Direct construction of chiral quaternary dihydropyranones through highly enantioselective organocatalytic hetero-Diels–Alder reactions of olefinic azlactones†
Tai-Ping
Gao
a,
Dan
Liu
a,
Jun-Bing
Lin
a,
Xiu-Qin
Hu
a,
Zhu-Yin
Wang
b and
Peng-Fei
Xu
*a
aState Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: xupf@lzu.edu.cn
bCollege of Mechanics and Materials, Hohai University, Nanjing 210098, P. R. China
First published on 3rd March 2016
Abstract
The first organocatalytic hetero-Diels–Alder reaction of an in situ generated diene with either α-keto esters or β,γ-unsaturated α-keto esters was successfully developed. The densely functionalized dihydropyranones with a highly congested quaternary center were readily obtained in moderate to good yields with excellent enantioselectivities under mild conditions.
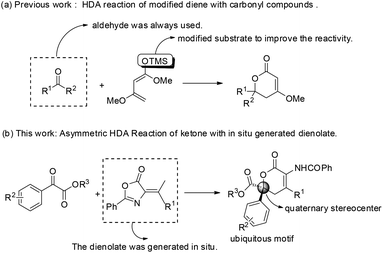
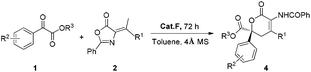
The catalytic asymmetric construction of quaternary stereocenters is one of the most challenging themes in modern organic chemistry, and therefore a great deal of effort has been devoted to this area.1 The hetero-Diels–Alder (HDA) reaction of ketones, as a very important subject for carbon–carbon bond formation, provides a convenient access to oxa-containing heterocycles bearing a quaternary stereocenter.2 Although a variety of asymmetric HDA reactions of aldehydes as dienophiles have been explored and developed, there are comparatively few reports using ketones as dienophiles in highly enantioselective HDA reactions.3 One of the major reasons is that the inherent reactivity of ketones is much lower in this kind of reaction. In addition, for the construction of chiral dihydropyranones, the traditional asymmetric HDA reactions were mainly limited to the use of pre-activated dienes such as Danishefsky-type dienes4 or Brassard-type dienes5 to enhance the reactivity (Scheme 1(a)).6 Therefore, a direct approach of the HDA reaction using catalyst-controlled reactivity is highly desired compared with the traditional approaches using substrate-influenced reactivity.
Azlactones or oxazolones7 which possess multiple reactive sites have already been proven to be powerful substrates to synthesize quaternary α-amino acids and other natural products.8 Recently, excellent progress has been made using olefinic azlactones as electron-poor acceptors by the LUMO-lowering strategy.9 However, using olefinic azlactones as carbon pronucleophiles through the HOMO-rising strategy still remains elusive. To the best of our knowledge, the vinylogous reactivity of azlactones has not been explored except that Jørgensen and co-workers first reported the organocatalyzed vinylogous Michael-type reaction between azlactones and unsaturated aldehydes via covalent aminocatalysis in 2013.10 Recently, we have reported an HDA reaction of olefinic azlactones with isatins for the construction of chiral spirooxindole dihydropyranones.11 And also recently we have reported an asymmetric vinylogous allylic–allylic alkylation reaction of MBH carbonates with olefinic azlactones by γ-position activitation.12 Despite these advances, the novel transformations utilizing the vinylogous reactivity of azlactones are highly demanding. So we developed and herein report the first highly enantioselective HDA reaction of olefinic azlactones with either α-keto esters or β,γ-unsaturated α-keto esters catalyzed by a bifunctional thiourea, delivering synthetically important dihydropyranone derivatives (Scheme 1(b)), which are ubiquitous scaffolds of many natural products possessing potent biological activities (Fig. 1).13
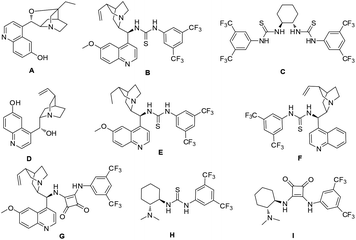
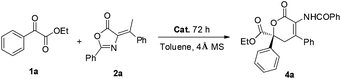
Initially, a HDA reaction of α-keto ester 1a and olefinic azlactone 2a was conducted as a model reaction to test our hypothesis and optimize the reaction parameters. Bifunctional cinchona alkaloid A (Fig. 2), the optimal catalyst in our previous similar HDA reaction of isatins and olefinic azlactones,11 was first investigated. The desired dihydropyranone 4a was obtained in good selectivity (84% ee), however, the reaction was very sluggish (72 h, 39% yield). This may be attributed to a relatively weak hydrogen-bonding interaction between catalyst A and α-keto ester 1a compared to isatin derivatives. To improve the reaction efficiency and selectivity, we further tested other commonly used bifunctional hydrogen bond donor catalysts B-I (Fig. 2). The representative quinine-thiourea B gave similar results compared to catalyst A, while the bifunctional catalysts C and D produced only a trace of the desired product (Table 1, entries 2–4). Gratifyingly, further investigation revealed that cinchona alkaloid derivatives E, F and G were promising catalysts with cinchonine derived tertiary amine-thiourea catalyst F providing the best enantioselectivity, although the yields were still unsatisfactory (entries 5–7). Takemoto's catalyst H could also be employed successfully in this HDA reaction (entry 8). Catalyst I proved to be ineffective in catalyzing this HDA reaction (Table 1, entry 9). Screening of solvents indicated that toluene was the optimal one (entries 10–15). Surprisingly, high yield was also achieved by using 100 mg of 4 Å molecular sieves (MS) (entry 16). MS could remove the trace amounts of water which might affect the hydrogen-bonding interaction between the catalyst and the substrate. Moreover, when 3.0 equivalents of 1a were used, a slightly improved yield was also obtained (entry 17).
| Entry | Cat. | Solvent | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.1 mmol), catalyst (0.02 mmol), solvent (1 mL) at rt. b Isolated yield based on 2a. c Determined by chiral HPLC analysis. d 100 mg of 4 Å MS were added. e 3.0 equiv. of 1a were used. | ||||
| 1 | A | DCM | 39 | 84 |
| 2 | B | DCM | 30 | 91 |
| 3 | C | DCM | Trace | n.d. |
| 4 | D | DCM | Trace | n.d. |
| 5 | E | DCM | 33 | 93 |
| 6 | F | DCM | 35 | 95 |
| 7 | G | DCM | 42 | 89 |
| 8 | H | DCM | 25 | 83 |
| 9 | I | DCM | Trace | n.d. |
| 10 | F | THF | 41 | 85 |
| 11 | F | CH3CN | 37 | 87 |
| 12 | F | Acetone | 53 | 80 |
| 13 | F | Toluene | 54 | 97 |
| 14 | F | Dioxane | n.r. | n.d. |
| 15 | F | Xylene | 45 | 96 |
| 16d | F | Toluene | 66 | 95 |
| 17d,e | F | Toluene | 73 | 95 |
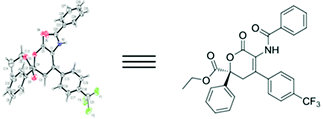
With the reaction conditions optimized, the substrate scope of the HDA reaction was then tested (Table 2). Generally, the desired products 4 were obtained in moderate to good yields (up to 83%) with excellent enantioselectivities (up to >99). Azlactones 2 with electron-donating substituents gave better yields than those with electron-withdrawing ones (entries 2–4 vs. entries 5–8). In addition, furyl and naphthyl substituents of olefinic azlactones 2 could also be tolerated in this HDA reaction, delivering the desired products 4i and 4j in moderated yields with high enantioselectivities (entries 9 and 10). With regard to the α-keto esters 1, those with electron-withdrawing substituents at the para-position of the phenyl moiety had better ee values than those with electron-donating substituents (entries 14–16 vs. entry 12). Notably, it was found that the dihalogen-substituted α-keto ester also worked very well and gave excellent enantioselectivity (entry 17). The influence of the R3 group of the benzoylformates 1 revealed that the steric effect played a crucial role in enantiocontrol, and it proved beneficial for the ee values when a bulky substituent such as the isopropyl group was used, although the yield of product 4s decreased dramatically (entries 1, 18 and 19). The structure and absolute configuration of the products 4 were unambiguously assigned by single crystal X-ray analysis of compound 4g (Fig. 3).
| Entry | R2 | R1 | R3 | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.3 mmol), 2 (0.1 mmol), catalyst F (0.02 mmol), toluene (1 mL) at rt. b Isolated yield based on 2. c Determined by chiral HPLC analysis. | |||||
| 1 | H | Ph | Et | 73(4a) | 95 |
| 2 | H | 3-MeC6H4 | Et | 79(4b) | >99 |
| 3 | H | 4-MeC6H4 | Et | 76(4c) | 95 |
| 4 | H | 4-MeOC6H4 | Et | 83(4d) | 92 |
| 5 | H | 3-BrC6H4 | Et | 62(4e) | 97 |
| 6 | H | 4-FC6H4 | Et | 66(4f) | 97 |
| 7 | H | 4-CF3C6H4 | Et | 61(4g) | 96 |
| 8 | H | 2,4-diClC6H3 | Et | 67(4h) | 98 |
| 9 | H | 2-Furyl | Et | 63(4i) | 91 |
| 10 | H | β-Naphthyl | Et | 63(4j) | 96 |
| 11 | 2-Me | Ph | Et | 72(4k) | 93 |
| 12 | 4-Me | Ph | Et | 71(4l) | 91 |
| 13 | 2-Br | Ph | Et | 62(4m) | 90 |
| 14 | 4-Br | Ph | Et | 69(4n) | 95 |
| 15 | 4-CF3 | Ph | Et | 80(4o) | 97 |
| 16 | 4-Cl | Ph | Et | 78(4p) | 96 |
| 17 | 2,4-diCl | Ph | Et | 77(4q) | >99 |
| 18 | H | Ph | Me | 70(4r) | 92 |
| 19 | H | Ph | Isopropyl | 47(4s) | 97 |
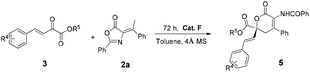
Compared with α-keto esters, β,γ-unsaturated α-keto esters were commonly used as the Michael acceptors in asymmetric synthesis. To further demonstrate the synthetic applications of the current methodology, we then explored the HDA reaction of olefinic azlactone 2a with unsaturated α-keto esters 3. When a double bond was present in the α-keto esters, the in situ generated dienolate reacted only with the carbonyl group under the standard reaction conditions. More importantly, the desired products 5 were isolated in excellent results (74–88% yields and 84–93% ee) (Table 3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | R4 | R5 | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a Reaction conditions: 3 (0.3 mmol), 2a (0.1 mmol), organocatalyst F (0.02 mmol), toluene (1 mL) at rt. b Isolated yield based on 2a. c Determined by chiral HPLC analysis. | ||||
| 1 | H | Et | 88(5a) | 89 |
| 2 | 3-Br | Et | 76(5b) | 93 |
| 3 | 4-Br | Et | 74(5c) | 91 |
| 4 | 4-Me | Et | 83(5d) | 86 |
| 5 | 4-OMe | Et | 88(5e) | 87 |
| 6 | 4-Cl | Et | 80(5f) | 93 |
| 7 | H | Me | 82(5g) | 84 |
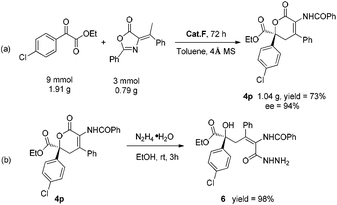
To further demonstrate the practical utility of this methodology, a gram-scale synthesis of product 4p was conducted (Scheme 2(a)). The expected product was obtained with only a slight decrease of yield while the enantiomeric excess was maintained. Hydrazinolysis of the product 4p with hydrazine hydrate afforded the ring-open product 6, a quaternary homoallyl alcohol, in quantitative yield (Scheme 2(b)).
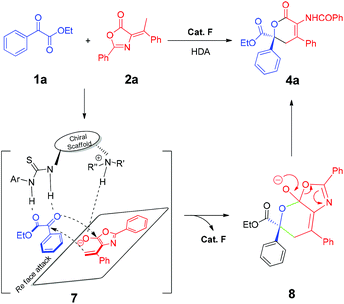
Based on the results, a possible catalytic cycle is depicted in Scheme 3. The dienolate from olefinic azlactone is generated by the γ-position deprotonation with the assistance of the catalyst. The bifunctional thiourea catalyst enabled the synergetic activation of both reaction partners to form the intermediate 7, and the in situ formed dienolate reacts with α-keto esters or β,γ-unsaturated α-keto esters in the γ-position through a way similar to the HDA reaction or a stepwise vinylogous aldol reaction by Re face attack to afford the intermediate 8, followed by tautomerization and protonation to give the desired product 4a and regenerate the catalyst.
Conclusions
In summary, we have developed an efficient asymmetric HDA reaction of olefinic azlactones with either α-keto esters or β,γ-unsaturated α-keto esters for the direct synthesis of chiral dihydropyranones using a tertiary amine-thiourea bifunctional catalyst. This also represents the first HDA reaction of keto esters with an in situ generated diene. With this protocol, a wide range of desired dihydropyranones with a quaternary stereocenter were produced in generally good yields with excellent enantioselectivities.Acknowledgements
We are grateful to the NSFC (20152087, 21372105, 21172097), the International S&T Cooperation Program of China (2013DFR70580), the National Natural Science Foundation from Gansu Province of China (no. 1204WCGA015), and the“111” program from MOE of P.R. China.Notes and references
- (a) Y. Liu, S.-J. Han, W.-B. Liu and B. M. Stoltz, Acc. Chem. Res., 2015, 48, 740 CrossRef CAS PubMed; (b) E. J. Corey and A. Guzman-Perez, Angew. Chem., Int. Ed., 1998, 37, 388 CrossRef; (c) B. M. Trost and C. Jiang, Synthesis, 2006, 369 CrossRef CAS; (d) N. A. Magnus, S. Campagna, P. N. Confalone, S. Savage, D. J. Meloni, R. E. Waltermire, R. G. Wethman and M. Yates, Org. Process Res. Dev., 2010, 14, 159 CrossRef CAS; (e) L. M. Repka and S. E. Reisman, J. Org. Chem., 2013, 78, 12314 CrossRef CAS PubMed; (f) M. Takeda, K. Takatsu, R. Shintani and T. Hayashi, J. Org. Chem., 2014, 79, 2354 CrossRef CAS PubMed; (g) I. Marek, Y. Minko, M. Pasco, T. Mejuch, N. Gilboa, H. Chechik and J. P. Das, J. Am. Chem. Soc., 2014, 136, 2682 CrossRef CAS PubMed.
- For selected reviews about HDA reactions, see: (a) K. A. Jørgensen, Angew. Chem., Int. Ed., 2000, 39, 3558 CrossRef; (b) V. Eschenbrenner-Lux, K. Kumar and H. Waldmann, Angew. Chem., Int. Ed., 2014, 53, 11146 CrossRef CAS PubMed; (c) V. Gouverneur and M. Reiter, Chem. – Eur. J., 2005, 11, 5806 CrossRef CAS PubMed; (d) B. S. Bodnar and M. J. Miller, Angew. Chem., Int. Ed., 2011, 50, 5630 CrossRef CAS PubMed.
- (a) H.-L. Cui and F. Tanaka, Chem. – Eur. J., 2013, 19, 6213 CrossRef CAS PubMed; (b) Y. Huang and V. H. Rawal, J. Am. Chem. Soc., 2002, 124, 9662 CrossRef CAS PubMed; (c) A. Landa, B. Richter, R. L. Johansen, A. Minkkilä and K. A. Jørgensen, J. Org. Chem., 2007, 72, 240 CrossRef CAS PubMed; (d) S. Yao, M. Johannsen, H. Audrain, R. G. Hazell and K. A. Jørgensen, J. Am. Chem. Soc., 1998, 120, 8599 CrossRef CAS; (e) K. Mori, T. Yamauchi, J. Maddaluno, K. Nakano, Y. Ichikawa and H. Kotsuki, Synlett, 2011, 14, 2080 Search PubMed; (f) L.-T. Shen, P.-L. Shao and S. Ye, Adv. Synth. Catal., 2011, 353, 1943 CrossRef CAS; (g) L. Zhu, C. Yu, T. Li, Y. Wang, Y. Lu, W. Wang and C. Yao, Org. Biomol. Chem., 2016, 14, 1485 RSC.
- (a) S. Danishefsky, T. Kitahara, C. F. Yan and J. Morris, J. Am. Chem. Soc., 1979, 101, 6996 CrossRef CAS; (b) L. Lin, X. Liu and X. Feng, Synlett, 2007, 2147 CAS.
- J. Savard and P. Brassard, Tetrahedron Lett., 1979, 20, 4911 CrossRef.
- (a) L. Lin, Q. Fan, B. Qin and X.-M. Feng, J. Org. Chem., 2006, 71, 4141 CrossRef CAS PubMed; (b) L. Lin, Y. Kuang, X. Liu and X.-M. Feng, Org. Lett., 2011, 13, 3868 CrossRef CAS PubMed; (c) L. Lin, Z. Chen, X. Yang, X. Liu and X.-M. Feng, Org. Lett., 2008, 10, 1311 CrossRef CAS PubMed; (d) J. Zheng, L. Lin, K. Fu, Y. Zhang, X. Liu and X. Feng, Chem. – Eur. J., 2014, 20, 14493 CAS; (e) Y. Hu, K. Xu, S. Zhang, F. Guo, Z. Zha and Z. Wang, Org. Lett., 2014, 16, 3564 CrossRef CAS PubMed; (f) Q. Fan, L. Lin, J. Liu, Y. Huang, X. Feng and G. Zhang, Org. Lett., 2004, 6, 2185 CrossRef CAS PubMed; (g) B. Zhao and T.-P. Loh, Org. Lett., 2013, 15, 2914 CrossRef CAS PubMed; (h) I.-H. Chen, K. Oisaki, M. Kanai and M. Shibasaki, Org. Lett., 2008, 10, 5151 CrossRef CAS PubMed.
- For recent reviews see: (a) A.-N. R. Alba and R. Rios, Chem. – Asian J., 2011, 6, 720 CrossRef CAS PubMed; (b) J. S. Fisk, R. A. Mosey and J. Tepe, Chem. Soc. Rev., 2007, 36, 1432 RSC; (c) R. A. Mosey, J. S. Fisk and J. J. Tepe, Tetrahedron: Asymmetry, 2008, 19, 2755 CrossRef CAS; (d) N. M. Hewlett, C. D. Hupp and J. J. Tepe, Synthesis, 2009, 2825 CAS.
- For selected representative examples, see: (a) W. Sun, G. Zhu, C. Wu, G. Li, L. Hong and R. Wang, Angew. Chem., Int. Ed., 2013, 52, 8633 CrossRef CAS PubMed; (b) B. M. Trost and L. C. Czabaniuk, Chem. – Eur. J., 2013, 19, 15210 CrossRef CAS PubMed; (c) S.-H. Shi, F.-P. Huang, P. Zhu, Z.-W. Dong and X. P. Hui, Org. Lett., 2012, 14, 2010 CrossRef CAS PubMed; (d) S. Peddibhotla and J. J. Tepe, J. Am. Chem. Soc., 2004, 126, 12776 CrossRef CAS PubMed; (e) P. Finkbeiner, N. M. Weckenmann and B. J. Nachtsheim, Org. Lett., 2014, 16, 1326 CrossRef CAS PubMed; (f) A. D. Melhado, G. W. Amarante, Z. J. Wang, M. Luparia and F. D. Toste, J. Am. Chem. Soc., 2011, 133, 3517 CrossRef CAS PubMed; (g) H. Jiang, M. W. Paixão, D. Monge and K. A. Jørgensen, J. Am. Chem. Soc., 2010, 132, 2775 CrossRef CAS PubMed.
- (a) Z.-C. Geng, N. Li, J. Chen, X.-F. Huang, B. Wu, G.-G. Liu and X.-W. Wang, Chem. Commun., 2012, 48, 4713 RSC; (b) L. Tian, G.-Q. Xu, Y.-H. Li, Y.-M. Liang and P.-F. Xu, Chem. Commun., 2014, 50, 2428 RSC; (c) M. Gonzales-Esguevillas, J. Adrio and J. C. Carretero, Chem. Commun., 2013, 49, 4649 RSC; (d) W.-Y. Han, S.-W. Li, Z.-J. Wu, X.-M. Zhang and W.-C. Yuan, Chem. – Eur. J., 2013, 19, 5551 CrossRef CAS PubMed; (e) K. S. Halskov, T. K. Johansen, R. L. Davis, M. Steurer, F. Jensen and K. A. Jørgensen, J. Am. Chem. Soc., 2012, 134, 12943 CrossRef CAS PubMed; (f) H. Jiang, B. Gschwend, Ł. Albrecht, S. G. Hansen and K. A. Jørgensen, Chem. – Eur. J., 2011, 17, 9032 CrossRef CAS PubMed; (g) J.-M. Yang, Y. Hu, Q. Li, F. Yu, J. Cao, D. Fang, Z.-B. Huang and D.-Q. Shi, ACS Comb. Sci., 2014, 16, 139 CrossRef CAS PubMed; (h) M.-Q. Zhou, J. Zuo, B.-D. Cui, J.-Q. Zhao, Y. You, M. Bai, Y.-Z. Chen, X.-M. Zhang and W.-C. Yuan, Tetrahedron, 2014, 70, 5787 CrossRef CAS; (i) B. M. Trost and P. J. Morris, Angew. Chem., Int. Ed., 2011, 50, 6167 CrossRef CAS PubMed; (j) B. M. Trost, P. J. Morris and S. J. Sprague, J. Am. Chem. Soc., 2012, 134, 17823 CrossRef CAS PubMed.
- L. D. Amico, L. Albrecht, T. Naicker, P. H. Poulsen and K. A. Jørgensen, J. Am. Chem. Soc., 2013, 135, 8063 CrossRef PubMed.
- T.-P. Gao, J.-B. Lin, X.-Q. Hu and P.-F. Xu, Chem. Commun., 2014, 50, 8934 RSC.
- S. Zhao, Y.-Y. Zhao, J.-B. Lin, T. Xie, Y.-M. Liang and P.-F. Xu, Org. Lett., 2015, 17, 3206 CrossRef CAS PubMed.
- (a) M. Carda, E. Castillo, S. Rodríguez and J. A. Marco, Tetrahedron Lett., 2000, 41, 5511 CrossRef CAS; (b) V. Boucard, G. Broustal and J. M. Campagne, Eur. J. Org. Chem., 2007, 225 CrossRef CAS; (c) G. Blay, L. Cardona, B. García, L. Lahoz and J. R. Pedro, Eur. J. Org. Chem., 2000, 2145 CrossRef CAS; (d) B. M. Trost and N. G. Andersen, J. Am. Chem. Soc., 2002, 124, 14320 CrossRef CAS PubMed; (e) H.-C. Lo, R. Entwistle, C.-J. Guo, M. Ahuja, E. Szewczyk, J.-H. Hung, Y.-M. Chiang, B. R. Oakley and C. C. C. Wang, J. Am. Chem. Soc., 2012, 134, 4709 CrossRef CAS PubMed; (f) Y. Matsuda, T. Awakawa, T. Wakimoto and I. Abe, J. Am. Chem. Soc., 2013, 135, 10962 CrossRef CAS PubMed; (g) T. M. Judge, G. Phillips, J. K. Morris, K. D. Lovasz, K. R. Romines, G. P. Luke, J. Tulinsky, J. M. Tustin, R. A. Chrusciel, L. A. Dolak, S. A. Mizsak, W. Watt, J. Morris, S. L. Vander Velde, J. W. Strohbach and R. B. Gammill, J. Am. Chem. Soc., 1997, 119, 3627 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectra for compounds 4 and 5. CCDC 1054787 (4g). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00018e |
| This journal is © the Partner Organisations 2016 |