Fermentation of bioactive solid lipid nanoparticles by human gut microflora
Ana Raquel
Madureira
 *a,
Débora
Campos
a,
Beatriz
Gullon
a,
Cláudia
Marques
b,
Luís M.
Rodríguez-Alcalá
a,
Conceição
Calhau
bcd,
Jose Luis
Alonso
g,
Bruno
Sarmento
cdef,
Ana Maria
Gomes
a and
Manuela
Pintado
a
*a,
Débora
Campos
a,
Beatriz
Gullon
a,
Cláudia
Marques
b,
Luís M.
Rodríguez-Alcalá
a,
Conceição
Calhau
bcd,
Jose Luis
Alonso
g,
Bruno
Sarmento
cdef,
Ana Maria
Gomes
a and
Manuela
Pintado
a
aCBQF – Centro de Biotecnologia e Química Fina – Laboratório Associado, Escola Superior de Biotecnologia, Universidade Católica Portuguesa/Porto, Rua Arquiteto Lobão Vital, Apartado 2511, 4202-401 Porto, Portugal. E-mail: rmadureira@porto.ucp.pt; Tel: +3515580044
bDepartamento de Bioquímica, Faculdade de Medicina, Universidade do Porto, 4200-319, Porto, Portugal
cCINTESIS, Centro de Investigação em Tecnologias e Sistemas de Informação em Saúde, 4200-450 Porto, Portugal
dCICS, Department of Pharmaceutical Sciences, Institute of Health Sciences-North, CESPU, Rua Central de Gandra, 1317, 4585-116 Gandra, Portugal
eI3S, Instituto de Investigação e Inovação em Saúde, 4150-180 Porto, Portugal
fINEB, Institute of Biomedical Engineering, University of Porto, Rua do Campo Alegre, 823, 4150-180 Porto, Portugal
gDepartment of Chemical Engineering, Faculty of Science, University of Vigo (Campus Ourense), As Lagoas, 32004 Ourense, Spain
First published on 19th October 2015
Abstract
Solid lipid nanoparticles (SLNs) can be used for oral delivery of phenolic compounds in order to protect them from the harsh conditions of digestion and improve their bioavailability in the intestinal epithelium. Recently, the production and characterization of SLNs loaded with rosmarinic acid (RA) and herbal extracts was performed for future use as functional food ingredients. Diet components have been shown to have a huge impact on gut microbiota viability and metabolic activity. Hence, SLNs loaded with RA, sage and savoury extracts have been evaluated for their effect on intestinal microbiota growth and the metabolic products generated. Fermentations in anaerobic batch cultures using volunteer human faeces were performed during 24 h. Dynamic bacterial population changes were analysed using PCR-real time, as well as the generation of fatty acids and the quantification of phenolic compounds by analytical methods. Solid lipid nanoparticles released phenolic compounds at non-inhibitory bacterial growth concentrations. Released herbal extract phenolic compounds showed a beneficial effect on gut microbiota growth (e.g. bifidogenic effects) and were used as substrates. Acetate, formate, lactate and butyrate were produced in higher concentrations. The released phenolic compounds also induced PUFA and trans fatty acids metabolic activity, the production of saturated fatty acids, as well of potential beneficial conjugated linoleic acid isomers. Solid lipid nanoparticles modulate gut microbiota and metabolic activities.
1. Introduction
Solid lipid nanoparticles (SLNs) were studied as delivery systems of rosmarinic acid (RA) and herbal extracts for the development of oral formulations.1,2 These systems can bring advantages such as protection from the harsh gastrointestinal conditions, a controlled compound release and an increase of compound bioavailability at the intestine. Rosmarinic acid is a natural polyphenol carboxylic acid, an ester of caffeic acid with 3,4-dihydroxyphenyllactate. A number of potential biological properties are associated with this compound, such as antioxidant, anti-mutagenic, anti-bacterial and anti-viral capabilities. Extracts from sage and savoury (common English names for Salvia sp. and Satureja montana, respectively) herbs can be used as natural sources of RA.3,4 The resulting SLNs loaded with RA at production time had mean diameters between 270 and 1000 nm, with ca. 99% association efficiencies and were highly stable.1,2 The production process used two different types of waxes – Witepsol® and carnauba. Witepsol® wax is usually used in the pharmaceutical industry as an excipient, but it is also approved for human consumption. Carnauba wax or Brazilian wax is naturally extracted from leaves of a palm tree known as Copernicia cerifera, a native plant of north-eastern Brazil, and used by several industries for different purposes, such as gelling, releasing and glazing agents.5–8Human gut microbiota metabolize nutrients and other compounds supplied through diet. In the specific case of RA, this phenolic compound is not absorbed in the stomach and reaches the colon, undergoing hydrolysis in the small intestine and releasing aglycones, oligomers by microbial glycosidases and esterases enhancing their absorption.9 Also, no studies are available on the effects on gut microbiota health and metabolic activities by lipidic nanoparticles. Animal and in vitro studies have shown that intestinal microbiota can regulate host lipid metabolism via numerous microbial activities.10 Modulation of gut microbiota through diet to enhance host health and to reduce the incidence of obesity and associated disorders are important research lines.11
Since the incorporation of SLNs in oral formulations is thought to the future, the effect of these systems on the metabolic activity of gut bacteria is an important subject that has to be evaluated. Few studies have been done concerning nanoparticle ingestion, but they are predominantly related to in vivo agricultural animals, with high variability levels in the results.12 Hence, it is imperative to assess in vitro the effects of administered nanoparticles on gut microbes from human faeces.13
Thus, for the first time the gut microbiota fermentation of SLNs produced with two different waxes, Witepsol® and carnauba, and loaded with RA and herbal extracts was performed. Evolution of the effects on growth of the major gut bacteria groups was followed, as well the metabolic activity on loaded phenolic compounds and the assessment of organic and fatty acids generated. In addition, free RA and herbal extracts effects on gut microbiota, which have never been studied, were analysed, bringing an additional output from the execution of this experimental research work.
2. Materials and methods
2.1. Solid lipid nanoparticle production
Solid lipid nanoparticles loaded with RA were produced according Campos et al.1 and Madureira et al.2 SLNs were produced with sage and savoury extracts according the same procedures. For extract preparation, leaves of sage (Salvia officinalis) and savoury (Satureja montana) were ground into a powder, then 1 g of powder was collected and added to 110 mL of boiling deionised water. The mixture was left to cool by mixing with a magnetic stirrer for 5 min. The solution was then filtered through no. 1 filter paper (V. Reis, Portugal), frozen and finally submitted to freeze-drying. The freeze-drying process was performed using a Vacuum Freeze Drier (Model FT33, Armefield, UK) under a vacuum pressure of 100 mTorr, with a freezing chamber temperature of −46 °C and 15 °C in the sample chamber. SLNs were prepared using a hot melt ultrasonication method by loading RA (Sigma-Aldrich Chemistry, St. Louis, Missouri, USA) and herbal extracts at a final concentration of 0.15 mg RA per mL; Witepsol® (Sasol, Hamburg, Germany) and carnauba (Sigma-Aldrich) waxes were added at 0.5% (w/v) and polysorbate 80 (Sigma-Aldrich) at 2% (v/v). Waxes were warmed up to a temperature of 5 °C above melting point – i.e. 36 °C for Witepsol® and 86 °C for carnauba wax – and then the RA solution (15 mg mL−1) and herbal extracts were added to the matrix. Herbal extracts were added taking into account that 1 g of extract contains ca. 0.58 mg RA per ml. Finally, the battery of testing samples was the following: free RA, free sage and savoury extract, empty Witepsol® SLNs (WSLNs), empty carnauba SLNs (CSLNs), loaded Witepsol® SLNs with RA (WSLN_RA), loaded carnauba SLNs with RA (CSLN_RA), loaded Witepsol® SLNs with sage extract (WSLN_Sage), loaded carnauba SLNs with sage extract (CSLN_Sage), loaded Witepsol® SLNs with savoury extract (WSLN_Savoury) and loaded carnauba SLNs with savoury extract (CSLN_Savoury). Production was performed in triplicate. The emulsions were added with manitol at 10% (m/v) as cryoprotectant and subject to freeze-drying with the same conditions used for the extracts. Finally, all the dried powders of all the samples (SLNs empty and loaded, free RA and extracts) were stored in opaque flasks, protected by light, at room temperature and in a desiccator to control humidity.2.2. In vitro fermentation assays
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 rpm, 10 min), and the pellets and supernatants were collected for further analysis (see below). All additions and inoculations were carried out inside an anaerobic cabinet (5% H2, 10% CO2 and 85% N2). All experiments were performed in compliance with the relevant laws and institutional guidelines.
200 rpm, 10 min), and the pellets and supernatants were collected for further analysis (see below). All additions and inoculations were carried out inside an anaerobic cabinet (5% H2, 10% CO2 and 85% N2). All experiments were performed in compliance with the relevant laws and institutional guidelines.
2.3. Gut microbiota evaluation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 1 min. Then, 25 μL of proteinase K was added to 200 μL of the supernatant for incubation at 70 °C for 10 min. The remaining steps followed the manufacturer's instructions. DNA purity and quantification were assessed with a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
000 g for 1 min. Then, 25 μL of proteinase K was added to 200 μL of the supernatant for incubation at 70 °C for 10 min. The remaining steps followed the manufacturer's instructions. DNA purity and quantification were assessed with a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratio was obtained by dividing the number of copies of Firmicutes divisions by the number of copies of Bacteroidetes divisions.
B ratio was obtained by dividing the number of copies of Firmicutes divisions by the number of copies of Bacteroidetes divisions.
| Target group | Primer sequence (5′–3′) | Genomic DNA standard | PCR product Size (bp) | AT | Ref. |
|---|---|---|---|---|---|
| AT, annealing temperature; b.p., base pairs. | |||||
| Universal | AAA CTC AAA KGA ATT GAC GG CTC ACR RCA CGA GCT GAC | Bacteroides vulgatus ATCC 8482 | 180 | 62 °C | 14 |
| Firmicutes | ATG TGG TTT AAT TCG AAG CA AGC TGA CGA CAA CCA TGC AC | Lactobacillus gasseri ATCC 33323 | 126 | 60 °C | 15 |
| Bacteroidetes | CAT GTG GTT TAA TTC GAT GAT AGC TGA CGA CAA CCA TGC AG | Bacteroides vulgatus ATCC 8482 | 126 | 60 °C | 15 |
| Lactobacillus spp. | GAG GCA GCA GTA GGG AAT CTT CGGC CAG TTA CTA CCT CTA TCC TTC TTC | Lactobacillus gasseri ATCC 33323 | 126 | 60 °C | 16 |
| Roseburia spp. | TAC TGC ATT GGA AAC TGT CG CGG CAC CGA AGA GCA AT | Roseburia hominis A2-183 | 230 | 60 °C | 17 |
| Bacteroides spp. | ATA GCC TTT CGA AAG RAA GAT CCA GTA TCA ACT GCA ATT TTA | Bacteroides vulgatus ATCC 8482 | 501 | 60 °C | 18 |
| Bifidobacterium spp. | CGC GTC YGG TGT GAA AG CCC CAC ATC CAG CAT CCA | Bifidobacterium longum subsp. Infantis ATCC 15697 | 244 | 60 °C | 16 |
| Clostridium leptum | GCA CAA GCA GTG GAG T CTT CCT CCG TTT TGT CAA | Clostridium leptum ATCC 29065 | 239 | 60 °C | 19 |
2.4. Determination of fermentation products
2.4.2.1. Fatty acid analyses assay. For the analysis of the total fatty acid composition, 500 mg of faeces and 10 mg of SLNs were accurately weighed and prepared as described elsewhere.20 For quantification purposes the samples were added to 50 μL of tritridecanoin (1.34 mg mL−1 in hexane) prior to derivatization and the extracts were added to 100 μL of methyl undecanoate (1.4 mg mL−1 in hexane). FAME were analysed in a gas chromatograph HP6890A (Hewlett-Packard, Avondale, PA, USA) equipped with a flame-ionization detector (GLC-FID) and a BPX70 capillary column (50 m × 0.32 mm × 0.25 μm; SGE Europe Ltd, Courtaboeuf, France). Analysis conditions were as follows: injector (split 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; injection volume 1 μL) and detector temperatures were 250 °C and 270 °C, respectively; carrier gas was hydrogen (11 psi) and the oven temperature program started at 60 °C (hold 2 min), raised 10 °C min−1 to 135 °C (hold 2 min), then 10 °C min−1 to 165 °C (hold 2 min) and finally 10 °C min−1 to 230 °C (hold 7 min). Supelco 37 and CRM-164 were used for the identification of FA. GLC-Nestlé36 was assayed for the calculation of response factors and the detection and quantification limits (LOD: 0.079 μg FA per mL; LOQ: 0.264 μg FA per mL).
1; injection volume 1 μL) and detector temperatures were 250 °C and 270 °C, respectively; carrier gas was hydrogen (11 psi) and the oven temperature program started at 60 °C (hold 2 min), raised 10 °C min−1 to 135 °C (hold 2 min), then 10 °C min−1 to 165 °C (hold 2 min) and finally 10 °C min−1 to 230 °C (hold 7 min). Supelco 37 and CRM-164 were used for the identification of FA. GLC-Nestlé36 was assayed for the calculation of response factors and the detection and quantification limits (LOD: 0.079 μg FA per mL; LOQ: 0.264 μg FA per mL).
2.4.2.2. Determination of short chain fatty acids and lactate. Supernatants from the test anaerobic culture tubes inoculated with samples were filtered through 0.20 μm cellulose acetate membranes. Aliquots of filtered samples were assayed for organic acids (lactic, acetic, propionic and butyric acids) by HPLC-RI using an Aminex HPX-87H column (BioRad, Hercules, CA, USA) operated at 60 °C (mobile phase 0.003 M H2SO4, flow rate 0.6 mL min−1).
2.5. Mathematical and statistical analysis
The relative difference to control % was calculated using the following equation:where SMC is the mean copy numbers of the sample at a certain time (8 or 24 h) and CMC is the mean copy numbers of the control sample at the same time as SMC. Positive % values means the occurrence of an increase in the number of copies relative to the control sample at that certain time. The higher the value, the higher increase.
Regarding fatty acids, all the results are expressed as the mean and standard deviations. In a first instance, an exploratory analysis of the data was performed to test the normal distribution and homogeneity of variance (Levene's test). The data were analysed according to Kruskal–Wallis’ test using Mann–Whitney's test post hoc. All analyses were performed using the SPSS Statistics software v22.0 for Mac (IBM, Armonk, NY, USA). Level of significance was set at P < 0.05.
Short chain fatty acids increment concentrations were calculated by the difference between the concentration of SCFA at a certain time (8 and 24 h) and the concentration of SCFA at time 0 h. Both microbiota groups and the SCFA evolution and production over time were subject to statistical analyses using non-parametric tests Mann–Witney U.
3. Results and discussion
The SLNs used in the present study were previously characterized for their particle size and zeta potential. At the time the WSLN particle sizes were in the range 310–950 nm and the CSLN in the range 300–1000 nm. The zeta potential values were in the range of −10 to −35 mV for WSLN and −12 to −39 mV for CSLN. The association efficiencies % of the RA and herbal extracts were ca. 98%.1,23.1. Evolution of the gut microbiota profile groups
In order to understand the evolution of the growth and metabolic activities during fermentation, samples at 0, 8 and 24 h were taken and analysed. In Table 2 is depicted the composition of average copy numbers obtained by PCR real time of the human gut microbiota main groups. Three of the four dominant phyla in the human gut were evaluated, viz. Firmicutes, Bacteroidetes and Actinobacteria.21 Numbers are according the ones found in healthy human volunteers’ faeces, e.g. Lactobacillus spp. which is usually found in low number in normal gut microbiota.22| Division (genus) | Number of copies (n = 3)a |
|---|---|
| a Values are presented as mean ± SD and expressed as log10 16S rRNA gene copies per 20 ng of DNA. | |
| Universal | 5.90 ± 0.416 |
| Firmicutes | 6.05 ± 0.487 |
| Clostridium leptum | 5.12 ± 1.06 |
| Lactobacillus | 2.63 ± 0.526 |
| Roseburia hominis | 4.92 ± 0.470 |
| Bacteroidetes | 5.34 ± 0.247 |
| Bacteroides | 5.06 ± 0.957 |
| Actinobacteria | |
| Bifidobacterium | 6.17 ± 0.277 |
F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratio B ratio |
1.13 ± 0.241 |
In Fig. 1 are represented the relative differences % between the microbiota groups of the tested and control faeces during 8 and 24 h of fermentation. Free RA exerted a positive effect in Universal, Lactobacillus and Bacteroidetes groups (Fig. 1a, d and f). Sage and savoury extracts only in Universal and Bacteroidetes groups (Fig. 1a and f). In some groups, the effect of the free extracts is positive and at the end of fermentation (24 h) is null or negative.
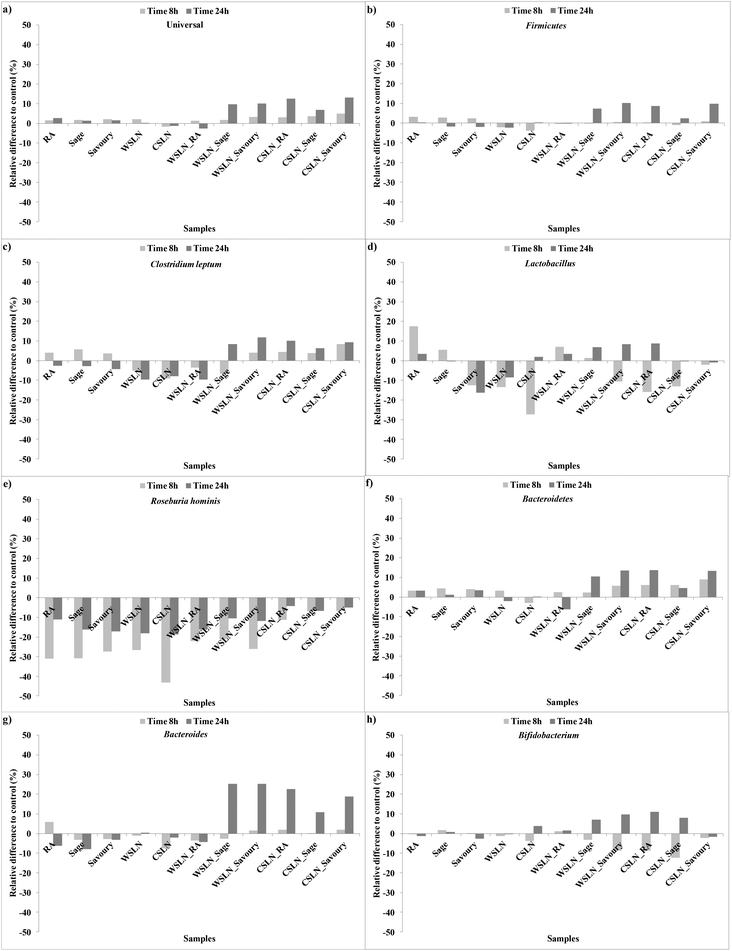 | ||
| Fig. 1 Evolution of the relative differences to control faeces (%) of the gut microbiota groups during fermentation times of 8 and 24 h. | ||
Empty SLNs were shown to have a negative effect on gut microbiota growth and viability, since in some cases the negative increment was higher than the control, which shows a bactericidal or toxic effect of the SLNs. Solid lipid nanoparticles loaded with RA induced growth of the Bifidobacterium and Lactobacillus groups until the end of fermentation (Fig. 1d and h). All SLNs loaded with herbal extracts had a positive effect on the growth of all the bacterial groups except Roseburia. The most expressive growth was in the specific case of Bacteroides (ca. 10–25%) in faeces with SLNs loaded with the herbal extracts and CSLN_RA (Fig. 1g). These results are very important since high fat diets have been related with decreases in Bacteroides and Bifidobacterium group bacteria.23 This is in agreement with the results obtained for empty SLNs, and the loading of SLNs with herbal extracts beneficiates the impact of the SLNs on gut bacteria.
In the special case of Roseburia hominis all the results were negative relative to the control (Fig. 1e). Decreases in Roseburia were already associated with low carbohydrate diets,23 but in the present study the control samples registered growth for this group, even not being supplemented with carbohydrate sources. Nevertheless, a lower bacteriostatic effect was detected for SLNs loaded with extracts. Hence, the negative effect could be attributed to concentrations of extracts phenolic compounds that are released from the SLNs.
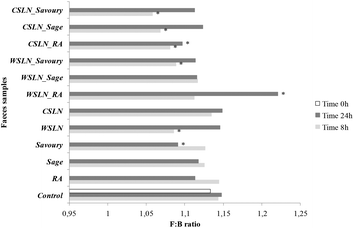
The F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratio evolution was also evaluated for all samples during the fermentation period (Fig. 2). This ratio is calculated using the number of Firmicutes and Bacteroidetes, since these two groups are the most predominant phyla in the human colon and together comprise 90% of the total gut microbiota.24 Hence, their proportion can give us a global idea of the total effect of SLNs and free compounds on gut microbiota. Up to 24 h of fermentation the F
B ratio evolution was also evaluated for all samples during the fermentation period (Fig. 2). This ratio is calculated using the number of Firmicutes and Bacteroidetes, since these two groups are the most predominant phyla in the human colon and together comprise 90% of the total gut microbiota.24 Hence, their proportion can give us a global idea of the total effect of SLNs and free compounds on gut microbiota. Up to 24 h of fermentation the F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratio values of empty SLNs and WSLN_RA increased and were higher than the F
B ratio values of empty SLNs and WSLN_RA increased and were higher than the F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B values of the control samples (P < 0.05). In the remaining samples, the ratios were similar or even lower. These ratio values increased after 24 h fermentations in faeces with encapsulated forms and decrease where free forms were used. F
B values of the control samples (P < 0.05). In the remaining samples, the ratios were similar or even lower. These ratio values increased after 24 h fermentations in faeces with encapsulated forms and decrease where free forms were used. F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratio values can be associated with obesity states, not without some controversial discussion around this subject, since obesity is associated with changes in abundance at the level of phylum, genus or species of gut microbiota. For example, in some human studies, weight loss in obese participants resulted in a decrease of the F
B ratio values can be associated with obesity states, not without some controversial discussion around this subject, since obesity is associated with changes in abundance at the level of phylum, genus or species of gut microbiota. For example, in some human studies, weight loss in obese participants resulted in a decrease of the F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratio from obese to lean patterns.25,26 The mutual influence of gut flora composition and overall weight conditions is related to differences in the energy-reabsorbing potential of different ratios of Firmicutes and Bacteroidetes, especially in the digestion of dietary fats and carbohydrates.26 In the present study, evaluation of the metabolic activity of the gut microbiota on phenolic compounds and lipids used in SLN production was done and is discussed below.
B ratio from obese to lean patterns.25,26 The mutual influence of gut flora composition and overall weight conditions is related to differences in the energy-reabsorbing potential of different ratios of Firmicutes and Bacteroidetes, especially in the digestion of dietary fats and carbohydrates.26 In the present study, evaluation of the metabolic activity of the gut microbiota on phenolic compounds and lipids used in SLN production was done and is discussed below.
3.2. Phenolic compound production during fermentation
In Table 3 are depicted the concentrations of phenolic compounds, viz. RA, rutin and ferulic acid present in fermented samples. Sage and savoury herbal extracts have these phenolic compounds in their composition justifying their use as a natural source of these ones.27| Samples | Concentration (mg mL−1) ± SD | |||
|---|---|---|---|---|
| Time | Rosmarinic acid | Rutin | Ferulic acid | |
| *The differences of the concentration means of each compound (rosmarinic, ferulic acids and rutin) in each sample as a function of time are statistically significant (P < 0.05). n.d – not detected. | ||||
| Free rosmarinic acid | 0 | 1.61 ± 0.0241 | n.d | n.d |
| 8 | 1.23 ± 0.0167 | n.d | n.d | |
| 24 | 0.900 ± 0.0191* | n.d | n.d | |
| Free sage extract | 0 | 0.981 ± 0.0291 | 0.472 ± 0.0189 | 0.0513 ± 0.00121* |
| 8 | 0.687 ± 0.0153* | 0.401 ± 0.246 | 0.023 ± 0.00151 | |
| 24 | 0.253 ± 0.0729* | n.d | 0.023 ± 0.00263 | |
| Free savoury extract | 0 | 0.901 ± 0.129 | 0.413 ± 0.0381 | 0.017 ± 0.0900 |
| 8 | 0.560 ± 0.0811* | 0.411 ± 0.0129 | 0.016 ± 0.00391 | |
| 24 | 0.240 ± 0.0113* | n.d | n.d | |
| WSLN_RA | 0 | n.d | n.d | n.d |
| 8 | 0.399 ± 0.0281 | n.d | n.d | |
| 24 | 0.413 ± 0.0761 | n.d | n.d | |
| CSLN_RA | 0 | n.d | n.d | n.d |
| 8 | 0.691 ± 0.0129 | n.d | n.d | |
| 24 | 0.687 ± 0.0101 | n.d | n.d | |
| WSLN_Sage | 0 | n.d | n.d | n.d |
| 8 | 0.164 ± 0.0281 | n.d | 0.0241 ± 0.0012* | |
| 24 | 0.163 ± 0.0156 | 0.310 ± 0.0691* | n.d | |
| CSLN_Sage | 0 | n.d | n.d | n.d |
| 8 | 0.314 ± 0.0390 | n.d | 0.0120 ± 0.0051 | |
| 24 | 0.235 ± 0.0392 | 0.280 ± 0.0520* | 0.0230 ± 0.00691 | |
| WSLN_Savoury | 0 | n.d | n.d | n.d |
| 8 | 0.133 ± 0.0293 | n.d | 0.0140 ± 0.0092* | |
| 24 | 0.144 ± 0.0250 | 0.290 ± 0.0391* | 0.0070 ± 0.001 | |
| CSLN_Savoury | 0 | n.d | n.d | n.d |
| 8 | 0.0796 ± 0.0102* | n.d | 0.035 ± 0.00200* | |
| 24 | 0.133 ± 0.0502 | 0.090 ± 0.0111* | 0.014 ± 0.00570 | |
In general, in faeces fermented with free RA and herbal extracts, a significant decrease in RA concentration was detected during 24 h of fermentation (P < 0.05), indicating that RA is used by gut bacteria metabolic activities. In the specific case of herbal extracts, concentrations of RA, rutin and ferulic acid significantly decreased during fermentation (P < 0.05). Nevertheless, these concentrations had a bacteriostatic effect on gut bacteria, as can be seen in Fig. 1, and as discussed before only Bacteroidetes showed slight increases in their numbers during fermentation. Taking into account that is supposed to have initially 2.14 mg RA per mL, the real amount of RA present in the faeces at time 0 h of fermentation was lower than desired. These obtained values could be a result of the extraction method. Nevertheless, all the samples underwent the same treatment, allowing conclusions to be made about their evolution during fermentation periods. Hence, RA is at ca. 1.61 mg mL−1 at the beginning of fermentation. Even if this concentration is lower than the minimal inhibitory concentration (MIC) found for several microorganisms (3.5–5.5 mg mL−1)28 no growth was observed, showing a bacteriostatic effect. The same results were observed when using extracts.
In the case of encapsulated forms, a release of RA is observed in all samples. In samples with SLN_RA, release of RA at 24 h reaches 0.41 and 0.69 mg mL−1 in WSLNs and CSLNs, respectively. In the case of sage extracts, WSLNs and CSLNs release are ca. 0.16 and 0.24 mg mL−1, respectively. Rutin is also released from SLNs loaded with herbal extracts, reaching ca. 0.30 mg mL−1 in all samples, with the exception of CSLN_Savoury (0.09 mg mL−1). Ferulic acid is released from WSLNs loaded with herbal extracts, especially after 8 h of fermentation and at very low concentration values, and disappears after 24 h of fermentation. In contrast, no inhibition was found when the same concentrations were encapsulated (Fig. 1). This can be due to the lower concentrations of phenolic compounds released by SLNs that do not have a bacteriostatic effect on bacterial growth.
Polyphenols could have beneficial effects like prebiotics (growth-promoting) on transient diet-derived beneficial bifidobacteria, which would in turn increase the retention of the bifidobacteria in the gut and could optimise the overall microbial balance.29 Some of these compounds can exert prebiotic-like effects or they can exert antimicrobial or bacteriostatic activities as already observed.30 In the case of the prebiotic-like effect, the numbers of Bifidobacterium are higher in faeces with SLNs loaded with herbal extracts (Fig. 1h), which indicates that the release of phenolic compounds at lower concentrations than the free compounds is beneficial for this group.
3.3. Fatty acid metabolic activity during fermentation
Since the present study uses nanoparticles produced with lipidic waxes, it is important to assess how gut microbiota metabolize these lipids or if they produce other lipid metabolites. Lipid metabolism by gut microbiota generates multiple fatty acid species, such as conjugated fatty acids and trans-fatty acids, that can affect the host lipid metabolism.31In Table 4 is depicted the composition of fatty acids of the Witepsol® and carnauba ingredients used in the production of SLNs. The composition is according to the manufacturers of the waxes. Saturated fatty acids have no double bonds between the individual carbon atoms of the fatty acids chain. Witepsol® SLNs contain more saturated fatty acids (SFAs) than CSLNs (34.42 and 9.59 μg fatty acids per mg sample, respectively), owing to the high concentration of lauric (C12) and myristc (C14) acids in Witepsol® wax. There is also a contribution of unsaturated fatty acids, i.e. with two carbon atoms in the chain that are bound next to either side of the double bond and by a cis or trans configuration. When fatty acids contain just one double bond, they are called monounsaturated fatty acids (MUFA). In both waxes the presence of oleic acid (C18:1 c9) is in the same range of concentrations (ca. 30 μg fatty acid per mg sample). In contrast, the polyunsaturated fatty acids (PUFAs), namely eicosapentaenoic acid (C22:5 n3, EPA) that contain more than one double bond, are in higher concentrations in carnauba than in Witepsol®. Since PUFAs are considered to have more potential toxicity than SFAs, the CSLNs have to be carefully studied in terms of toxicity effects.32
| Witepsol® | Carnauba | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| c: cis double bond; t: trans double bond; SFAs: total saturated fatty acids; MUFAs: total monounsaturated fatty acids; PUFAs: total polyunsaturated fatty acids; n.d – not detected; n.a – not applicable. | ||||
| C12 | 13.06 | 0.03 | n.d | n.a |
| C14 | 5.71 | 0.07 | n.d | n.a |
| C16 | 7.20 | 0.07 | 2.44 | 0.13 |
| C16:1 c9 | 0.49 | 0.08 | 0.45 | 0.02 |
| C18 | 6.26 | 0.01 | 0.92 | 0.06 |
| C18:1 c9 | 30.81 | 0.05 | 32.21 | 1.46 |
| C18:1 11c | 1.31 | 0.02 | 1.42 | 0.15 |
| C22 | 2.20 | 0.25 | 2.96 | 0.09 |
| C18:4 | 1.81 | 0.06 | 1.98 | 0.10 |
| C22:5 n3 | n.d | n.a | 1.07 | 0.03 |
| C24 | n.d | n.a | 3.26 | 0.11 |
| μg mg−1 | 68.84 | 0.30 | 46.73 | 2.15 |
| SFAs | 34.42 | 0.23 | 9.59 | 0.40 |
| MUFAs | 32.60 | 0.01 | 34.09 | 1.62 |
| PUFAs | 1.81 | 0.06 | 3.05 | 0.13 |
In terms of fatty acid size chains, WSLNs contain a mixture of short, medium and long fatty acids below stearic acid (C18), while CSLNs contain fatty acids with a number of carbons higher than caproic acid (C6).
| Time (h) | Empty SLN | WSLN_RA | WSLN_Sage | WSLN_Savoury | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 24 | 8 | 24 | 8 | 24 | 8 | 24 | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| (c: cis double bond; t: trans double bond; SFAs: saturated fatty acids; MUFAs: monounsaturated fatty acids; PUFAs: polyunsaturated fatty acids). *Values within a column for significant differences during 8 vs. 24 h; A, B, C, D, E, F and G within a row for significant differences among groups. n.a – not applicable. | ||||||||||||||||
| SFA | A 2700 | 278.9 | AB 2994 | 317.4 | B 2062 | 200.41 | A 2643 | 412.3 | B 1888 | 188.7 | B 2296 | 268.9 | B 2295 | 345.6 | AB 2955 | 193.8 |
| C4 | B 64.14* | 8.09 | 560.83* | 117.5 | B 77.60* | 9.71 | 403.7* | 79.25 | AB 98.74* | 13.34 | 374.9* | 55.38 | A 117.9* | 20.51 | 659.4* | 66.73 |
| C6 | A 4.44* | 1.36 | 12.41 | 4.71 | A 3.98 | 0.27 | 4.71 | 1.07 | B < LOQ* | n.a | 7.56 | 2.84 | A 4.56 | 1.23 | 6.91 | 3.43 |
| C7 | A 3.72 | 0.08 | 3.74 | 0.06 | B < LOD | n.a | < LOQ | n.a | C < LOD* | n.a | 3.70 | 0.27 | A 3.76 | 0.37 | 3.80 | 0.09 |
| C8 | A 6.98 | 0.19 | E 6.04 | 0.85 | B 4.60 | 0.20 | F 3.70 | 0.06 | C < LOQ | n.a | F < LOQ | n.a | B 5.00 | 0.27 | F 4.94 | 0.19 |
| C10 | 5.77 | 0.31 | 5.23 | 0.33 | 4.74 | 0.72 | 4.30 | 0.77 | 3.83 | 0.17 | 3.88 | 0.34 | 4.40 | 0.37 | 4.62 | 0.18 |
| C12 | A 1199 | 124.9 | 1074 | 68.96 | B 900.4 | 89.06 | 975.5 | 169.6 | B 817.3* | 70.76 | 849.1* | 80.94 | AB 909.4 | 122.2 | 968.0 | 41.65 |
| C14 | 459.6 | 54.83 | 425.2 | 37.52 | 332.6 | 37.90 | 396.5 | 62.05 | 308.9 | 27.30 | 351.4 | 34.67 | 358.1 | 61.83 | 386.8 | 23.44 |
| C16 | A 536.4 | 43.62 | 500.1 | 42.51 | AB 425.8 | 33.48 | 469.7 | 53.42 | AB 367.9 | 45.01 | 385.1 | 49.40 | A 518.8 | 72.64 | 528.8 | 34.15 |
| C17i | <LOQ | n.a | <LOQ | n.a | <LOD | n.a | <LOQ | n.a | <LOQ | n.a | <LOQ | n.a | 3.80 | 0.34 | <LOQ | n.a |
| C17 | AB 4.35 | 0.12 | 4.11 | 0.33 | B 4.06 | 0.25 | 3.74 | 0.08 | B < LOQ | n.a | <LOQ | n.a | A 4.99 | 0.31 | 5.44 | 0.79 |
| C18 | A 400.0 | 44.83 | 387.2 | 43.15 | AB 296.0 | 28.07 | 368.7 | 44.54 | B 275.0 | 26.52 | 314.3 | 44.16 | AB 343.5 | 62.79 | 365.9 | 21.08 |
| C20 | 9.44 | 0.26 | 9.11 | 0.74 | 7.90 | 0.34 | 7.94 | 0.34 | 7.64 | 2.89 | 6.12 | 0.88 | 10.85 | 1.25 | 10.59 | 0.64 |
| C22 | <LOQ | n.a | <LOQ | n.a | <LOQ | n.a | <LOQ | n.a | 4.54 | 1.83 | <LOQ | n.a | 4.17 | 0.43 | 3.91 | 0.22 |
| C26 | 5.93 | 0.26 | 5.88 | 0.74 | 4.23 | 0.41 | 4.05 | 1.07 | 4.00 | 0.88 | <LOQ | n.a | 5.98 | 1.07 | 6.10 | 1.21 |
| MUFA | 2723 | 16.92 | 2439 | 324.8 | 2698 | 112.2 | B 2311 | 72.08 | 1967 | 293.2 | C 1580 | 252.8 | B 3661 | 270.2 | B 3365 | 164.8 |
| C14:1 c9 | 3.91 | 0.19 | 3.72 | 0.07 | 4.23 | 0.24 | 3.85 | 0.10 | <LOQ | n.a | <LOQ | n.a | 5.47 | 0.21 | 5.41 | 0.35 |
| C16:1 t9 | A 9.37 | 0.21 | 8.44 | 0.94 | D <LOD | n.a | <LOQ | n.a | C < LOQ | n.a | <LOD | n.a | B 3.76 | 0.37 | 3.69 | 0.01 |
| C16:1 c7 | BC 5.35 | 0.06 | F 4.98 | 0.62 | B 6.18* | 0.46 | F 4.92* | 0.51 | C 4.29 | 0.64 | F 3.85 | 0.05 | A 7.79 | 0.28 | E 7.40 | 0.39 |
| C16:1 c9 | BC 41.28 | 0.20 | FG 36.18 | 5.14 | B 48.62* | 1.87 | F 40.19* | 2.15 | C 35.36 | 4.91 | G 29.33 | 4.33 | A 63.60 | 3.16 | E 59.77 | 2.75 |
| C17:1 c10 | BC 7.20 | 0.23 | 7.95 | 1.53 | B 8.30 | 0.32 | 8.41 | 1.33 | C 6.15 | 0.91 | 6.12 | 0.16 | A 10.83 | 0.62 | 10.93 | 0.87 |
| C18:1 t9 | 467.2 | 4.06 | 428.8 | 45.76 | 74.88 | 1.54 | 68.74 | 4.22 | 59.71 | 18.10 | 1.33 | 0.05 | 176.9 | 18.25 | 172.6 | 13.58 |
| C18:1 t10 | A 28.40 | 1.43 | 26.33 | 4.12 | C 12.01 | 1.95 | 10.39 | 2.27 | C 9.02 | 1.42 | 10.33 | 3.82 | B 18.58 | 2.32 | 20.64 | 1.95 |
| C18:1 c9 | BC 2064 | 9.64 | F 1835 | 254.73 | B 2419* | 100.4 | F 2068* | 57.81 | C 1761.9 | 255.4 | FG 1453 | 233.8 | A 3214 | 234.9 | E 2934 | 137.6 |
| C18:1 c11 | BC 85.76 | 0.80 | FG 77.04 | 10.55 | B 99.99* | 4.19 | F 86.14* | 3.29 | C 72.45 | 9.30 | G 61.74 | 9.20 | A 132.6 | 7.92 | E 124.9 | 6.46 |
| C20:1 c7 | B < LOQ | n.a | F < LOQ | n.a | A 7.22* | 0.61 | E 5.77 | 0.12 | A 5.55* | 0.78 | F 3.98* | 0.14 | A 7.30 | 0.77 | E 6.28 | 0.06 |
| C20:1 c9 | C 11.06 | 0.10 | G 10.12 | 1.38 | B 17.42* | 0.66 | F 14.74 | 0.28 | C 12.60 | 1.72 | G 10.27 | 1.26 | A 20.62 | 1.41 | E 19.23 | 0.79 |
| PUFA | A 79.59 | 2.800 | 69.22 | 13.37 | B 226.47 | 9.55 | C 187.71 | 12.72 | 161.2 | 21.7 | 133.3 | 20.38 | 202.3 | 16.88 | 184.0 | 16.89 |
| C18:2 c9c12 | 4.48 | 1.25 | 4.27 | 1.16 | 4.62 | 0.35 | 4.54 | 1.49 | 4.24 | 0.80 | 4.10 | 0.99 | 5.85 | 1.43 | 5.77 | 1.36 |
| C18:2 c9t11 | C 8.12 | 0.35 | G 6.80 | 1.41 | A 56.30 | 3.75 | E 45.92* | 2.05 | B 45.06* | 4.62 | F 29.83* | 0.53 | AB 52.96* | 1.39 | E 42.79* | 3.99 |
| C18:2 c11t13 | B 8.79 | 0.20 | F 7.56 | 1.32 | A 12.88 | 0.51 | EF 10.00* | 0.86 | B 8.02 | 1.98 | F 7.86 | 2.03 | A 11.96 | 1.70 | E 12.03 | 0.92 |
| C18:2 t10c12 | C 8.22 | 0.44 | 7.26 | 1.45 | A 55.98 | 4.08 | E 46.25* | 1.53 | B 44.65* | 3.98 | F 30.65* | 0.41 | AB 51.90* | 1.48 | E 43.07* | 4.25 |
| C18:2 CLA tt | C 49.98 | 0.56 | F 43.33 | 8.03 | A 96.69 | 0.86 | E 81.00* | 6.79 | BC 59.23 | 10.32 | EF 60.83 | 16.42 | AB 79.66 | 10.88 | E 80.36 | 6.37 |
| μg mL−1 | A 5514.2 | 279.7 | E 5513 | 540.5 | E 4999 | 287.8 | EF 5154 | 421.0 | B 4036 | 493.9 | F 4082 | 610.3 | A 6159 | 597.3 | E 6507 | 284.9 |
| Time (h) | Empty SLN | CSLN_RA | Sage | Savoury | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 24 | 8 | 24 | 8 | 24 | 8 | 24 | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| (c: cis double bond; t: trans double bond; SFAs: saturated fatty acids; MUFAs: monounsaturated fatty acids; PUFAs: polyunsaturated fatty acids). *Values within a column for significant differences during 8 vs. 24 h; A, B, C, D, E, F and G within a row for significant differences among groups. n.a – not applicable. | ||||||||||||||||
| SFA | A 464.58 | 40.59 | 694.14 | 288.7 | A 365.1 | 39.2 | 749.3 | 177.7 | 395.9 | 66.03 | 770.2 | 158.3 | 399.6 | 142.08 | C 944.7 | 210.3 |
| C4 | 79.52* | 3.63 | 415.18* | 82.83 | 63.30* | 21.41 | 491.6* | 129.9 | 91.30 | 39.74 | 504.3 | 99.57 | 130.0* | 51.20 | 617.8* | 156.1 |
| C6 | 4.14* | 0.78 | 12.48* | 3.24 | 3.93 | 0.32 | 8.02 | 4.94 | 3.85 | 0.02 | 9.81 | 7.93 | 4.09 | 0.90 | 14.35 | 7.31 |
| C7 | B < LOD | n.a | <LOQ | n.a | A 3.81 | 0.15 | <LOQ | n.a | A 3.77 | 0.12 | 3.70 | 0.01 | A < LOQ | n.a | <LOQ | n.a |
| C8 | <LOQ | n.a | <LOQ | n.a | 3.75 | 0.05 | <LOQ | n.a | 3.79 | 0.02 | <LOQ | n.a | <LOQ | n.a | <LOQ | n.a |
| C12 | B < LOQ | n.a | <LOQ | n.a | B 3.70 | 0.01 | <LOQ | n.a | B 3.72 | 0.11 | <LOQ | n.a | A 5.59 | 1.06 | 6.62 | 1.00 |
| C14 | 13.28 | 0.54 | 10.45 | 5.85 | 11.29 | 0.72 | 10.02 | 0.83 | 10.04 | 1.01 | 8.91 | 0.64 | 10.88 | 3.60 | 12.26 | 0.19 |
| C16 | 189.6 | 2.69 | 140.6 | 84.98 | 159.9 | 6.77 | 138.0 | 15.12 | 151.8 | 10.74 | 127.9 | 16.63 | 144.87 | 49.26 | 158.21 | 5.09 |
| C17i | <LOQ | n.a | <LOQ | n.a | 3.78 | 0.15 | <LOQ | n.a | 3.70 | 0.02 | <LOQ | n.a | <LOQ | n.a | <LOQ | n.a |
| C17 | <LOQ | n.a | <LOQ | n.a | <LOQ | n.a | <LOQ | n.a | 3.86 | 0.15 | <LOQ | n.a | <LOQ | n.a | <LOQ | n.a |
| C18 | 66.28 | 4.92 | 48.70 | 37.18 | 57.27 | 5.48 | 49.60 | 11.69 | 58.07 | 9.51 | 47.30 | 12.38 | 52.77 | 20.94 | 58.64 | 9.36 |
| C20 | A 20.14 | 1.60 | 13.53 | 11.46 | B 12.29 | 0.50 | 11.19 | 2.01 | B 13.60 | 0.77 | 13.07 | 3.06 | B 12.06 | 3.58 | 14.48 | 3.69 |
| C22 | A 16.37 | 2.70 | 9.83 | 10.46 | B 8.33 | 0.71 | 7.94 | 2.04 | B 9.57 | 0.27 | 10.05 | 2.88 | B 8.24 | 2.17 | 11.23 | 4.41 |
| C24 | 39.80 | 9.82 | 23.20 | 28.90 | 17.84 | 1.46 | 17.45 | 5.69 | 21.44 | 1.49 | 24.93 | 8.26 | 17.49 | 4.70 | 28.00 | 12.69 |
| C26 | A 18.21 | 6.33 | 9.70 | 12.70 | B 8.27 | 0.69 | 7.42 | 2.66 | AB 9.03 | 0.96 | 9.88 | 3.41 | B 7.28 | 2.33 | 11.47 | 5.23 |
| C28 | 17.24 | 7.58 | 10.47 | 11.13 | 7.64 | 0.78 | 8.02 | 2.81 | 8.40 | 1.10 | 10.30 | 3.54 | 6.34 | 2.34 | 11.91 | 5.24 |
| MUFA | 2664.14 | 92.42 | 2447.48 | 208.57 | 2242.7 | 102.08 | B 1913.98 | 223.33 | 2081.65 | 63.31 | A 1766.12 | 225.69 | 2005.12 | 394.76 | 2188.58 | 123.99 |
| C14:1 c9 | 3.85 | 0.10 | 3.82 | 0.41 | 3.86 | 0.15 | <LOQ | n.a | 3.84 | 0.06 | <LOQ | n.a | <LOQ | n.a | <LOQ | n.a |
| C16:1 t9 | A 4.87 | 0.29 | 4.33 | 0.86 | C < LOD | n.a | <LOD | n.a | B 3.78 | 0.22 | <LOQ | n.a | C < LOD | n.a | <LOD | n.a |
| C16:1 c7 | 5.48 | 0.17 | 4.67 | 1.40 | 4.65 | 0.23 | 4.32 | 0.59 | 4.65 | 0.46 | 4.01 | 0.50 | 4.30 | 1.51 | 4.90 | 0.15 |
| C16:1 c9 | 44.44 | 1.43 | 32.85 | 20.00 | 39.37 | 1.67 | 33.99 | 4.40 | 36.94 | 0.97 | 31.46 | 4.26 | 35.53 | 12.55 | 39.09 | 2.22 |
| C17:1 c10 | 7.88 | 0.18 | 6.79 | 3.01 | 6.85 | 0.13 | 7.37 | 0.35 | 6.85 | 0.27 | 6.70 | 0.25 | 6.49 | 2.19 | 7.69 | 0.18 |
| C18:1 t9 | A 242.97 | 16.98 | 234.3 | 7.51 | B 71.72 | 5.17 | 66.12 | 7.62 | B 77.55 | 3.33 | 71.05 | 7.42 | B 69.39 | 24.92 | 79.40 | 0.75 |
| C18:1 t10 | A 19.71 | 0.87 | 19.59 | 2.44 | B 11.13 | 1.54 | 11.35 | 3.21 | B 11.04 | 2.76 | 11.01 | 3.59 | B 9.27 | 4.02 | 11.86 | 3.15 |
| C18:1 c9 | 2224 | 69.84 | E 2040 | 160.1 | 2004 | 89.31 | EF 1702 | 197.3 | 1841 | 51.74 | F 1559 | 199.4 | 1787 | 318.1 | EF 1943 | 112.0 |
| C18:1 c11 | 92.74 | 2.40 | 87.24 | 6.31 | 82.24 | 3.32 | 73.33 | 8.15 | 77.38 | 3.01 | 67.77 | 8.35 | 75.20 | 25.54 | 83.79 | 4.44 |
| C20:1 c7 | 4.42* | 0.12 | F 3.83* | 0.35 | 5.44* | 0.30 | EF 4.04* | 0.43 | 5.53 | 0.07 | EF 4.13 | 0.50 | 5.27 | 1.51 | E 5.09 | 0.27 |
| C20:1 c9 | 13.78 | 0.04 | 10.06 | 6.18 | 13.44 | 0.26 | 11.46 | 1.28 | 13.09 | 0.42 | 10.99 | 1.42 | 12.67 | 4.42 | 13.76 | 0.83 |
| PUFA | 168.99 | 9.73 | 145.46 | 36.4 | 161.54 | 22.42 | 134.45 | 24.51 | 166.82 | 15.08 | 139.71 | 21.61 | 156.41 | 55.12 | 170.14 | 10.46 |
| C18:2 c9c12 | 5.92 | 1.32 | 4.83 | 2.73 | 5.00 | 1.65 | 4.74 | 1.62 | 5.52 | 1.77 | 4.70 | 1.90 | 5.17 | 2.15 | 5.64 | 1.67 |
| C18:2 c9t11 | 23.24 | 1.42 | F 16.30 | 9.76 | 38.06* | 3.77 | EF 25.58* | 2.71 | 39.10 | 2.27 | E 29.27 | 2.27 | 41.57 | 13.23 | E 36.75 | 2.17 |
| C18:2 c11t13 | 15.25 | 0.97 | 11.91 | 7.33 | 9.98 | 1.79 | 9.58 | 2.50 | 10.14 | 1.50 | 9.01 | 2.03 | 8.15 | 3.08 | 10.88 | 0.77 |
| C18:2 t10c12 | 23.88 | 1.51 | F 16.63 | 9.93 | 38.22* | 4.50 | EF 26.45* | 3.10 | 39.53 | 2.18 | E 29.69 | 2.23 | 41.55 | 13.50 | E 37.22 | 2.25 |
| C18:2 CLA tt | A 100.7 | 4.51 | 95.79 | 6.65 | AB 70.28 | 10.71 | 68.10 | 14.58 | AB 72.53 | 7.36 | 67.04 | 13.18 | B 59.97 | 23.16 | 79.65 | 3.60 |
| μg mL−1 | 3313 | 58.03 | 3304 | 531.0 | 2774 | 95.49 | 2819 | 403.4 | 2645 | 57.57 | 2697 | 342.2 | 2580 | 839.3 | 3322 | 330.3 |
3.3.2.1. Short chain fatty acids. Short-chain fatty acids, also referred to as volatile fatty acids, are fatty acids with an aliphatic tail of less than 6 C atoms. In all faeces samples an increase in SCFAs (≤6 carbons, C6) is observed at 24 h of fermentation (Tables 5 and 6). Higher concentrations were detected in faeces loaded with SLNs (e.g. with savoury) rather than without. During fermentation, the fatty acids with medium and longer chains, i.e. MCFAs and LCFAs, were metabolized producing SCFAs. The SLN_Sage and SLN_Savoury showed a higher concentration of SCFAs at 8 h than the remaining SLNs (P < 0.05).
In Fig. 2 is represented the evolution of the SCFAs during the time of fermentation. Short chain fatty acids are produced when dietary fibre is fermented in the colon. In the present study, no dietary fibre was added with the intention to evaluate the metabolic activity of gut bacteria only in phenolic compound extracts and SLNs.
Fig. 3 shows the increment of acids produced during fermentation, and values are expressed as the increments of acid concentrations relative to the control samples. In normal conditions, SCFAs produced in higher concentrations are propionate, acetate and butyrate.33 In the present work, these acids were not the ones produced in higher concentrations, indicating changes in gut microbiota metabolic activity. Instead, significant concentrations of acetate, lactate and formate were found, and no succinate was detected. This result is important, since succinate is involved in several intestinal disturbances, such as impact in large bowel lumen pH and as an inducer of bacterial low motility, diarrhoeal inducer and others.34
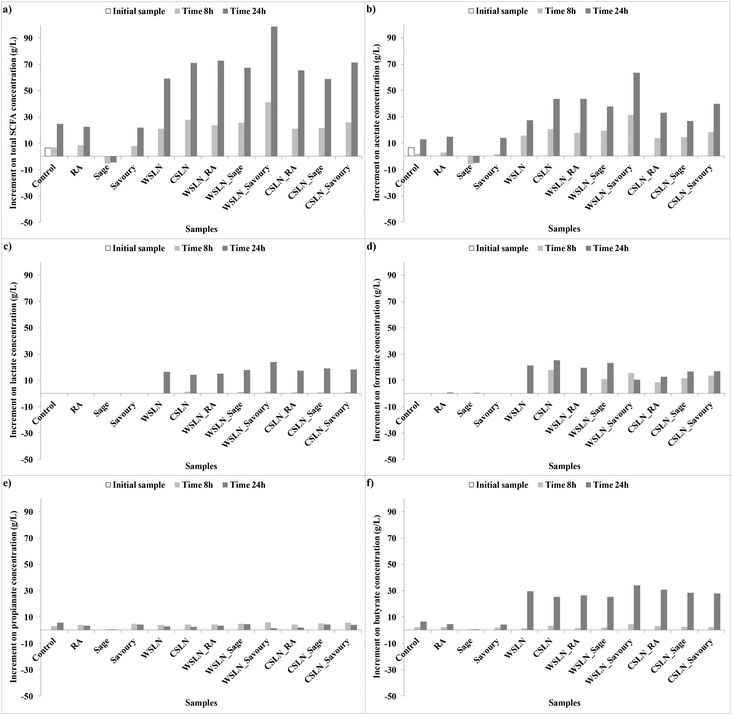 | ||
| Fig. 3 Increment of short chain fatty acid (SCFA) concentrations (g L−1) relative to the control faeces samples during fermentation. | ||
In general, in faeces with free compounds, the production of SCFAs was lower, or even null, than in samples with loaded SLNs. Samples with RA showed a significant increase in total SCFAs until the end of the fermentation time, with acetate, formate or butyrate as the major contributors. Samples with free extracts, sage and savoury also presented low concentrations of SCFAs. Faeces fermented with sage have negligible concentrations of SCFAs, while with savoury a significant production of acetate, formate and butyrate was observed. Production of these compounds in faeces with RA and savoury can be attributed mainly to the Bacteroidetes and Lactobacillus groups which are the gut microbiota groups that suffer higher increases in growth numbers.
In faeces with SLNs the production of SCFAs was significant. For the total SFCAs an increase in production was observed in faeces with SLNs (P < 0.05), especially for WSLN_Savoury. Lactate and formate (Fig. 2ac and d) were only produced in samples with SLNs, and the first one was only produced after 24 h of fermentation. These SCFAs can be the result of major length fatty acids metabolic activity by gut bacteria. Acetate and butyrate were the acids with higher expressions (Fig. 2b and f), in contrast with propionate (Fig. 2e), and in the case of butyrate the production was more expressive after 24 h. Careful attention has to be given to the high concentration of lactate, since this SCFA has been reported at high concentrations in faeces from individuals who have undergone gut resections (short bowel syndrome) or that suffer from ulcerative colitis.35 Nevertheless, the abundance of this fatty acid is positive as an intermediate for production of butyrate, a beneficial fatty acid.36–38 Butyrate was detected at high concentrations by the two techniques employed, GC and HPLC (Tables 5 and 6), especially for WSLN_Savoury. This SCFA is important for colon health as it is the primary37 energy source for colonic cells, anti-carcinogenic as well as anti-inflammatory properties.39,40 In addition, it stimulates motility, it activates propulsive ileal motor patterns in humans and ensures that bacteria are propelled from the ileum to the colon.41 The Roseburia bacterial group is known to be one of the most important butyrate producers.37 Even with a negative growth of Roseburia hominis in faeces in all samples (except for the control), the level of this acid increased.
Correlation between the bacterial growth and the production of fatty acids was observed. As an example, acetate is produced in high concentrations in all samples, except in samples with sage extract (Fig. 2b). Bacteroidetes and Bacteroides are the main produces of acetate,34 contributing to the production. Also, Firmicutes phyla are indicated as the major producers of butyrate.34,37 This phylum is positively correlated with acetate, lactate and butyrate acids (R = 0.495, R = 0.647, R = 0.597, P < 0.001). This significant and positive correlation means that this group growth number is correlated with an increase of concentration of these acids. In addition, the F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratio is high after 24 h of fermentation for faeces samples where butyrate is found at higher concentrations, i.e. SLNs.
B ratio is high after 24 h of fermentation for faeces samples where butyrate is found at higher concentrations, i.e. SLNs.
3.3.2.2. Medium chain fatty acids. Medium chain fatty acids (MCFAs) are acids with aliphatic tails with 6–12 carbons. Samples with WSLNs have a higher concentration of fatty acids with 12 carbons (C12) such as lauric acid (C12:0, dodecanoic acid), than the ones with CSLNs. This observation results from the composition of Witepsol® wax which is rich in C12 fatty acids (Table 4). This fatty acid does not suffer any change during fermentation. Medium chain triglycerides are generally considered a good biologically inert source of energy that the human body easily metabolizes. Some of them when ingested are quickly metabolized by saliva and stomach enzymes, without needing pancreatic enzymes. Nevertheless, the maintenance of concentrations during fermentation, suggest that for some reason this fatty acid was not metabolized during fermentation or that long chain fatty acids (LCFAs) were metabolized and gave raise to MCFAs. Short chain fatty acids and MCFAs are primarily absorbed through the portal vein during lipid digestion, while long chain fatty acids (LCFAs) are packed into chylomicrons and enter lymphatic capillaries, entering the blood stream first at the subclavian vein.42,43
3.3.2.3. Long chain fatty acids. Long chain fatty acids are fatty acids with aliphatic tails of 13–21 carbons. Very long chain fatty acids (VLCFAs) are fatty acids with aliphatic tails longer than 22 carbons. In samples with WSLNs, a high concentration of acids with 14 and 16 C is observed. One fatty acid of interest is myristoletic acid (C14:1 c9, 9-tetradecenoic acid), an omega-5 fatty acid, biosynthesized from myristic acid by the enzyme delta-9 desaturase, but uncommon in nature. This fatty acid, resembles lauric acid and the concentrations are maintained in samples with both type of SLNs (Tables 4 and 5). This confirms that LCFAs were metabolized and generate fatty acids such as those with 12 C, since carnauba wax is not composed of these fatty acids and during the fermentation process these fatty acids are produced (Table 4). Palmitoleic acid (C16:1 c9, (9Z)-hexadec-9-enoic acid) also appears in high concentrations in samples with WSLNs. This fatty acid is an omega-7 MUFA, a common constituent of the glycerides of human adipose tissues, and biosynthesized from palmitic acid by the action of the enzyme delta-9 desaturase. These acids are called beneficial and have shown to increase insulin sensitivity by suppressing inflammation, as well as to inhibit the destruction of insulin-secreting pancreatic beta cells.
A decrease in the concentration of fatty acids with more than 16 C until the end of fermentation is observed in all samples, in particular C18 fatty acids such as oleic acid (C18:1 cis-9, MUFA omega-9), which is present at the highest concentration of all (ca. 2000 μg mL−1) and elaidic acid (C18:1 t9) (ca. 200–400 μg mL−1), the trans fatty acid isomer of oleic acid. In fact, in samples with loaded SLNs, oleic acid is the one that suffers more metabolic activity by gut bacteria, achieving concentrations of ca. 70 μg mL−1. The presence of phenolic compounds entrapped in SLNs induces an increase in the metabolic activity by gut bacteria on this fatty acid. Trans fatty acid intake is associated with a risk factor for metabolic diseases, such as diabetes mellitus and coronary heart disease, associated with systemic or localized inflammation and increased plasma levels of several pro-inflammatory cytokines, C-reactive protein, triglycerides and low-density lipoprotein (LDL) cholesterol.44 Hence, the intake of phenolic compounds loaded in SLNs can be beneficial by promoting the metabolism and decrease of these specific fatty acids in the gut.
Other C18:1 fatty acids detected are called essential fatty acid, that are required by the human body and must be obtained from food. There are two types of essential fatty acids: one has a double bond 3 carbon atom removed from the methyl end; the other has a double bond six carbon atom removed from the methyl end. Humans lack the ability to introduce double bonds in fatty acids beyond carbons 9 and 10, as counted from the carboxylic acid side.45 SLNs provide these essential fatty acids, viz. linoleic acid (C18:2 c9c12), and are not metabolized during the fermentation process.
With high expression in samples with CSLNs are the following fatty acids, C18:2 c9t11, C18:2 c11t13, C18:2 t10c12, C18:2 CLA tt, the first a conjugated linolenic acid (CLA) or rumenic acid, and the other three CLA isomers. CLA (C18:2 c9t11) and isomers are present in higher concentrations in samples with CSLNs than WSLNs, especially in those loaded with RA and herbal extracts compared to the empty ones. Again the presence of phenolic compounds induces the production of these beneficial fatty acids. In fact, CLA inhibits the growth of several cancers and is anti-atherosclerotic, whereas the cis-9 and trans-11 forms of CLA can reduce the risk for cardiovascular disease and help fight inflammation.46
Fatty acids with 20 C are called arachidic acids (C20, eicosanoic acid) and are present in higher concentrations in CSLNs, and suffer a significant decrease during fermentation (P < 0.05). Long chain fatty acids include the ones with more than 22 C and are present in higher concentrations in CSLNs, and suffer a considerable decrease during fermentation, without any differences between the empty and loaded ones.
4. Conclusions
The evaluation of the effect of SLNs loaded with phenolic compounds, pure and from herbal extracts, on human gut microbiota viability and metabolic activity was performed for the first time. In general, SLNs do not exert any toxic effect in gut microbiota. Human gut bacteria are able to maintain or even grow in the presence of these nanoparticles, when these are loaded with pure RA or herbal extracts and even without the presence of a carbohydrate source to use as a substrate. The only toxic effect in all groups was observed for the empty nanoparticles. In addition, the Roseburia hominis was affected by the addition of all the samples tested.The most expressive bacterial growth was observed in samples fermented with SLNs loaded with the herbal extracts. The herbal extract phenolic compounds that were released by the SLNs showed a beneficial effect on gut bacteria growth and were used as a substrate in contrast with free RA. This growth is associated with the release of non-inhibitory and prebiotic concentrations of phenolic compounds, as in the case of Bifidobacterium. The presence of phenolic compounds also induces the metabolic activity of potential toxic and dangerous PUFAs and trans fatty acids, the production of less toxic SFAs and potentially beneficial CLA isomers. In terms of SCFAs, considerable amounts of acetate, lactate, formate and butyrate are produced, and low concentrations of propionate are produced. The impact of using different waxes in the production of SLNs was mainly observed in the fatty acids resulting from the microbiota metabolic activity. These results will help to predict the effects on human gut microbiota, when using these ingredients in oral formulations.
Conflict of Interest
There are no conflict of interests.Acknowledgements
Authors acknowledge the FCT (Fundação para a Ciência e Tecnologia) for funding research work through project NANODAIRY (PTDC/AGR-ALI/117808/2010) and the National Funds from FCT through project PEst-OE/EQB/LA0016/2013 and PTDC/AGR-TEC/2227/2012. This work was financed by the European Regional Development Fund (ERDF) through the Programa Operacional Factores de Competitividade – COMPETE, the Portuguese funds through FCT, in the framework of the project PEst-C/SAU/LA0002/2013, and co-financed by the North Portugal Regional Operational Programme (ON.2 – O Novo Norte) in the framework of project SAESCTN-PIIC&DT/2011, under the National Strategic Reference Framework (NSRF). Author Ana Raquel Madureira acknowledges the FCT for the post-doctoral scholarship SFRH/BPD/71391/2010 and Cláudia Marques acknowledges the FCT for the doctoral scholarship SFRH/BD/93073/2013.References
- D. A. Campos, A. R. Madureira, A. M. Gomes, B. Sarmento and M. M. Pintado, Colloids Surf., B, 2014, 115, 109–117 CrossRef CAS PubMed.
- A. R. Madureira, D. A. Campos, P. Fonte, S. Nunes, F. Reis, A. M. Gomes, B. Sarmento and M. M. Pintado, RSC Adv., 2015, 5, 22665–22673 RSC.
- M. S. Gião, C. I. Pereira, S. C. Fonseca, M. E. Pintado and F. X. Malcata, Food Chem., 2009, 117, 412–416 CrossRef.
- M. S. Giao, D. Pestana, A. Faria, J. T. Guimaraes, M. E. Pintado, C. Calhau, I. Azevedo and F. X. Malcata, J. Med. Food, 2010, 13, 131–136 CrossRef CAS PubMed.
- M. Chiumarelli and M. D. Ferreira, Hortic. Bras., 2006, 24, 381–385 Search PubMed.
- M. Chiumarelli, L. M. Pereira, C. C. Ferrari, C. I. Sarantopoulos and M. D. Hubinger, J. Food Sci., 2010, 75, E297–E304 CrossRef CAS PubMed.
- L. C. Garcia, L. M. Pereira, C. I. de Luca Sarantópoulos and M. D. Hubinger, Food Bioprocess Technol., 2010, 3, 834–842 CrossRef CAS.
- C. F. da Silva, P. Severino, F. Martins, M. V. Chaud and M. H. A. Santana, J. Microencapsulation, 2009, 26, 523–528 CrossRef PubMed.
- M. V. Selma, J. C. Espin and F. A. Tomas-Barberan, J. Agric. Food Chem., 2009, 57, 6485–6501 CrossRef CAS PubMed.
- F. Fava, J. Lovegrove, R. Gitau, K. Jackson and K. Tuohy, Curr. Med. Chem., 2006, 13, 3005–3021 CrossRef CAS PubMed.
- C. Grootaert, M. Marzorati, P. Van den Abbeele, T. Van de Wiele and S. Possemiers, Benefic. Microbes, 2011, 2, 305–318 CrossRef CAS PubMed.
- I. L. Bergin and F. A. Witzmann, Int. J. Biomed. Nanosci. Nanotechnol., 2013, 3, 163–210 CrossRef CAS PubMed.
- V. B. Young, Curr. Opin. Gastroenterol., 2012, 28, 63–69 CrossRef CAS PubMed.
- T. B. De Gregoris, N. Aldred, A. S. Clare and J. G. Burgess, J. Microbiol. Methods, 2011, 86, 351–356 CrossRef PubMed.
- M. I. Queipo-Ortuno, L. M. Seoane, M. Murri, M. Pardo, J. M. Gomez-Zumaquero, F. Cardona, F. Casanueva and F. J. Tinahones, PLoS One, 2013, 8, e65465 CAS.
- J. M. Delroisse, A. L. Boulvin, I. Parmentier, R. D. Dauphin, M. Vandenbol and D. Portetelle, Microbiol. Res., 2008, 163, 663–670 CrossRef CAS PubMed.
- N. Larsen, F. K. Vogensen, F. W. van den Berg, D. S. Nielsen, A. S. Andreasen, B. K. Pedersen, W. A. Al-Soud, S. J. Sorensen, L. H. Hansen and M. Jakobsen, PLoS One, 2010, 5, e9085 Search PubMed.
- T. Matsuki, K. Watanabe, J. Fujimoto, Y. Miyamoto, T. Takada, K. Matsumoto, H. Oyaizu and R. Tanaka, Appl. Environ. Microbiol., 2002, 68, 5445–5451 CrossRef CAS PubMed.
- T. Matsuki, K. Watanabe, J. Fujimoto, T. Takada and R. Tanaka, Appl. Environ. Microbiol., 2004, 70, 7220–7228 CrossRef CAS PubMed.
- P. Castro-Gómez, J. Fontecha and L. M. Rodríguez-Alcalá, Talanta, 2014, 128, 518–523 CrossRef PubMed.
- S. Khanna and P. K. Tosh, Mayo Clinic Proc., 2014, 89, 107–114 CrossRef CAS PubMed.
- F. Guarner and J.-R. Malagelada, Lancet, 2003, 361, 512–519 CrossRef.
- L. Yuan-Kun, Bioscience of Microbiota, Food and Health, 2013, 32, 1–12 CrossRef PubMed.
- P. B. Eckburg, E. M. Bik, C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson and D. A. Relman, Science, 2005, 308, 1635–1638 CrossRef PubMed.
- P. J. Turnbaugh, R. E. Ley, M. A. Mahowald, V. Magrini, E. R. Mardis and J. I. Gordon, Nature, 2006, 444, 1027–1131 CrossRef PubMed.
- R. E. Ley, P. J. Turnbaugh, S. Klein and J. I. Gordon, Nature, 2006, 444, 1022–1023 CrossRef CAS PubMed.
- M. Dent, V. Dragović-Uzelac, M. Penić, T. Bosiljkov and B. Levaj, Food Technol. Biotechnol., 2013, 51, 84–91 CAS.
- A. R. Madureira, A. Pereira, P. Castro and M. Pintado, J. Food Eng., 2015, 167, 210–216 CrossRef CAS.
- X. Tzounis, J. Vulevic, G. G. Kuhnle, T. George, J. Leonczak, G. R. Gibson, C. Kwik-Uribe and J. P. Spencer, Br. J. Nutr., 2008, 99, 782–792 CrossRef CAS PubMed.
- H. C. Lee, A. M. Jenner, C. S. Low and Y. K. Lee, Res. Microbiol., 2006, 157, 876–884 CrossRef CAS PubMed.
- J. Fernandes, W. Su, S. Rahat-Rozenbloom, T. Wolever and E. Comelli, Nutrition & Diabetes, 2014, 4, 1–7 Search PubMed.
- S. Kishino, M. Takeuchi, S.-B. Park, A. Hirata, N. Kitamura, J. Kunisawa, H. Kiyono, R. Iwamoto, Y. Isobe and M. Arita, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17808–17813 CrossRef CAS PubMed.
- M. Romo-Vaquero, M.-V. Selma, M. Larrosa, M. Obiol, R. García-Villalba, R. González-Barrio, N. Issaly, J. Flanagan, M. Roller and F. A. Tomás-Barberán, PLoS One, 2014, 9, 1–11 Search PubMed.
- S. Macfarlane and G. T. Macfarlane, Proc. Nutr. Soc., 2003, 62, 67–72 CrossRef CAS PubMed.
- T. Kaneko, Y. Bando, H. Kurihara, K. Satomi, K. Nonoyama and N. Matsuura, J. Clin. Microbiol., 1997, 35, 3181–3185 CAS.
- C. Bourriaud, S. Akoka, S. Goupry, R. Robins, C. Cherbut and C. Michel, Reprod., Nutr., Dev., 2002, 42, S55 CrossRef.
- P. Louis and H. J. Flint, FEMS Microbiol. Lett., 2009, 294, 1–8 CrossRef CAS PubMed.
- S. Prasanna Kumar, G. Thippeswamy, M. L. Sheela, B. T. Prabhakar and B. P. Salimath, Arch. Biochem. Biophys., 2008, 478, 85–95 CrossRef CAS PubMed.
- J. B. Greer and S. J. O'Keefe, Front. Physiol., 2010, 1, 168 Search PubMed.
- W. Scheppach, Gut, 1994, 35, S35–S38 CrossRef CAS PubMed.
- M. Simrén, G. Barbara, H. J. Flint, B. M. Spiegel, R. C. Spiller, S. Vanner, E. F. Verdu, P. J. Whorwell and E. G. Zoetendal, Gut, 2013, 62, 159–176 CrossRef PubMed.
- A. Kuksis, A. Christophe and S. D. Vriese, Fat Dig. Absorpt., 2000, 119–181 CAS.
- P. W. van der Wielen, S. Biesterveld, S. Notermans, H. Hofstra, B. A. Urlings and F. van Knapen, Appl. Environ. Microbiol., 2000, 66, 2536–2540 CrossRef CAS PubMed.
- R. Micha and D. Mozaffarian, Nat. Rev. Endocrinol., 2009, 5, 335–344 CrossRef CAS PubMed.
- S. R. Bolsover, E. A. Shephard, H. A. White and J. S. Hyams, Cell biology: a short course, John Wiley & Sons, 2011 Search PubMed.
- S. Benjamin and F. Spener, Nutr. Metab., 2009, 6, 36 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |