Hydraulic circuit (tanks, pipes, valves, distributors, accessories, supports, etc.) (€) | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| Collection field CPC including the tubular reactor (€) | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
159![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
253![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Foundations and civil engineering (€) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| Transport of material and equipment (€) | 1500 | 2400 | 3600 |
| Total cost of installation (€) | 113![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
306![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
483![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| Cost per m2 of CPC (€ per m2) | 1139 | 612 | 484 |
According to Sánchez-Pérez et al., the investment costs may be found26 by scaling eqn (6) based on the rule of six tenths.27
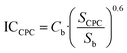 | (6) |
| CostO3 = 1719.5 · q0.6143 | (7) |
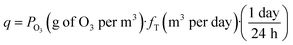 | (8) |
• Feeding section (FS): feed tank, cleaning system and pump are included in the feeding section costs, CFS. This parameter is directly proportional to annual treatment flow, Qa.
• Membrane section (MS): the recirculation line, pump, pipes, membranes and support structure are included in the membrane section costs, CMS. This parameter is directly proportional to membrane surface, Ambn.
• Automation section: the instrumentation and control system are included in the automation factor fautomation.
The investment in the membrane system was found from eqn (9)–(11):
| ICmbn = (CFS + CMS) · fautomation | (9) |
| CFS = 5000€ · Qa(m3 h−1) | (10) |
| CMS = 1000€ · Ambn(m2) · fmodule | (11) |
| f automation | Operation type |
|---|---|
| 1.0 | Manual |
| 1.3 | Semi-automatic |
| 1.5 | Automatic |
| f module | Module type |
|---|---|
| 1.0 | Spiral wound |
| 1.2 | Tubular |
| 1.3 | Plate and frame |
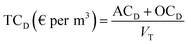 | (12) |
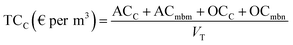 | (13) |
3. Results
The treatment technologies selected for this study were: (i) EDDS (iron complexing agent)-assisted solar photo-Fenton at neutral pH and (ii) ozonation at natural pH directly applied to real wastewater effluents (D) and to the same effluents after NF concentration (C). The best operating conditions for both treatments (see sections 2.5.1 and 2.5.2) had been previously evaluated and published by the same authors.30,313.1. Pilot plant experiment results: energy and reagent consumption for each treatment
For solar photo-Fenton experiments, bicarbonates and carbonates (HCO3−/CO32−) were stripped under agitation (to avoid interaction with hydroxyl radicals thereby reducing efficiency) by adding 0.11 g L−1 of sulfuric acid to effluent D (110 g in 1000 L of water). Carbonates contained in the nanofiltration (NF) effluent (C) were stripped by adding 65 g of sulfuric acid to 250 L of concentrated water (from 1000 L of MWTP effluent). 0.065 g L−1 of sulfuric acid should be considered for correct comparison of D and C. It should be stressed that as less than 60% of carbonates were retained by NF, acid consumption decreased substantially from D to C. In both cases, bicarbonate concentration decreased to 25 mg L−1 without significantly affecting pH (pH 6.8). Ozonation was applied without any pretreatment (see Table 4).| Solar photo-Fenton | Ozonation | |||
|---|---|---|---|---|
| D | C | D | C | |
| Time, min | — | — | 6.7 | 7.4 |
| Fe(III), mM | 0.1 | 0.2 | — | — |
| H2O2, mg L−1 | 55.8 | 105.4 | — | — |
| Q UV, kJ L−1 | 2.9 | 3.8 | — | — |
| O3, mg L−1 | — | — | 6.1 | 10.1 |
| H2SO4, mg L−1 | 110 | 65 | — | — |
| EDDS, mL L−1 | 0.2 | 0.1 | — | — |
In all cases, MC degradation detected in real wastewater effluent was over 90%, the percentage selected for process comparison. Solar photo-Fenton and ozonation were carried out with two different real wastewater effluent samples. The starting MC concentration containing 35 different MCs detected in D treated by solar photo-Fenton was 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 ng L−1. In the NF effluent, the starting MC concentration was 136
900 ng L−1. In the NF effluent, the starting MC concentration was 136![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 (see Fig. 1). In effluent D, only 9 of the 35 MCs were detected in concentrations over 800 ng L−1, which is over 90% of total MCs (43
000 ng L−1 (see Fig. 1). In effluent D, only 9 of the 35 MCs were detected in concentrations over 800 ng L−1, which is over 90% of total MCs (43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ng L−1). These compounds were dipyrone metabolites (4-AAA, 4300 ng L−1 and 4-FAA, 2300 ng L−1), antipyrine (1300 ng L−1), caffeine (20
200 ng L−1). These compounds were dipyrone metabolites (4-AAA, 4300 ng L−1 and 4-FAA, 2300 ng L−1), antipyrine (1300 ng L−1), caffeine (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ng L−1), carbamazepine (6600 ng L−1), naproxen (4500 ng L−1), norfloxacin (2300 ng L−1), ofloxacin (860 ng L−1) and sulfamethoxazole (870 ng L−1).
200 ng L−1), carbamazepine (6600 ng L−1), naproxen (4500 ng L−1), norfloxacin (2300 ng L−1), ofloxacin (860 ng L−1) and sulfamethoxazole (870 ng L−1).
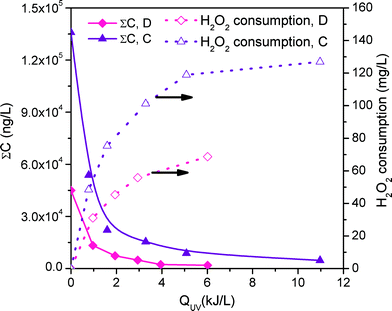 | ||
| Fig. 1 Degradation profile for the sum of MCs in effluents D and C treated by EDDS-assisted solar-photo-Fenton at neutral pH. Hydrogen peroxide consumption is also shown. | ||
2.9 kJ L−1 of UV accumulated energy was required to remove 90% of all MCs in D with hydrogen peroxide consumption of 1.64 mM (55.7 mg L−1), while treatment of 90% of all MCs in C required 3.8 kJ L−1 of energy and 3.10 mM (105.4 mg L−1) of hydrogen peroxide (see Table 4).
Degradation by ozonation of the sum of MCs is shown in Fig. 2. MWTP effluent samples were different from those used for photo-Fenton. In this case, 40 MCs were detected in the starting concentration of 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 ng L−1 in D and 188
600 ng L−1 in D and 188![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ng L−1 in C (see Fig. 2). Ten of the 40 MCs were detected in concentrations over 800 ng L−1, that is, over 90% of the total MC (47
500 ng L−1 in C (see Fig. 2). Ten of the 40 MCs were detected in concentrations over 800 ng L−1, that is, over 90% of the total MC (47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ng L−1) load. These compounds were dipyrone metabolites (4-AAA, 6000 ng L−1 and 4-FAA, 10
500 ng L−1) load. These compounds were dipyrone metabolites (4-AAA, 6000 ng L−1 and 4-FAA, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 ng L−1), azithromycin (7800 ng L−1), amitriptyline (3200 ng L−1), ciprofloxacin (5800 ng L−1), citalopram (2100 ng L−1), nicotine (1300 ng L−1), ofloxacin (4200 ng L−1) and paraxanthine (2000 ng L−1). Ozone consumption for removing 90% of the total MCs was 6.1 mg L−1 in 6.4 min and 10.1 mg L−1 of ozone in 7.4 min for D and C, respectively (see Table 4).
400 ng L−1), azithromycin (7800 ng L−1), amitriptyline (3200 ng L−1), ciprofloxacin (5800 ng L−1), citalopram (2100 ng L−1), nicotine (1300 ng L−1), ofloxacin (4200 ng L−1) and paraxanthine (2000 ng L−1). Ozone consumption for removing 90% of the total MCs was 6.1 mg L−1 in 6.4 min and 10.1 mg L−1 of ozone in 7.4 min for D and C, respectively (see Table 4).
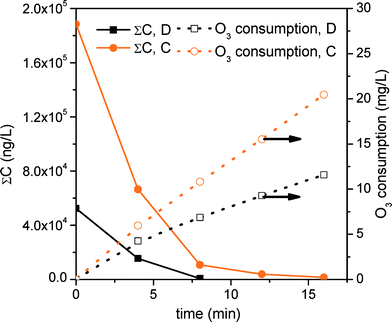 | ||
| Fig. 2 Degradation profile for the sum of MCs in effluents D and C treated by ozonation at pH 8. Ozone consumption is also shown. | ||
Total reagent consumption is shown in Table 4. It should be mentioned that in EDDS-assisted solar photo-Fenton, carbonates had to be removed because they are known hydroxyl radical “scavengers”,32 but this was unnecessary for ozonation as based on previous studies under the same operating conditions,31 the reaction rate was known not to be affected.
4. Discussion
4.1. Investment costs
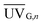 would be 18.6 W m−2. This is the annual average local global solar UV irradiation at Plataforma Solar de Almería (Spain) as used in this study, but it would also be valid throughout the Mediterranean region. The ICCPC was found using Cb = 483
would be 18.6 W m−2. This is the annual average local global solar UV irradiation at Plataforma Solar de Almería (Spain) as used in this study, but it would also be valid throughout the Mediterranean region. The ICCPC was found using Cb = 483![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000€ and Sb = 1000 m2, and the ACCPC was calculated using eqn (4). Table 5 summarizes these results, but it should be mentioned that in effluent C, NF membranes reduced the total volume to be treated by solar photo-Fenton or ozonation by a factor of 4, that is, the total volume treated in C was estimated at 91
000€ and Sb = 1000 m2, and the ACCPC was calculated using eqn (4). Table 5 summarizes these results, but it should be mentioned that in effluent C, NF membranes reduced the total volume to be treated by solar photo-Fenton or ozonation by a factor of 4, that is, the total volume treated in C was estimated at 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 m3 per year, while in D, the treatment volume was 365
250 m3 per year, while in D, the treatment volume was 365![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per year (see Table 5).
000 m3 per year (see Table 5).
| VCF | V T (m3) | Q UV (kJ L−1) | S CPC (m2) | € per m2 | ICCPC (€) | ACCPC |
|---|---|---|---|---|---|---|
| 1 (D) | 365![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.9 | 3600 | 290 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043 043![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
| 4 (C) | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
3.8 | 1200 | 450 | 539![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| VCF | q (kg of O3 h−1) | CostO3 (€) | ICO3 (€) | ACO3 (€) |
|---|---|---|---|---|
| 1 (D) | 1.00 | 119![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
681![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| 4 (C) | 0.25 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
290![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| Q a (m3 h−1) | A mbn (m2) | C FS (€) | C MS (€) | ICmbn (€) | ACmbm (€) |
|---|---|---|---|---|---|
| 41.6 | 78 | 208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
4.2. Operating costs
Operating costs (OCs) include all costs related to staff, reagents for both facility operation and maintenance, electricity costs, etc. The operating staff for a 500 m2 solar plant was estimated at only 0.1 workers per year, since the plant would be fully automated.24Therefore, for 3600 m2, 0.72 workers would be required, and for 1200 m2, 0.24 would be needed. Basing from the Spanish annual gross technician's salary of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000€ per year, labor would cost 21
000€ per year, labor would cost 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600€ (0.06€ per m3) and 7200€ (0.07€ per m3) per year for 3600 m2 and 1200 m2, respectively. Prieto-Rodríguez et al. published20 an estimate of personnel costs of 0.05€ per m3 for ozonation, which is in the same range as for photo-Fenton in this study. Thus the personnel cost would be 18
600€ (0.06€ per m3) and 7200€ (0.07€ per m3) per year for 3600 m2 and 1200 m2, respectively. Prieto-Rodríguez et al. published20 an estimate of personnel costs of 0.05€ per m3 for ozonation, which is in the same range as for photo-Fenton in this study. Thus the personnel cost would be 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250€ per year for D and 4600€ per year for C treated by ozonation. Personnel costs for NF membrane system operation must also be considered. Samhaber et al. estimated this cost as twice the maintenance costs, which would be 8100€ per year.18 Costs of reagents and electricity to be consumed for each treatment are outlined in Fig. 3.
250€ per year for D and 4600€ per year for C treated by ozonation. Personnel costs for NF membrane system operation must also be considered. Samhaber et al. estimated this cost as twice the maintenance costs, which would be 8100€ per year.18 Costs of reagents and electricity to be consumed for each treatment are outlined in Fig. 3.
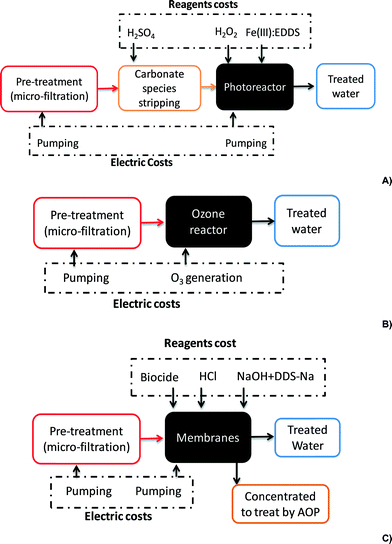
The cost of electricity was estimated by calculating water pumping requirements for pretreatment, reactor circulation, NF membrane system and ozone generation for the consumption calculated for each treatment, as shown in Fig. 3. Electricity requirements are described below:
• Pretreatment: 11.4 kW for the centrifugal pump to treat 1000 m3 per day (41.6 m3 h−1). Electric consumption of 0.27 kW h m−3.
• Solar photo-reactor: a 23 kW centrifugal pump for circulation or flow through the collector field. At 12 plant operating hours per day, the flow would be 83.3 m3 h−1, which means an electric consumption of 0.28 kW h m−3.
• NF membrane system: 10 bar high-pressure pump (11.6 kW). Considering a treatment flow rate of 41.6 m3 h−1, the power required by the pump would be 11.6 kW, costing 0.28 kW h m−3.
Total operating costs are summarized in Table 8 with reactor maintenance costs (CPC and ozone reactor), which were estimated as 2% of the ACme. Maintenance costs of the NF membranes were estimated18 as 10% of the ACmbm.
| Reagents | Solar photo-Fenton | Ozonation | ||
|---|---|---|---|---|
| OCD | OCC+mbn | OCD | OCC+mbn | |
| € | € | € | € | |
| Fe(III) | 7200 | 3600 | — | — |
| H2O2 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
— | — |
| H2SO4 | 2200 | 1300 | — | — |
| EDDS | 255![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
— | — |
| Electric cost | ||||
| Pre-treatment | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Solar photo-reactor | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2600 | — | — |
| NF membrane | — | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
— | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
| O3 generation | — | — | 202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| Maintenance | ||||
| Reactors | 1800 | 900 | 1200 | 500 |
| NF membrane | — | 4100 | — | 4100 |
| Personnel | ||||
| CPC and ozone reactor | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
7200 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
4600 |
| NF membrane | 8100 | 8100 | ||
| Total OCs | 336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
188![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
231![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
113![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| € per m3 | 0.92 | 0.51 | 0.64 | 0.24 |
The highest operating costs are for EDDS and hydrogen peroxide for photo-Fenton, pumping through the NF membrane and O3 generation for ozonation. Note that in both treatments (solar photo-Fenton and ozonation), operating costs are substantially lower when combined with nanofiltration (case C). Total operating costs for ozonation fell from 0.64€ per m3 for treatment of D to 0.24€ per m3 for treatment of C, mainly because electricity costs were significantly less for effluent C, since the volumetric concentration factor was only 4. For solar photo-Fenton, total operating costs also fell from 0.92€ per m3 for D to 0.51€ per m3 for C as the concentration of EDDS (most expensive reagent) was substantially lower in C.
4.3. Total treatment costs
Table 9 shows the total costs for each process studied, where the unitary cost (per m3) was calculated taking into account the total annual volume of treated water (eqn (12) and (13)).| Solar photo-Fenton | Ozonation | |||||||
|---|---|---|---|---|---|---|---|---|
| D | C | D | C | |||||
| € | € per m3 | € | € per m3 | € | € per m3 | € | € per m3 | |
| AC | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.25 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.13 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.16 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.07 |
| ACmbm | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.11 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.11 | ||||
| OC | 336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.92 | 188![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.52 | 231![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.64 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.24 |
| TC | 427![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.17 | 276![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.76 | 291![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.80 | 154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.42 |
| TC (€ per m3), excluding the NF membrane system | 0.65 | 0.31 | ||||||
The results show that the treatment costs of effluent C were lower when both processes were applied (EDDS-assisted solar photo-Fenton and ozonation). These reductions result from the smaller reactor size and lower reagent consumptions, mainly due to the lower total volume in C than in D (VCF = 4) (see Table 9). The very considerable reduction in EDDS-assisted photo-Fenton was due to the smaller reactor size and lower EDDS consumption. Reduction of ozonation costs was mainly due to the reactor size needed and the ozone generator as these parameters are directly proportional to ozone treatment capability.28 TCs exclude the membrane system, as many MWTPs already have a tertiary treatment membrane system installed. Therefore, TCs are for the elimination of MCs in the membrane rejection stream already installed in the MWTP, avoiding accumulation of MCs in MWTPs and/or disposal into the environment. But costs are also related to flow rate and all costs included in Table 9 were estimated to be 1000 m3 per day, which means a low treatment flow rate corresponding to an equivalent of a population of 5000. If other treatment flows were used, TCs would be considerably lower, as shown in Table 10. These costs were calculated following the same sequence as in section 2.6. The highest cost reduction was observed in treatment of C where increasing photo-Fenton treatment capacity from 500 to 3000 m3 per day resulted in a 25% reduction in TC. Cost of ozonation of C was also substantially lower when treatment capacity was increased (41% from 500 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per day).
000 m3 per day).
| Flow (m3 per day) | Solar photo-Fenton | Ozonation | ||||
|---|---|---|---|---|---|---|
| € per m3 | € per m3 | € per m3 | € per m3 | € per m3 | € per m3 | |
| D | C | Excluding NF membrane system | D | C | Excluding NF membrane system | |
| 500 | 1.31 | 0.86 | 0.71 | 0.85 | 0.49 | 0.34 |
| 1000 | 1.17 | 0.76 | 0.65 | 0.80 | 0.42 | 0.31 |
| 2000 | 1.08 | 0.68 | 0.60 | 0.76 | 0.37 | 0.29 |
| 3000 | 1.04 | 0.64 | 0.57 | 0.74 | 0.35 | 0.27 |
| 5000 | 1.00 | 0.61 | 0.55 | 0.72 | 0.32 | 0.26 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.96 | 0.57 | 0.53 | 0.70 | 0.29 | 0.25 |
5. Conclusions
Economic evaluation of both chemical oxidation processes studied shows that: (i) use of a membrane system combined with photo-Fenton or ozonation leads to a reduction in total cost and operating cost of the oxidation process mainly due to the reduction in total AOP treatment volume; (ii) reductions in TCs when NF is combined with solar photo-Fenton and ozonation were similar, as the energy consumption for ozone generation and reagent consumption were substantially reduced in photo-Fenton by VFC = 4; (iii) photo-Fenton at neutral pH was more expensive than ozonation in both effluents (D and C); and (iv) the larger the treatment plant is, the more significant is the reduction in cost of NF retentate (C) treatment compared to direct treatment of the secondary effluent (D).Total costs depend on wastewater type, water flow rate and specific operating conditions of each treatment. Therefore, the procedure for calculating treatment costs included in this paper should be carefully applied case by case. Ozonation depends mainly on ozone generation, and therefore, ozone generator selection in the system design stage is extremely important. In solar photo-Fenton, minimizing reagent consumption, such as EDDS to work at circumneutral pH and hydrogen peroxide, has been proven to be effective. Finally, combination of these technologies is a suitable strategy for cost reduction.
Acknowledgements
The authors wish to thank the Regional Government of Andalusia (Project ref. RNM-1739) and the European Regional Development Fund (ERDF). Sara Miralles Ph.D. wishes to thank the Solar Energy Research Center for her post-doctoral position in Arica, Chile under the SERC-Chile FONDAP Project (Reference: 15110019).Notes and references
- R. Lopez-Serna, M. Petrovic and D. Barceló, Chemosphere, 2011, 85, 1390 CrossRef CAS PubMed.
- M. Gros, S. Rodriguez-Mozaz and D. Barceló, J. Chromatogr. A, 2012, 1248, 104 CrossRef CAS PubMed.
- F. Hernández, M. Ibáñez, R. Bade, L. Bijlsma and J. V. Sancho, TrAC, Trends Anal. Chem., 2014, 63, 140 CrossRef.
- M. Huerta-Fontela, M. T. Galceran and F. Ventura, J. Chromatogr. A, 2010, 1217, 4212 CrossRef CAS PubMed.
- B. Petrie, R. Barden and B. Kasprzyk-Hordern, Water Res., 2015, 72, 3 CrossRef CAS PubMed.
- L. D. Nghiem, A. I. Schäfer and M. Elimelech, Environ. Sci. Technol., 2004, 38, 1888 CrossRef CAS PubMed.
- A. Subramani and J. G. Jacangelo, Sep. Purif. Technol., 2014, 122, 472 CrossRef CAS.
- A. W. Mohammad, Y. H. Teow, W. L. Ang, Y. T. Chung, D. L. Oatley-Radcliffe and N. Hilal, Desalination, 2015, 356, 226 CrossRef CAS.
- M. Pontié, H. Dach, J. Leparc, M. Hafsi and A. Lhassani, Desalination, 2008, 221, 174 CrossRef.
- J. Fang and B. Deng, J. Membr. Sci., 2014, 453, 42 CrossRef CAS.
- F. Chang, W.-L. Liu and X.-M. Wang, Desalination, 2014, 334, 10 CrossRef CAS.
- K. Kimura, S. Toshima, G. Amy and Y. Watanabe, J. Membr. Sci., 2004, 245, 71 CrossRef CAS.
- A. J. C. Semião and A. I. Schäfer, J. Membr. Sci., 2011, 381, 132 CrossRef.
- L. D. Nghiem, A. I. Schäfer and M. Elimelech, Sep. Sci. Technol., 2005, 40, 2633 CrossRef CAS.
- D. B. Luiz, A. K. Genena, H. J. José, R. F. Moreira and H. F. Schröder, Water Sci. Technol., 2009, 60, 1869 CrossRef CAS PubMed.
- I. Oller, S. Malato and J. A. Sánchez-Pérez, Sci. Total Environ., 2011, 409, 4141 CrossRef CAS PubMed.
- J. O. Tijani, O. O. Fatoba, G. Madzivire and L. F. Petrik, Water, Air, Soil Pollut., 2014, 225, 2102 CrossRef.
- W. M. Samhaber and M. T. Nguyen, J. Nanotechnol., 2014, 5, 476 Search PubMed.
- H. T. Madsen, E. G. Søgaard and J. Muff, Chem. Eng. J., 2015, 276, 358 CrossRef CAS.
- L. Prieto-Rodríguez, I. Oller, N. Klamerth, A. Agüera, E. M. Rodríguez and S. Malato, Water Res., 2013, 47, 1521 CrossRef PubMed.
- Y. Yoon, Y. Hwang, M. Kwon, Y. Jung, T.-M. Hwang and J.-W. Kang, J. Ind. Eng. Chem., 2014, 20, 2801 CrossRef CAS.
- S. Miralles-Cuevas, A. Arqués, M. I. Maldonado, J. A. Sánchez-Pérez and S. Malato Rodríguez, Chem. Eng. J., 2013, 224, 89 CrossRef CAS.
- S. Malato, J. Blanco, A. Vidal, D. Alarcón, M. I. Maldonado, J. Cáceres and W. Gernjak, Sol. Energy, 2003, 75, 329 CrossRef CAS.
- S. Miralles-Cuevas, I. Oller, A. Ruiz Aguirre, J. A. Sánchez Pérez and S. Malato Rodríguez, Chem. Eng. J., 2014, 239, 68 CrossRef CAS.
- CADOX project: A coupled advanced oxidation-biological process for recycling of industrial wastewater containing persistent organic contaminants, http://www.psa.es/webeng/projects/cadox/index.php, (accessed December 2015) Search PubMed.
- J. A. Sánchez-Pérez, I. M. Román Sánchez, I. Carra, A. Cabrera Reina, J. L. Casas López and S. Malato, J. Hazard. Mater., 2013, 244–245, 195 CrossRef PubMed.
- W. D. Seider, J. D. Seader and D. R. Lewin, Product and Process Design Principles: Synthesis, Analysis and Evaluation, 2nd edn, Wiley, New York, 2004 Search PubMed.
- P. Cañizares, R. Paz, C. Sáez and M. A. Rodrigo, J. Environ. Manage., 2009, 90, 410 CrossRef PubMed.
- M. Nilsson, F. Lipnizki, G. Trägårdh and K. Östergren, Sep. Purif. Technol., 2008, 60, 36 CrossRef CAS.
- S. Miralles-Cuevas, I. Oller, J. A. S. Pérez and S. Malato, Water Res., 2014, 64, 23 CrossRef CAS PubMed.
- S. Miralles-Cuevas, F. Audino, I. Oller, R. Sánchez-Moreno, J. A. Sánchez Pérez and S. Malato, Sep. Purif. Technol., 2014, 122, 515 CrossRef CAS.
- J. J. Pignatello, E. Oliveros and A. MacKay, Crit. Rev. Environ. Sci. Technol., 2006, 36, 1 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |