Effect of head/tail groups on molecular induced aggregation of polycationic cyclodextrin towards anionic surfactants†
Rui-Juan
Shi
,
Yong
Chen
,
Xiao-Fang
Hou
and
Yu
Liu
*
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin 300071, P. R. China. E-mail: yuliu@nankai.edu.cn
First published on 29th January 2016
Abstract
The molecular induced aggregation behaviors of a polycationic cyclodextrin, i.e. per-6-deoxy-6-(1-methylimidazol-3-ium-3-yl)-β-cyclodextrin (1), towards five anionic surfactants, i.e. sodium decyl sulfonate (SDES), sodium undecyl sulfonate (SUS), sodium dodecyl sulfate (SDS), sodium lauryl sulfate (SLS) and sodium dodecyl benzene sulfonate (SDBS), were comprehensively investigated in aqueous solution. The results showed that the introduction of 1 could efficiently decrease the critical aggregation concentrations (CAC) of the selected surfactants by a factor of 14–467, leading to the formation of nanoscale spherical particles with a diameter of 200–400 nm and nearly neutral or moderate positive zeta potential. Significantly, the resultant 1/SLS and 1/SDBS aggregates exhibited the ability of loading both anionic and neutral model substrates.
Introduction
Recently, increasing attention has been paid to the construction and application of supramolecular assemblies due to their advantages of convenient preparation, controllable morphology, multi-stimuli response towards light, temperature, pH, redox, and enzymes, and wide applications in the chemistry, materials and biomedicine fields.1–4 Among the various supramolecular assemblies, supramolecular amphiphiles possessing good functional versatility as well as biocompatibility and being tunably responsive to various external stimuli, are widely regarded as very outstanding candidates to build delivery systems for biologically important matters.5–7 For this aim, various water-soluble macrocyclic molecules, including cucurbiturils,8 sulfonatocalixarenes,9 pillararene carboxylates,10 and cyclodextrins,11 were widely employed to construct supramolecular amphiphiles via the molecular induced aggregation strategy. However, most of these researches were focused on the neutral or polyanionic macrocyclic molecules, the related studies on the polycationic macrocyclic molecules are still rare. In a preliminary work, we found that the polycationic cyclodextrins derivative bearing seven methylimidazoliumyl arms could promote the molecular aggregation of anionic guest molecules by lowering the critical aggregation concentration (CAC).12 In this work, we wish to report a comparative study on the molecular induced aggregation behaviors of this positively charged β-cyclodextrin towards several anionic surfactants with different hydrophilic heads and hydrophobic tails (Scheme 1) by means of UV-vis, zeta-potential, dynamic light scattering (DLS), scanning electron microscope (SEM) and transmission electron microscopy (TEM). The obtained results will serve to increase our understanding of the factors, especially the type of head groups and the length of tail groups, governing the molecular induced aggregation behaviors of this positively charged β-cyclodextrin towards anionic molecules. Furthermore, the encapsulation ability of induced aggregates towards the anionic or neutral model substrates was also investigated.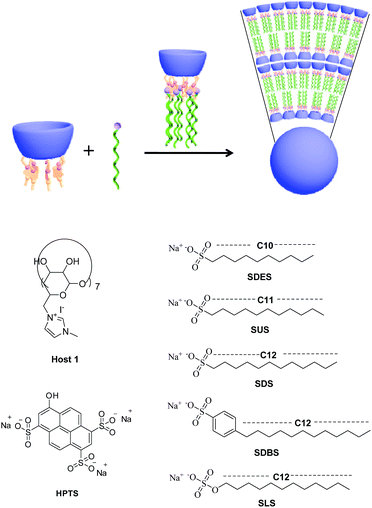 | ||
| Scheme 1 Chemical structures of hepta-imidazoliumyl-β-cyclodextrin (1), different anionic surfactants and HPTS. | ||
Results and discussion
Induced aggregation towards anionic surfactants with different hydrophobic tails
Possessing the same hydrophilic sulfonate head and different hydrophobic tails in length, sodium decyl sulfonate (SDES), sodium undecyl sulfonate (SUS) and sodium dodecyl sulfate (SDS) were able to form self-aggregate in water, and their critical aggregation concentrations (CACs) at 25 °C were measured as 39.9 mmol L−1, 20 mmol L−1 and 9.7 mmol L−1, respectively.13 However, the CAC values of these guest anionic surfactants sharply decreased with the addition of polycationic host 1, which could be quantitatively measured by observing the dependence of the optical transmittance at 450 nm on the concentration of anionic surfactants in the absence and presence of 1. Without 1, the optical transmittance of anionic surfactant at 450 nm nearly unchanged in the experimental concentration range, indicating that the anionic surfactant could not self-aggregate at these concentrations. With the addition of 1, the optical transmittance of anionic surfactants gradually decreased (Fig. 1).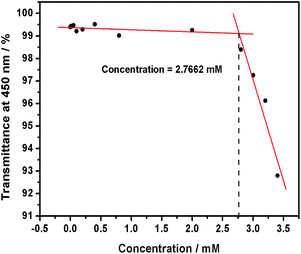 | ||
| Fig. 1 Dependence of optical transmittance of SDES at 450 nm at different concentrations in the presence of 1 (0.4 mmol L−1) at 25 °C. | ||
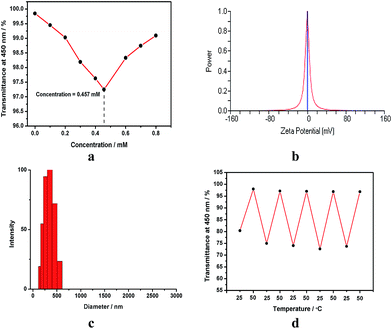
Moreover, the preferable mixing ratios between the polycationic host 1 and the selected anionic surfactants were also determined. By gradually adding 1 to the solution of SDES at a fixed concentration of 3.20 mmol L−1, the optical transmittance of resulting mixture at 450 nm decreased rapidly and then gradually increased thereafter to a quasi-plateau, and a minimum was reached at concentration of 1 at 0.457 mmol L−1 (Fig. 2a), referring to a 1/SDES ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, i.e. a imidazolium cation/sulfonate anion ratio of 1
7, i.e. a imidazolium cation/sulfonate anion ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The rapid decrease of optical transmittance indicated the formation of large aggregates between 1 and SDES. Subsequently, the further addition of an excess amount of 1 resulted in the formation of a simple inclusion complex, which led to the disassembly of the aggregates, accompanied by the decrease of turbidity. Control experiments showed that the optical transmittance of anionic surfactants at 450 nm showed no appreciable changes in the experimental concentration range in the absence of 1, indicating that no self-aggregation happened at these concentrations of anionic surfactants. Similar preferable mixing ratio of 1
1. The rapid decrease of optical transmittance indicated the formation of large aggregates between 1 and SDES. Subsequently, the further addition of an excess amount of 1 resulted in the formation of a simple inclusion complex, which led to the disassembly of the aggregates, accompanied by the decrease of turbidity. Control experiments showed that the optical transmittance of anionic surfactants at 450 nm showed no appreciable changes in the experimental concentration range in the absence of 1, indicating that no self-aggregation happened at these concentrations of anionic surfactants. Similar preferable mixing ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 was also found in the case of 1/SUS or 1/SDS system.
7 was also found in the case of 1/SUS or 1/SDS system.
The induced aggregates were characterized by characterized by Tyndall effect, zeta potential, DLS, and TEM. The solutions of 1/anionic surfactant systems exhibited the obvious Tyndall effect, clearly indicating the existence of abundant large nanoparticles. In the control experiments, no Tyndall effect was observed in the solution of free 1 or surfactant, revealing that 1 or anionic surfactant alone could not form the nano-scaled self-aggregate under the same condition. Moreover, the zeta potential of 1/SDES, 1/SUS or 1/SDS systems was measured to nearly neutral (Fig. 2b). This phenomenon manifested that the charges of both 1 and anionic surfactant were packaged inside the aggregate, and the outside surface of the aggregate should expose the uncharged wide open rim limiting the β-cyclodextrin cavity. In addition, TEM images of 1/SDES and 1/SUS aggregates gave a number of spherical particles like the reported ones of 1/SDS aggregates.12 Because there was no critical evidence to prove that such assemblies were micelles or vesicles, thus we classified them as a kind of nanoparticle. However, the average diameters of 1/surfactant aggregates measured by DLS were ca. 291 nm for 1/SDES, ca. 256 nm for 1/SUS and ca. 239 nm for 1/SDS, accompanied by a relatively narrow size distribution (Fig. 2c). Moreover, no appreciable DLS signals were observed for free 1 and surfactants at the same concentrations. Therefore, we could deduce that the polycationic host 1 tended to form the more compact nanoparticles with the anionic surfactant with a longer hydrophobic tail.
In addition, the thermal stability of these nanoparticles was also measured by monitoring the optical transmittance at different temperatures. At the room temperature, the optical transmittance of 1/surfactant aggregate at 450 nm could keep unchanged for at least 6 h. With increasing the temperature from 25 °C to 50 °C, the optical transmittance of 1/surfactant aggregate gradually enhanced, indicating the disassembly of aggregates. Thereafter, the enhanced optical transmittance nearly decreased to the original level when reducing the temperature from 50 °C to 25 °C, and these cycles could be repeated for several times (Fig. 2d), demonstrating a satisfactory temperature responsiveness of 1/surfactant aggregates. A further comparison showed that the aggregate formed by 1 and SDES, which had the shortest hydrophobic tail, presented the highest thermal stability, giving a very small changes (<2.5%) of optical transmittance when varying the temperature between 25 °C and 50 °C.
Induced aggregation towards anionic surfactants with different hydrophilic heads
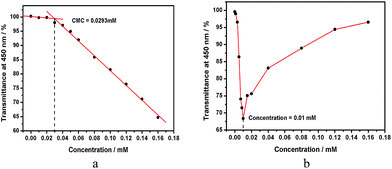
In addition to the effect of hydrophobic tail, the effect of hydrophilic head on the induced aggregation was also investigated. Therein, three anionic surfactants possessing the same C12-alkyl tail and different hydrophilic heads, i.e. sodium lauryl sulfate (SLS), sodium dodecyl benzene sulfonate (SDBS) and SDS were selected as model substrates, and their CAC values were reported to be 5.5 mmol L−1 for SLS, 14.0 mmol L−1 for SDBS and 9.7 mmol L−1 for SDS.14 However, in the presence of 1 (0.04 mmol L−1), their CAC values significantly decreased to 0.029 mmol L−1 for SLS, 0.030 mmol L−1 for SDBS and 0.14 mmol L−1 for SDS, which were respectively 190, 467 and 69 times lower than the corresponding CAC value of free surfactants, by monitoring the dependence of the optical transmittance at 450 nm on the concentration of anionic surfactants (Fig. 3a). Moreover, the preferable 1/surfactant mixing ratios were measured to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for 1/SLS, 1
6 for 1/SLS, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for 1/SDBS, and 1
6 for 1/SDBS, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 for 1/SDS (Fig. 3b), respectively. These 1/surfactant mixing ratios indicated that there would exist an excess of positive charges in 1/SLS or 1/SDBS, which was subsequently verified by the positive zeta potential of 1/SLS (+9.57 mV) or 1/SDBS (+7.01 mV).
7 for 1/SDS (Fig. 3b), respectively. These 1/surfactant mixing ratios indicated that there would exist an excess of positive charges in 1/SLS or 1/SDBS, which was subsequently verified by the positive zeta potential of 1/SLS (+9.57 mV) or 1/SDBS (+7.01 mV).
The formation of large nanoparticles was confirmed by the Tyndall effect, TEM, SEM and DLS. The solution of 1/SLS or 1/SDBS exhibited stronger Tyndall effects than that of 1/SDS. Moreover, TEM and SEM images of 1/SLS or 1/SDBS also presented a number of spherical particles with a diameter ca. 300–400 nm (Fig. 4), which was a bit larger than that of 1/SDS (100–200 nm).12 This result was also consistent with the diameter order of 1/SDBS (ca. 341) > 1/SLS (327 nm) > 1/SDS (ca. 239 nm), measured by DLS. In the control experiments, no appreciable Tyndall effect or DLS signal were observed for free 1 and anionic surfactants alone at the same concentrations. In addition, the 1/SLS or 1/SDBS aggregates also showed the good thermal stability and temperature responsiveness, like the case of 1/SDS.
Guest encapsulation
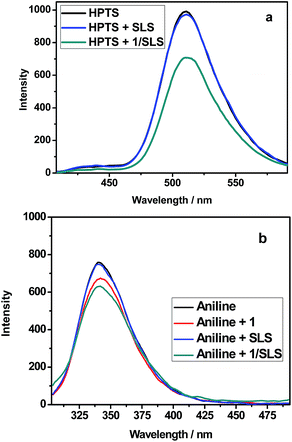
Possessing a moderate positive zeta potential, the 1/SLS or 1/SDBS aggregate may have the possibility of loading anionic guests. Herein, HPTS was selected as a model molecule, because HPTS was reported unable to be included in β-CD cavity.11e,14 As seen in Fig. 5a, the fluorescence intensity of HPTS nearly unchanged with the addition of SLS, but obviously decreased by 28% after loading HPTS to the 1/SLS aggregate. Moreover, after the anionic dye HPTS was loaded, the zeta potential of the 1/SLS aggregate decreased to −11.30 mV. The decreased fluorescence and zeta potential may indicate that HPTS was encapsulated by the 1/SLS aggregate mainly through the electrostatic interactions between the positively charged aggregate and the anionic substrate. In addition to the anionic substrates, the 1/SLS aggregate also showed the ability to load the neutral substrates. As seen in Fig. 5b, the fluorescence intensity of aniline, a neutral substrate, obviously decreased with the addition of 1/SLS aggregate, which was consistent with the fluorescence behavior of aniline in the presence of 1. The control experiment showed that either the change of ion strength or the addition of simple sulfate anion could not decrease the fluorescence intensity of aniline under the same condition. Therefore, we deduced that the decreased fluorescence of aniline may be attributed to the inclusion of aniline by the β-cyclodextrin cavity of 1. This phenomenon consequently demonstrated the capability of β-cyclodextrin cavities in the aggregate for including neutral model substrates.Conclusions
In conclusion, the effect of head/tail groups on the molecular induced aggregation of polycationic cyclodextrin towards anionic surfactants was sufficiently investigated, giving an order of ability on lowering the original CAC of anionic surfactants as SDBS > SLS > SDS > SUS > SDES as well as an order of induced aggregate diameter as 1/SDBS > 1/SLS > 1/SDES > 1/SUS > 1/SDS. That is, changing the hydrophilic head group led to the more effective host-induced aggregation than changing the hydrophobic tail group of surfactants. Significantly, the induced aggregated constructed from polycationic cyclodextrin and SLS (or SDBS) showed the ability of loading anionic and neutral substrates. On this basis, further studies on the delivery system cooperatively utilizing cyclodextrin/substrate inclusion complexation and aggregate/substrate electrostatic interactions are in progress.Experimental
Material
All aqueous solutions were prepared in distilled water. Anhydrous N,N-dimethylfomamide (DMF) was prepared by stirring commercially available DMF with calcium hydride (CaH2) over 24 h and distilled under reduced pressure. Host 1 was prepared according to the literature procedure12,15 (Scheme S1†). All of the anionic surfactants were purchased from Aladdin or Alfa Aesar. Aniline was purchased from Aladdin, and HPTSwere purchased from Sigma-Aldrich.UV-vis spectroscopy
UV-vis spectra and the optical transmittance were measured in a quartz cell (light path 10 mm) on a Shimadzu UV-3600 spectrophotometer equipped with a PTC-348WI temperature controller.Fluorescence spectroscopy
Steady-state fluorescence spectra were recorded in a conventional quartz cell (light path 10 mm) on a Varian Cary Eclipse equipped with a Varian Cary single-cell Peltier accessory to control temperature (HPTS: λex = 355 nm, bandwidth (ex) 5 nm, bandwidth (em) 5 nm; aniline: λex = 280 nm, bandwidth (ex) 10 nm, bandwidth (em) 10 nm, 25 °C).TEM
High-resolution TEM images were recorded on a Tecnai G2 F20 high-resolution transmission electron microscope operating at an accelerating voltage of 200 kV. The samples for high-resolution TEM measurements were prepared by dropping the solution onto a copper grid, and then air-dried the grid.SEM
SEM images were received from a JSM-7500F scanning electron microscope operating at an accelerating voltage of 0.1–30 kV. The samples for SEM measurements were prepared by dropping the solution onto a cover slip, followed by evaporating the liquid in air.DLS and zeta potential measurements
The sample solution for DLS measurement was prepared by filtering solution through a 200 nm Millipore filter into a clean scintillation vial. The samples were examined on a laser light scattering spectrometer (BI-200SM) equipped with a digital correlator (TurboCorr) at 636 nm at a scattering angle of 90°. Zeta potential was measured on a Zeta PALS + BI-90 instrument (Brookhaven Co. USA).Acknowledgements
We thank NNSFC (21432004, 21272125 and 91527301) for financial support.References
-
(a) M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889–896 CrossRef CAS PubMed
; (b) D. M. Vriezema, M. C. Aragonès, J. A. A. W. Elemans, J. J. L. M. Cornelissen, A. E. Rowan and R. J. M. Nolte, Chem. Rev., 2005, 105, 1445–1489 CrossRef CAS PubMed
; (c) X. Zhang and C. Wang, Chem. Soc. Rev., 2011, 40, 94–101 RSC
.
- P. Hu, Y. Chen and Y. Liu, Chem. Commun., 2015, 51, 10839–10842 RSC
.
-
(a) J. Du, Y. Tang, A. L. Lewis and S. P. Armes, J. Am. Chem. Soc., 2005, 127, 17982 CrossRef CAS PubMed
; (b) Y. Wang, N. Ma, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2823–2826 CrossRef CAS PubMed
; (c) Y. X. Wang, Y. M. Zhang and Y. Liu, J. Am. Chem. Soc., 2015, 137, 4543–4549 CrossRef CAS PubMed
; (d) Y. J. Jeon, P. K. Bharadwaj, S. Choi, J. W. Lee and K. Kim, Angew. Chem., Int. Ed., 2002, 41, 4474–4476 CrossRef CAS
; (e) P. D. Thornton, R. J. Mart and R. V. Ulijn, Adv. Mater., 2007, 19, 1252–1256 CrossRef
.
-
(a) K. R. Raghupathi, M. A. Azagarsamy and S. Thayumanavan, Chem.–Eur. J., 2011, 17, 11752–11760 CrossRef CAS PubMed
; (b) H. L. Sun, Y. Chen, J. Zhao and Y. Liu, Angew. Chem., Int. Ed., 2015, 54, 9376–9380 CrossRef CAS PubMed
.
-
(a) Y. Wang, H. Xu and X. Zhang, Adv. Mater., 2009, 21, 2849–2864 CrossRef CAS
; (b) C. Wang, Z. Wang and X. Zhang, Acc. Chem. Res., 2012, 45, 608–618 CrossRef CAS PubMed
.
-
(a) K. Wang, D. S. Guo and Y. Liu, Chem.–Eur. J., 2010, 16, 8006–8011 CrossRef CAS PubMed
; (b) K. Wang, D. S. Guo, X. Wang and Y. Liu, ACS Nano, 2011, 5, 2880–2894 CrossRef CAS PubMed
.
-
(a) Y. Chen, N. Li, Y. Yang and Y. Liu, RSC Adv., 2015, 5, 8938–8941 RSC
; (b) K. M. Cai, X. He, Z. Y. Song, Q. Yin, Y. F. Zhang, F. M. Uckun, C. Jiang and J. J. Cheng, J. Am. Chem. Soc., 2015, 137, 3458–3461 CrossRef CAS PubMed
; (c) Y. Kang, C. Wang, K. Liu, Z. Wang and X. Zhang, Langmuir, 2012, 28, 14562–14566 CrossRef CAS PubMed
.
-
(a) U. Rauwald and O. A. Scherman, Angew. Chem., Int. Ed., 2008, 47, 3950–3953 CrossRef CAS PubMed
; (b) Z. Huang, L. Yang, Y. Liu, Z. Wang, O. A. Scherman and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 5351–5355 CrossRef CAS PubMed
; (c) T. Lee, E. Kalenius, A. I. Lazar, K. I. Assaf, N. Kuhner, C. H. Grun, J. Janis, O. A. Scherman and W. M. Nau, Nat. Chem., 2013, 5, 376–382 CrossRef CAS PubMed
; (d) J. Tian, T. Y. Zhou, S. C. Zhang, S. Aloni, M. V. Altoe, S. H. Xie, H. Wang, D. W. Zhang, X. Zhao, Y. Liu and Z. T. Li, Nat. Commun., 2014, 5, 5574 CrossRef CAS PubMed
; (e) K. D. Zhang, J. Tian, D. Hanifi, Y. B. Zhang, A. C. H. Sue, T. Y. Zhou, L. Zhang, X. Zhao, Y. Liu and Z. T. Li, J. Am. Chem. Soc., 2013, 135, 17913–17918 CrossRef CAS PubMed
.
-
(a) D. S. Guo and Y. Liu, Acc. Chem. Res., 2014, 47, 1925–1934 CrossRef CAS PubMed
; (b) K. P. Wang, Y. Chen and Y. Liu, Chem. Commun., 2015, 51, 1647–1649 RSC
; (c) Z. B. Qin, D. S. Guo, X. N. Gao and Y. Liu, Soft Matter, 2014, 10, 2253–2263 RSC
.
-
(a) H. Zhang, X. Ma, K. T. Nguyen and Y. Zhao, ACS Nano, 2013, 7, 7853–7863 CrossRef CAS PubMed
; (b) Y. Cao, X. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762–10769 CrossRef CAS PubMed
; (c) Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542–10549 CrossRef CAS PubMed
; (d) J. Yang, G. Yu, D. Xia and F. Huang, Chem. Commun., 2014, 50, 3993–3995 RSC
; (e) D. Xia, G. Yu, J. Li and F. Huang, Chem. Commun., 2014, 50, 3606–3608 RSC
; (f) Y. W. Yang, Y. L. Sun and N. Song, Acc. Chem. Res., 2014, 47, 1950–1960 CrossRef CAS PubMed
.
-
(a) Y. Tang, L. P. Zhou, J. X. Li, Q. A. Luo, X. Huang, P. Wu, Y. G. Wang, J. Y. Xu, J. C. Shen and J. Q. Liu, Angew. Chem., Int. Ed., 2010, 49, 3920–3924 CrossRef CAS PubMed
; (b) X. T. Fan, L. Wang, Q. Luo, L. L. Zhao, J. Y. Xu, J. Q. Liu and Q. C. Zheng, Chem. Commun., 2015, 51, 6512–6514 RSC
; (c) Y. Wang, N. Ma, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2823–2826 CrossRef CAS PubMed
; (d) T. Bojinova, Y. Coppel, N. Lauth-de Viguerie, A. Milius, I. Rico-Lattes and A. Lattes, Langmuir, 2003, 19, 5233–5239 CrossRef CAS
; (e) X. F. Hou, Y. Chen and Y. Liu, Soft Matter, 2015, 11, 2488–2493 RSC
.
- D. Zhao, Y. Chen and Y. Liu, Chin. Chem. Lett., 2015, 26, 829–833 CrossRef CAS
.
-
(a) J. E. Bujake and E. D. Goddard, Trans. Faraday Soc., 1965, 61, 190–195 RSC
; (b) A. Chatterjee, S. P. Moulik, S. K. Sanyal, B. K. Mishra and P. M. Puri, J. Phys. Chem. B, 2001, 105, 12823–12831 CrossRef CAS
.
- K. Uekama, F. Hirayama and T. Irie, Chem. Rev., 1998, 98, 2045–2076 CrossRef CAS PubMed
.
- A. Gadelle and J. Defaye, Angew. Chem., Int. Ed., 1991, 30, 78–80 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra28043e |
| This journal is © The Royal Society of Chemistry 2016 |