Metal–nitrogen (Co-g-C3N4) doping of surface-modified single-walled carbon nanohorns for use as an oxygen reduction electrocatalyst
Li Denga and
Mingyuan Zhu*ab
aSchool of Chemistry and Chemical Engineering of Shihezi University, Shihezi, Xinjiang 832000, P.R. China
bKey Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, Shihezi, Xinjiang 832000, P.R. China. E-mail: zhuminyuan@shzu.edu.cn; Fax: +86 9932057210; Tel: +86 9932057270
First published on 2nd March 2016
Abstract
A cobalt-doped graphitic carbon nitride (g-C3N4) polymer was supported on surface-modified single-walled carbon nanohorns (SWCNHs) to produce a new Co-g-C3N4 catalyst for the oxygen reduction reaction (ORR). X-ray photoelectron spectroscopy results show that Co–N bonds are formed and that strong electronic coupling occurs between the SWCNHs and Co-g-C3N4. The as-fabricated catalysts exhibit reasonable ORR activity, high selectivity, and outstanding electrochemical stability in alkaline media. These results are attributed to the large number of stable Co–Nx active sites, high percentage of pyridinic N and rapid mass transport on the “Dahlia flower”-like SWCNHs surface. The fabricated Co-g-C3N4/SWCNHs catalyst has potential for use as a non-precious metal cathodic catalyst in fuel cell applications.
1. Introduction
The slow kinetics of the oxygen reduction reaction (ORR) is the main factor preventing fuel cells from becoming an efficient, environmentally friendly power source. Commercial platinum-based electrocatalysts are currently considered to be the most effective cathodic catalysts, but the large-scale commercialization of these catalysts is hindered by their high cost and inferior durability.1–3 Consequently, extensive efforts are underway to develop cheap electrocatalysts with high activity and durability as alternatives to precious metal catalysts for ORR.Of the non-precious metal electrocatalysts employed in ORR, heteroatom-doped carbon materials, especially N-doped carbon materials, are a promising alternative.4–6 These materials perform comparably to Pt/C in ORR in alkaline media because of their distinct electronic properties and structural features. Recently, graphitic carbon nitride (g-C3N4) has attracted considerable attention as a non-precious metal catalyst for ORR. In particular, its graphene-like sp2 bonding structure and the abundant in-plane nitrogen dopants and defects makes its electronic structure attractive for ORR. Nevertheless, the widespread use of g-C3N4 is limited by two problems: the poor electroconductivity and low specific surface area of bulk g-C3N4 hinder electron transport during the ORR process. Therefore, researchers have combined carbon materials (carbon black, mesoporous carbon, and graphene)7–9 to improve the electrical conductivity, which leads to greatly enhanced catalytic activity. These materials also have more active sites than other N–carbon materials. However, the specific surface areas of these different carbon matrices are still too small, and their surface smoothness leads to interactions between the active sites and carrier that are too weak; therefore, their mass activities are much lower than those of Pt-based catalysts.
Chen et al. reported the doping of g-C3N4 with transition metals. The rich pyridine-like nitrogen in g-C3N4 can trap many transition metal atoms to form potential active sites.10–12 The formation of metal–nitrogen bonds (Me–Nx) is crucial for reducing the oxygen molecule, and metal doping is thus used to enhance the ORR catalytic activity of g-C3N4. Two key factors dominate the performance of non-precious metal catalysts: (1) the interactions between different transition metals and heteroatom compositions, which control the inherent nature of the active sites, and (2) the accessible surface area and porosity, which control the number of accessible active sites and the transport of ORR-relevant species.13,14 Therefore, this work aims to synthesize catalysts with higher active sites (Me–Nx) concentrations using suitable nitrogen/non-precious metal precursors and carbon supports, thereby improving the catalyst activity and durability.
Single-walled carbon nanohorns (SWCNHs) are a new type of carbon material synthesized by the arc discharge method.15 These materials are desirable over other carbon morphologies because of their easily tunable surface area and microporosity. The most advantageous characteristic of this material is its distinct “Dahlia flower”-like morphology formed via aggregation of the angled structure. This morphology results in more defects and an open architecture, which are beneficial for supporting dispersed metal particles. More number of Me–Nx sites can be obtained by tuning the surface area and microporosity to achieve higher activity and durability without sacrificing the physical and chemical characteristics of the carbon matrix.16,17 Hence, several previous studies demonstrated the excellent electrochemical performance of SWCNHs doped with noble metals (Pt, Ru, and Se).18,19 To facilitate charge transfer/gas diffusion and increase the active site density, SWCNHs were used as a support for Co-doped g-C3N4, which was employed due to the doping effect of the transition metal Co and the good catalytic performance of g-C3N4 in ORR. Therefore, the SWCNHs and Co-g-C3N4 structures were combined to exploit their unique properties to obtain a composite material with the desired properties. The resulting Co-g-C3N4/SWCNHs material is shown to be an active, cost-effective, robust cathodic catalyst in alkaline fuel cells.
2. Experimental section
2.1. Materials
SWCNHs were purchased from XFNANO. Dicyandiamide (DCD), Co(OAc)2·4H2O, and carbon black (Vulcan XC-72R) were obtained from Cabot Corporation.2.2. Catalyst preparation
The SWCNHs were thermally treated at 400 °C for 4 h in air to remove amorphous carbon impurities and to open the nanohorns.20 The Co-g-C3N4/SWCNHs material was synthesized as follows. A preprocessed SWCNHs water suspension (100 mg, 10 mg mL−1) was treated ultrasonically for 30 min. Subsequently, a solution of DCD (200 mg) and Co(OAc)2·4H2O (30 mg) was slowly added to the suspension. The resulting mixture was mechanically stirred at 80 °C for 10 h and then dried overnight to obtain a black solid. Finally, the sample was annealed at 600 °C under flowing Ar for 5 h, and the heat-treated sample was collected.For comparison, g-C3N4/SWCNHs, g-C3N4/C, and Co-g-C3N4/C were prepared via the same process.
2.3. Catalyst characterization
Powder X-ray diffraction (XRD) data were collected using a D8 ADVANCE X-ray diffractometer (Bruker Biosciences Corporation, USA) with Cu Kα radiation (λ = 0.15406 nm) at 40 kV and 40 mA. Transmission electron microscopy (TEM) was performed using a Tecnai F30 field emission transmission electron microscope (Hillsboro, OR, USA). X-ray photoelectron spectroscopy (XPS) data were collected using an ESCA 3400 instrument (Kratos Analytical Ltd., Manchester, UK). Raman spectra analyses were performed using a Raman spectrometer (Renishaw) with a 514.3 nm Ar laser.2.4. Electrocatalytic measurements
The electrochemical properties were evaluated using an electrochemical work station (CHI760D) and rotating disk electrode (RDE) instrument with a three-electrode test cell. Ag/AgCl (saturated KCl), modified glass carbon (GC) (3 mm in diameter), and a Pt pole were used as the reference, working, and counter electrodes, respectively. The catalyst-coated working electrode was prepared as follows. Approximately 5 mg of the sample were dissolved in 1 mL of ethanol, and 50 μL of 5% Nafion were added to give a homogeneous suspension after ultrasonication for 30 min. Approximately 20 μL of the Nafion catalyst ink were loaded onto a GC electrode surface and directly dried at room temperature.Cyclic voltammetry (CV), linear sweep voltammetry (LSV), and stability experiments were performed in an N2- or O2-saturated 0.1 M KOH solution at different sweep rates.
3. Results and discussion
The TEM images of all of the SWCNHs, g-C3N4/SWCNHs, and Co-g-C3N4/SWCNHs catalysts are shown in Fig. 1. The distinct “Dahlia flower”-like morphology of the SWCNHs, which is not affected by the high-temperature treatment to produce the g-C3N4/SWCNHs and Co-g-C3N4/SWCNHs, is easily observed (Fig. 1a–d). Cobalt oxide nanoparticles are also clearly observed in Fig. 1c and d. This result conflicts with those in the literature21 and might be attributed the fact that only some of the Co atoms are into doped in g-C3N4, whereas the rest form CoOx. In order to prove that Co can be doped into g-C3N4 with excellent distribution, Co and N element mapping images were obtained and are shown in Fig. 1e–g. It was found that the Co atoms are often adjacent or close to N atoms, suggesting that Co interacts with g-C3N4 by forming Co–N bonds.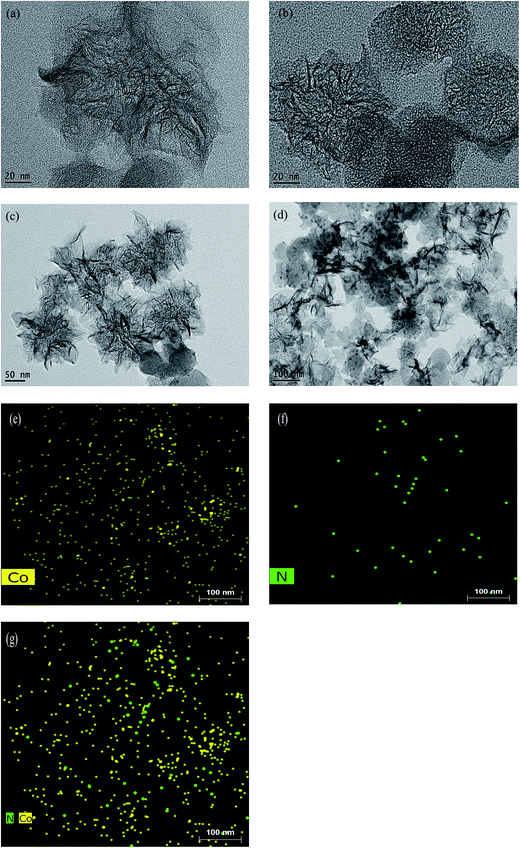 | ||
| Fig. 1 HR-TEM images of the (a) SWCNHs, (b) g-C3N4/SWCNHs, and (c and d) Co-g-C3N4/SWCNHs. (e and f) Corresponding Co and N elemental maps. (g) An overlaid Co and N elemental maps. | ||
The sample crystal structures were characterized by XRD, and the results are shown in Fig. 2a. Two obvious diffraction peaks corresponding to the characteristic peaks of g-C3N4 are observed at 27.71° and 43.63°, suggesting that g-C3N4 is present in all of the synthesized catalysts.7,22 The Raman results confirm that the Co is successfully doped into the g-C3N4 polymer. Fig. 2b shows that the ID/IG ratio for the SWCNHs, g-C3N4/SWCNHs and Co-g-C3N4/SWCNHs samples are 1.24, 1.49 and 1.75, respectively, indicating that the SWCNHs in the Co-g-C3N4/SWCHNs have the most defects. After the SWCNHs are coupled to the undoped g-C3N4, the G band hardly shifts. In contrast, when Co-g-C3N4 is supported on the SWCNHs, G band of the SWCNHs is obviously red-shifted, suggesting that electron transfer occurs between the Co species and g-C3N4 matrix.23 This result indicates that the Co species is chemically coordinated to the g-C3N4 matrix through Co–N bonds. The explicit structure of Co-g-C3N4 was shown in Fig. 2c.
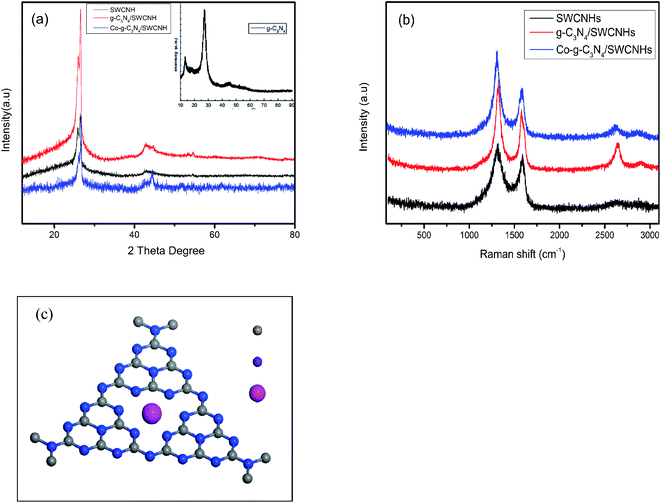 | ||
| Fig. 2 (a) XRD patterns and (b) Raman spectra of the as-prepared SWCNHs, g-C3N4/SWCNHs and Co-g-C3N4/SWCNHs catalysts. (c) The structure of the Co-g-C3N4 complex. | ||
XPS was employed to determine the catalyst chemical composition and elemental content as well as the states of the cobalt and nitrogen atoms in the prepared catalysts. In the XPS survey spectrum of the Co-g-C3N4/SWCNHs in Fig. 3a, C1s, O1s, N1s, and Co2p signals are observed, proving that C, O, N, and Co are present in the catalyst. Table 1 lists the elemental contents of the catalysts. After introducing Co into the SWCNHs, the N doping level increases from 7.17% for the g-C3N4/SWCNHs to 8.08% for the Co-g-C3N4/SWCNHs, indicating that Co and N interact. Moreover, the Co-g-C3N4/SWCNHs capture more N atoms to create more Co–Nx species, which might be beneficial for the ORR catalytic activity.
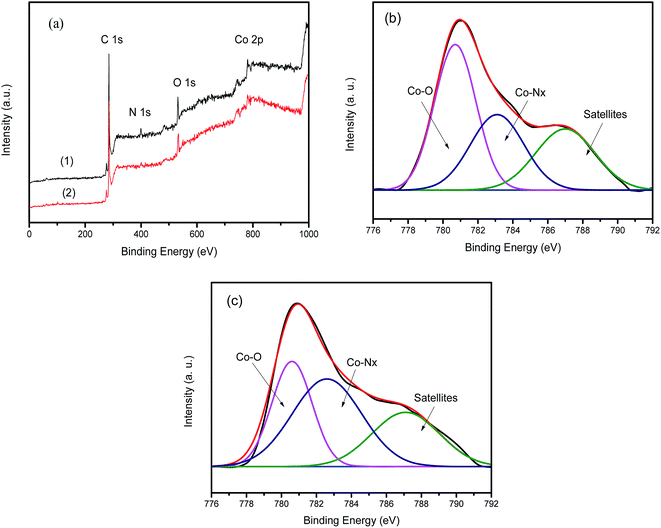 | ||
| Fig. 3 (a) XPS survey scan and (b and c) high-resolution Co spectra of the Co-g-C3N4/C and Co-g-C3N4/SWCNHs catalysts. | ||
| Catalyst | C | N | O | Co |
|---|---|---|---|---|
| g-C3N4/C | 81.89 | 6.89 | 11.22 | |
| Co-g-C3N4/C | 76.56 | 7.15 | 10.40 | 5.89 |
| g-C3N4/SWCNHs | 81.71 | 7.17 | 11.12 | |
| Co-g-C3N4/SWCNHs | 76.21 | 8.08 | 9.52 | 6.19 |
High-resolution surveys of the metal compounds demonstrate the presence of various Co species (Fig. 3b and c). The high-resolution Co2p spectra of the catalysts can be deconvoluted into two peaks with binding energies of 782 and 780.2 eV corresponding to Co–Nx and CoOx, respectively.24–26 As discussed previously, CoOx nanoparticles are observed in the TEM images in Fig. 1. These results provide further evidence that not only does Co coordinate to the g-C3N4 matrix by Co–N bonding, but it is also present in the form of CoOx in the synthesized catalysts.
The N1s spectra of all of the N-coordinated metal catalysts have four component peaks (Fig. 4), namely the pyridinic N (398.2 eV), pyrrolic N (399.6 eV), graphitic N (401.2 eV), and N oxides (404.2 eV) peaks.27–29 The percentages of the various nitrogen types are listed in Table 2. It was reported that pyridinic N, pyrrolic N and graphitic N all exhibit high ORR catalytic activities, and of these N species, pyridinic N has the highest activity.26 As shown in Table 2, the Co-g-C3N4/SWCNHs catalyst has the highest percentage of pyridinic N, indicating that it might exhibit excellent ORR catalytic activity.
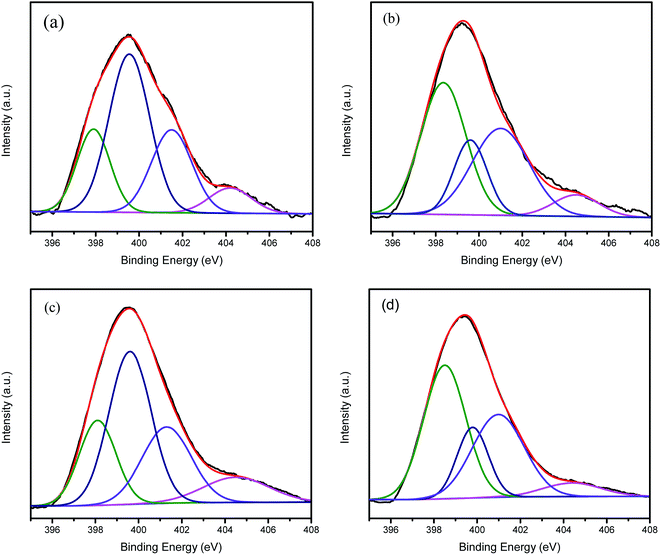 | ||
| Fig. 4 High-resolution N1s spectra for the (a) g-C3N4/C, (b) Co-g-C3N4/C, (c) g-C3N4/SWCNHs and (d) Co-g-C3N4/SWCNHs catalysts. | ||
| Catalyst | Percentage of nitrogen type | |||
|---|---|---|---|---|
| Pyridinic N | Pyrrolic N | Graphitic N | N oxides | |
| g-C3N4/C | 20.2 | 47.2 | 24.7 | 7.9 |
| Co-g-C3N4/C | 41.4 | 19.0 | 34.5 | 5.2 |
| g-C3N4/SWCNHs | 19.4 | 41.7 | 25.0 | 13.9 |
| Co-g-C3N4/SWCNHs | 43.7 | 17.0 | 33.2 | 6.1 |
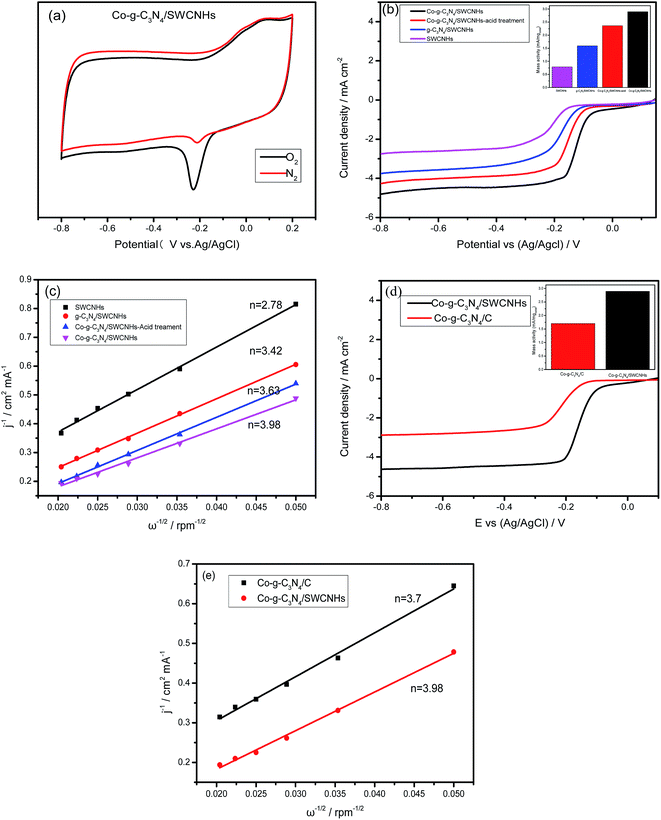
The ORR catalytic activity of the Co-g-C3N4/SWCNHs was investigated by CV. As shown in Fig. 5a, when the electrolyte solution is saturated with N2, the cathodic current in the CV has a featureless slope. In contrast, a well-defined cathodic peak appears in the CV when the electrolyte solution is saturated with O2, suggesting that the Co-g-C3N4/SWCNHs exhibit significant catalytic activity. The electrocatalytic properties of the as-prepared catalysts were compared using the RDE curves obtained in an O2-saturated 0.1 M KOH solution at a sweep rate of 5 mV s−1, rotation rate of 1600 rpm and room temperature. The corresponding Koutecky–Levich (K–L) plots were obtained by collecting the ORR polarization curves at different rotation rates. According to K–L theory,30 the electron transfer numbers (n) for a reaction can be calculated using the following equations:
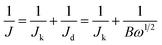 | (1) |
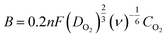 | (2) |
As shown in Fig. 5b, the half-wave potentials (E1/2) of the Co-g-C3N4/SWCNHs, g-C3N4/SWCNHs and SWCNHs are −0.157, −0.184, and −0.197 V, respectively, and their electron transfer numbers are 3.98, 3.42 and 2.78, respectively. The g-C3N4/SWCNHs exhibit higher catalytic activity than the SWCNHs, indicating that the g-C3N4 matrix has ORR active sites, which is consistent with literature results.7 However, it should be noted that both the E1/2 and n values of the Co-g-C3N4/SWCNHs are higher than those of the g-C3N4/SWCNHs, showing that the presence of Co is important for achieving high catalytic activity. As shown by the TEM and XPS results, the synthesized Co-g-C3N4/SWCNHs catalyst contains both CoOx and Co–Nx. It was reported that both of these species can act as effective ORR catalysts.31,32 To elucidate the effect of Co doping on the Co-g-C3N4/SWCNHs catalytic activity, the synthesized catalyst was treated in 0.1 M H2SO4 at 60 °C for 6 h to remove the CoOx species from the catalyst, and the ORR catalytic activity was subsequently evaluated under the same conditions. The acid-treated Co-g-C3N4/SWCNHs catalyst exhibits higher activity than the g-C3N4/SWCNHs and lower activity than the Co-g-C3N4/SWCNHs catalyst. This result indicates that doping Co into the g-C3N4 matrix can effectively enhance its ORR catalytic activity. Fig. 4d and 5b show that Co–Nx is present in the Co-g-C3N4/SWCNHs catalyst and that the percentage of pyridinic N in this catalyst is higher. Jiang et al. reported that cobalt species that form Co–Nx sites in the carbon shells are ORR active sites.32 Nonetheless, pyridinic N is more active for ORR than pyrrolic N and graphitic N. Therefore, it can be concluded that the improved catalytic activity of the Co-g-C3N4/SWCNHs catalyst is due to the presence of both Co–N bonds and pyridinic N.
To determine the effect of the SWCNHs support on the Co-g-C3N4 active species, the ORR catalytic activity of the Co-g-C3N4/SWCNHs is compared to that of the Co-g-C3N4/C catalyst in Fig. 5d. The Co-g-C3N4/SWCNHs exhibit higher ORR catalytic activity than the Co-g-C3N4/C catalyst as shown by their onset potentials (E0), half-wave potentials (E1/2), and electron transfer numbers (Fig. 5e). A comparison of these results to previous literature results shows that the onset potential (−0.066 V) of the Co-g-C3N4/SWCNHs is more positive than those of Co–N-GN (−0.098 V) and Co/PANI-PPYR (−0.13) catalysts.33,34 These results suggest that the SWCNHs act as a synergistic, conductive support with a high surface area and microporosity, which allow the creation of more metal active sites and provide the reactants with sufficient access to the active sites to enhance the charge and mass transfer. Therefore, the catalyst and support exhibit beneficial chemical interactions that improve the catalyst efficiency and prevent the loss of active sites.
The catalyst stability was tested by cycling over the potential range from −0.8 V to 0.2 V in O2-saturated 0.1 M KOH. The ORR current densities on the catalyst surface were recorded at −0.16 V after 1200 cycles (12 h). Compared to the Co-g-C3N4/C and commercial Pt/C catalysts, which exhibit a rapid current loss of approximately 20% after 4 h, the Co-g-C3N4/SWCNHs catalyst has a much more stable performance in alkaline media (Fig. 6), demonstrating that the Co-g-C3N4 and SWCNHs are strongly coupled in the Co-g-C3N4/SWCNHs catalyst.33
 | ||
| Fig. 6 Effect of the scanning cycles on the ORR current density of the Co-g-C3N4/SWCNHs, Co-g-C3N4/C and Pt/C catalysts. The current density was measured at −0.15 V (vs. Ag/AgCl). | ||
4. Conclusions
In summary, a Co-g-C3N4/SWCNHs material was synthesized via polycondensation and shown to exhibit reasonable ORR catalytic activity and outstanding electrochemical stability in alkaline media. The excellent ORR performance of this catalyst is attributed to its high surface area and microporosity, which provides the reactants with suitable access to the active sites to enhance the charge and mass transfer. Furthermore, more Co–Nx active sites are homogeneously distributed on the distinct flower-like SWCNHs structure, and the percentage of pyridinic N is higher. It is expected that Co-g-C3N4/SWCNHs can act as efficient non-precious metal catalysts and should be further developed in the future.Acknowledgements
This work was supported by the National Natural Science Funds of China (NSFC, 21366027), the Doctor Foundation of Bingtuan (2013BB010), the Young Scientific and Technological Innovation Leaders of Bingtuan (2015BC001), and the Foundation of Young Scientists at Shihezi University (No. 2013ZRKXJQ03).References
- D. Wang, H. L. Xin, R. Hovden, H. Wang, Y. Yu, D. A. Muller, F. J. DiSalvo and H. D. Abruña, Nat. Mater., 2013, 12, 81–87 CrossRef CAS PubMed.
- S. M. Alia, K. O. Jensen, B. S. Pivovar and Y. Yan, ACS Catal., 2012, 2, 858–863 CrossRef CAS.
- J.-G. Oh, H.-S. Oh, W. H. Lee and H. Kim, J. Mater. Chem., 2012, 22, 15215–15220 RSC.
- D. S. Su, S. Perathoner and G. Centi, Chem. Rev., 2013, 113, 5782–5816 CrossRef CAS PubMed.
- M. Liu, R. Zhang and W. Chen, Chem. Rev., 2014, 114, 5117–5160 CrossRef CAS PubMed.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760–764 CrossRef CAS PubMed.
- J. Liang, Y. Zheng, J. Chen, J. Liu, D. Hulicova-Jurcakova, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2012, 51, 3892–3896 CrossRef CAS PubMed.
- K. Kwon, Y. J. Sa, J. Y. Cheon and S. H. Joo, Langmuir, 2012, 28, 991–996 CrossRef CAS PubMed.
- L. Xu, W.-Q. Huang, L.-L. Wang, Z.-A. Tian, W. Hu, Y. Ma, X. Wang, A. Pan and G.-F. Huang, Chem. Mater., 2015, 27, 1612–1621 CrossRef CAS.
- X. Wang, X. Chen, A. Thomas, X. Fu and M. Antonietti, Adv. Mater., 2009, 21, 1609–1612 CrossRef CAS.
- Y. Di, X. Wang, A. Thomas and M. Antonietti, ChemCatChem, 2010, 2, 834–838 CrossRef CAS.
- X. Chen, J. Zhang, X. Fu, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 11658–11659 CrossRef CAS PubMed.
- F. Jaouen, E. Proietti, M. Lefèvre, R. Chenitz, J.-P. Dodelet, G. Wu, H. T. Chung, C. M. Johnston and P. Zelenay, Energy Environ. Sci., 2011, 4, 114–130 CAS.
- M. Lefèvre and J.-P. Dodelet, ECS Trans., 2012, 45, 35–44 CrossRef.
- N. Li, Z. Wang, K. Zhao, Z. Shi, Z. Gu and S. Xu, Carbon, 2010, 48, 1580–1585 CrossRef CAS.
- S. M. Unni, S. Ramadas, R. Illathvalappil, S. N. Bhange and S. Kurungot, J. Mater. Chem. A, 2015, 3, 4361–4367 CAS.
- L. Brandão, C. Passeira, D. M. Gattia and A. Mendes, J. Mater. Sci., 2011, 46, 7198–7205 CrossRef.
- T. Yoshitake, Y. Shimakawa, S. Kuroshima, H. Kimura, T. Ichihashi, Y. Kubo, D. Kasuya, K. Takahashi, F. Kokai and M. Yudasaka, Phys. B, 2002, 323, 124–126 CrossRef CAS.
- K. M. Eblagon and L. Brandao, J. Fuel Cell Sci. Technol., 2015, 12, 021006 CrossRef.
- L. Zhang, N. Zheng, A. Gao, C. Zhu, Z. Wang, Y. Wang, Z. Shi and Y. Liu, J. Power Sources, 2012, 220, 449–454 CrossRef CAS.
- Q. Liu and J. Zhang, Langmuir, 2013, 29, 3821–3828 CrossRef CAS PubMed.
- S. Maldonado and K. J. Stevenson, J. Phys. Chem. B, 2005, 109, 4707–4716 CrossRef CAS PubMed.
- Q. Yu, J. Xu, C. Wu and L. Guan, RSC Adv., 2015, 5, 65303–65307 RSC.
- H.-W. Liang, W. Wei, Z.-S. Wu, X. Feng and K. Müllen, J. Am. Chem. Soc., 2013, 135, 16002–16005 CrossRef CAS PubMed.
- G. Wu, Z. Chen, K. Artyushkova, F. H. Garzon and P. Zelenay, ECS Trans., 2008, 16, 159–170 CAS.
- K. Niu, B. Yang, J. Cui, J. Jin, X. Fu, Q. Zhao and J. Zhang, J. Power Sources, 2013, 243, 65–71 CrossRef CAS.
- J. Casanovas, J. M. Ricart, J. Rubio, F. Illas and J. M. Jiménez-Mateos, J. Am. Chem. Soc., 1996, 118, 8071–8076 CrossRef CAS.
- M. Vikkisk, I. Kruusenberg, U. Joost, E. Shulga, I. Kink and K. Tammeveski, Appl. Catal., B, 2014, 147, 369–376 CrossRef CAS.
- Z. Lin, G. H. Waller, Y. Liu, M. Liu and C.-p. Wong, Nano Energy, 2013, 2, 241–248 CrossRef CAS.
- P. J. Kulesza, B. Grzybowska, M. A. Malik and M. T. Galkowski, J. Electrochem. Soc., 1997, 144, 1911–1917 CrossRef CAS.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780–786 CrossRef CAS PubMed.
- Y. Jiang, Y. Lu, X. Wang, Y. Bao, W. Chen and L. Niu, Nanoscale, 2014, 6, 15066–15072 RSC.
- S. Jiang, C. Zhu and S. Dong, J. Mater. Chem. A, 2013, 1, 3593–3599 CAS.
- Q. Yi, Y. Zhang, X. Liu and Y. Yang, Sci. China: Chem., 2014, 57, 739–747 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |